Scanning Rate Extension of Conventional DSCs through Indirect Measurements
Abstract
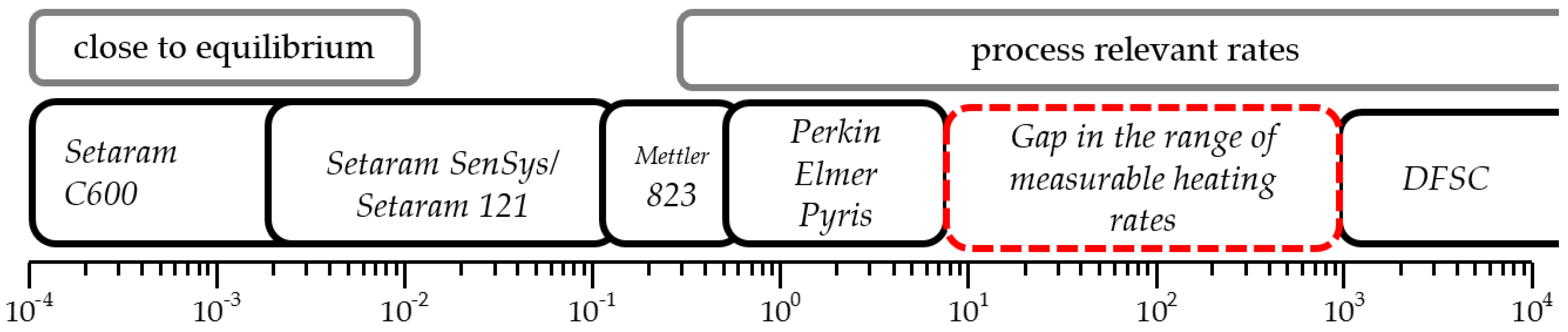
:1. Introduction
2. Materials and Methods
2.1. DSC Curve Reconstruction for (Fast) Linear Heating
- The precipitation state of the material may not change during the cooling step. Overcritical quenching is necessary to suppress precipitation reactions during cooling.
- The precipitation state of the material may not change during the intermediate time between initial heat treatment and reheating. Store the samples in a freezer at a low temperature.
2.2. Assessment of a Non-Linear Heat Treatment by DSC on the Example of a Laser Heating and a Welding Process
2.3. Investigated Aluminium Alloy
2.4. Differential Scanning Calorimetry (DSC)
2.5. Data Processing of Raw Measured Heat Flow Curves
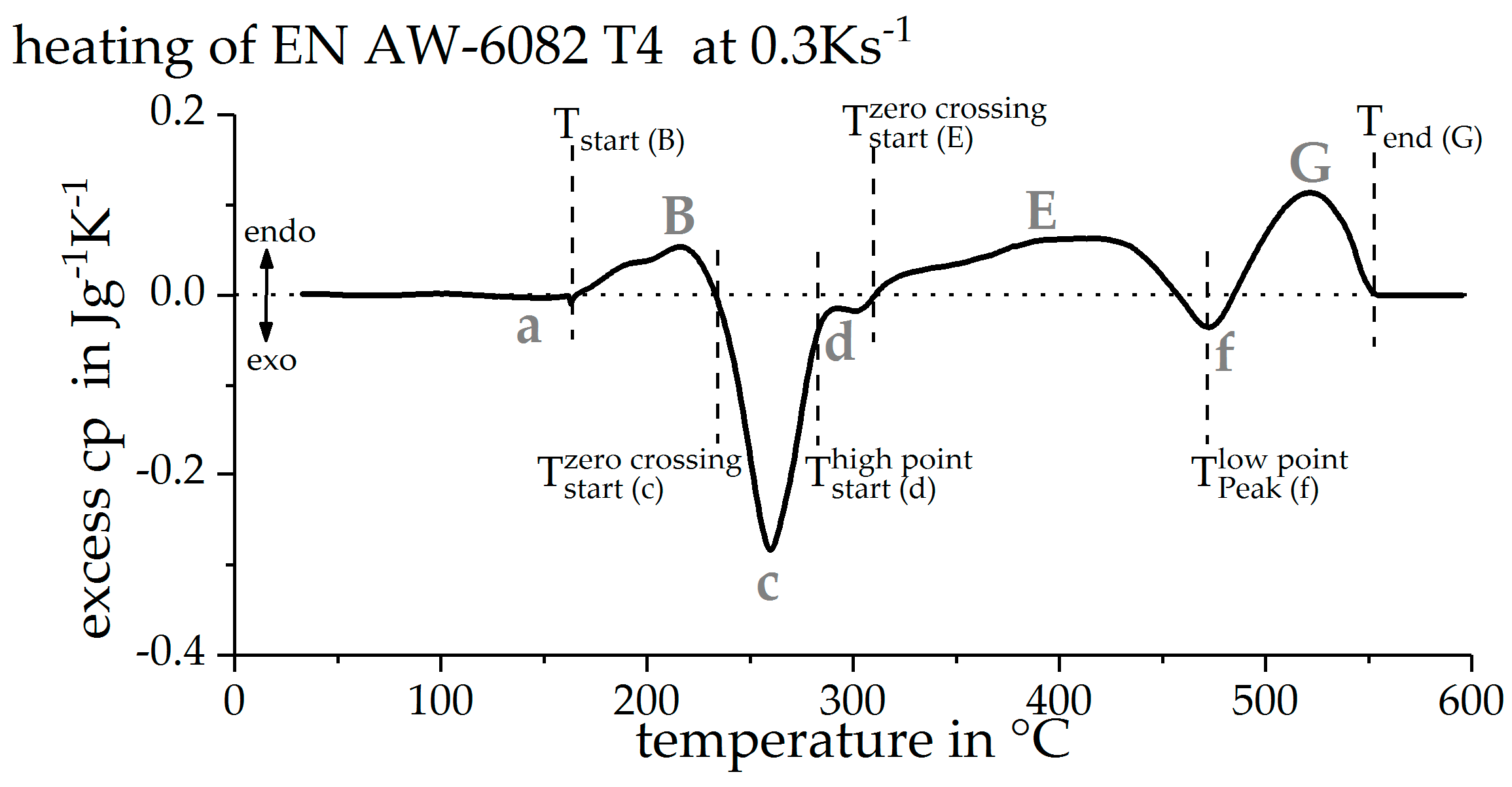
2.6. Precipitation and Dissolution Reactions
2.7. Previous Heat Treatment in a Quenching Dilatometer
3. Results and Discussion
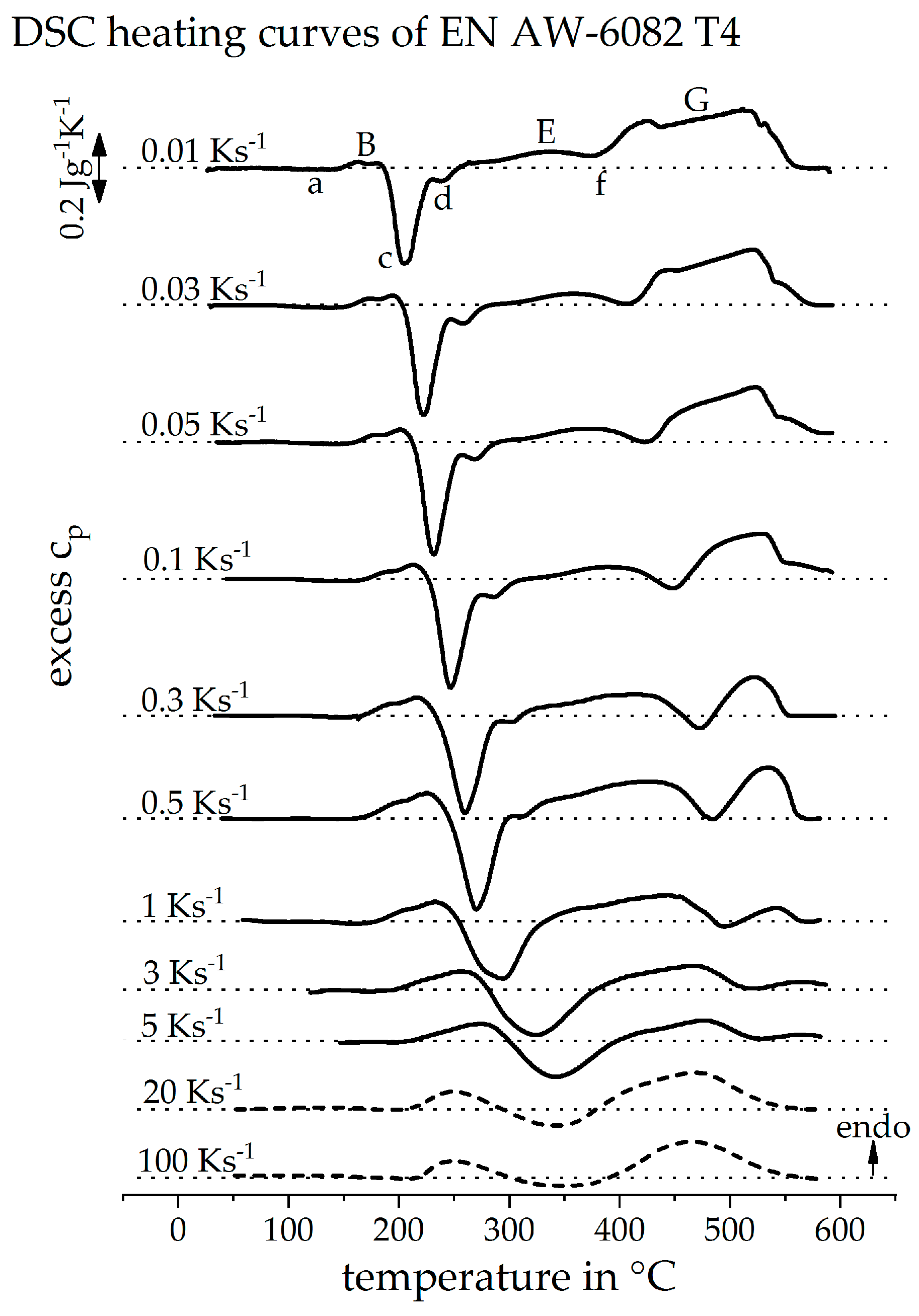
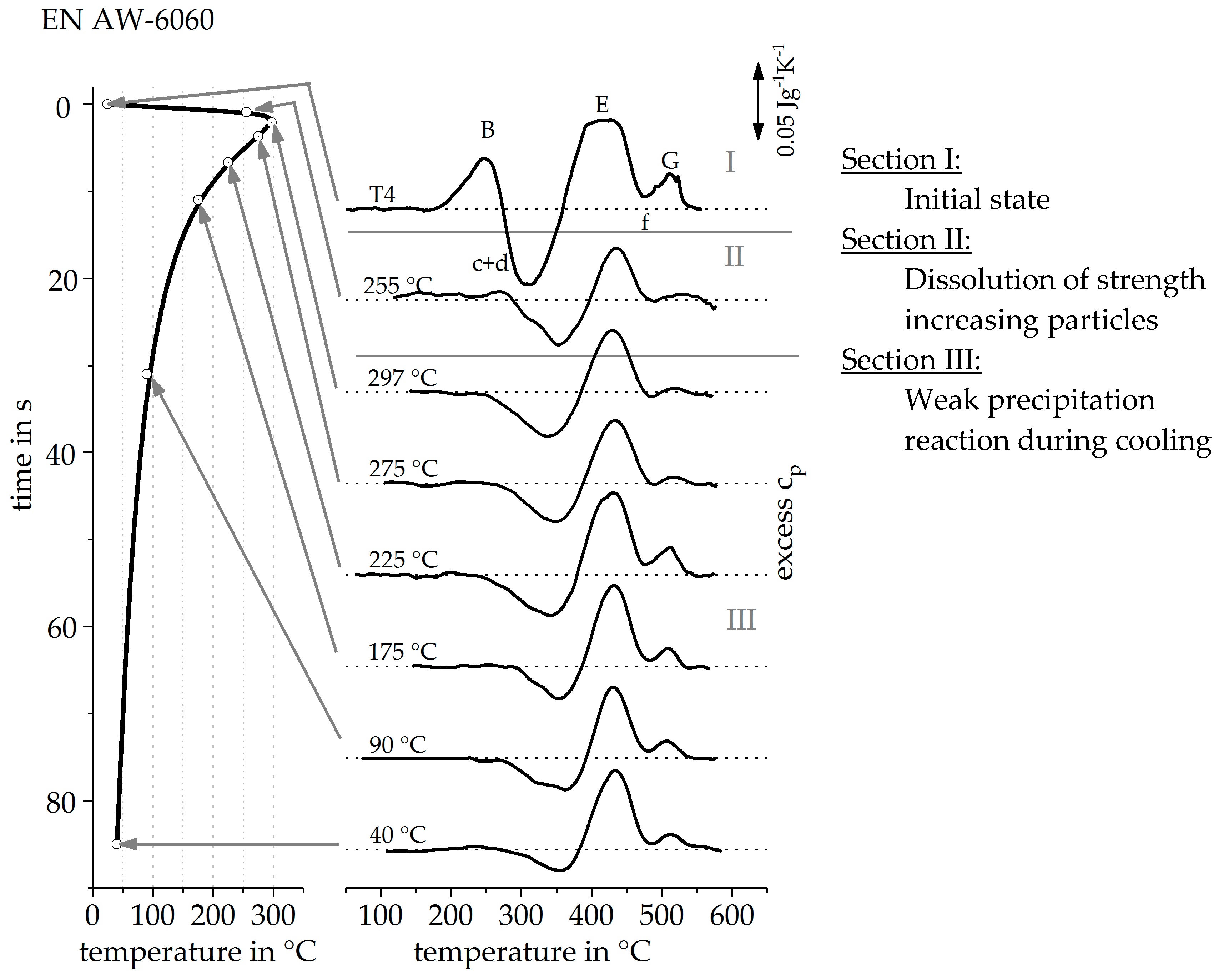
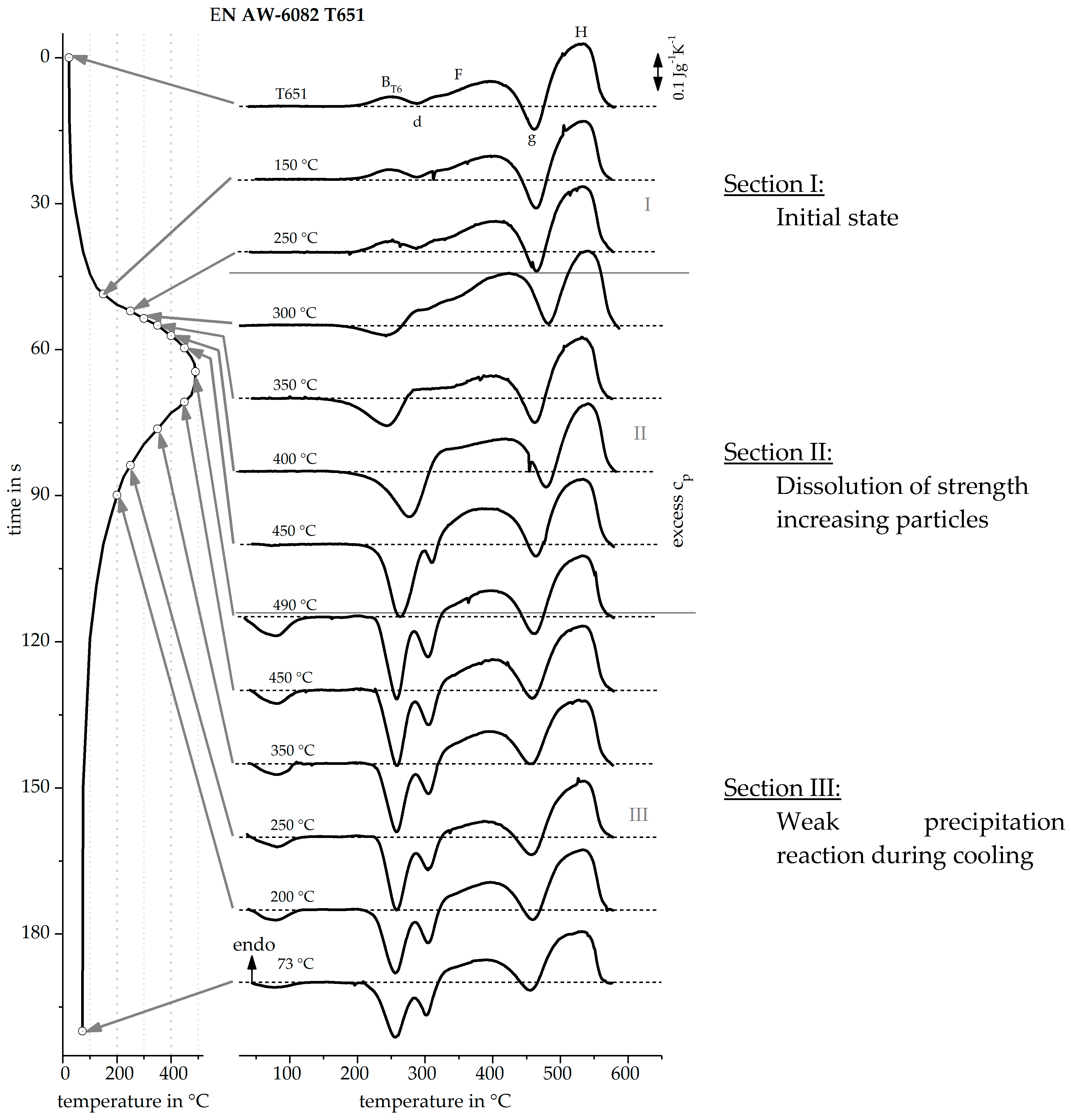
3.1. Direct DSC-Measurements
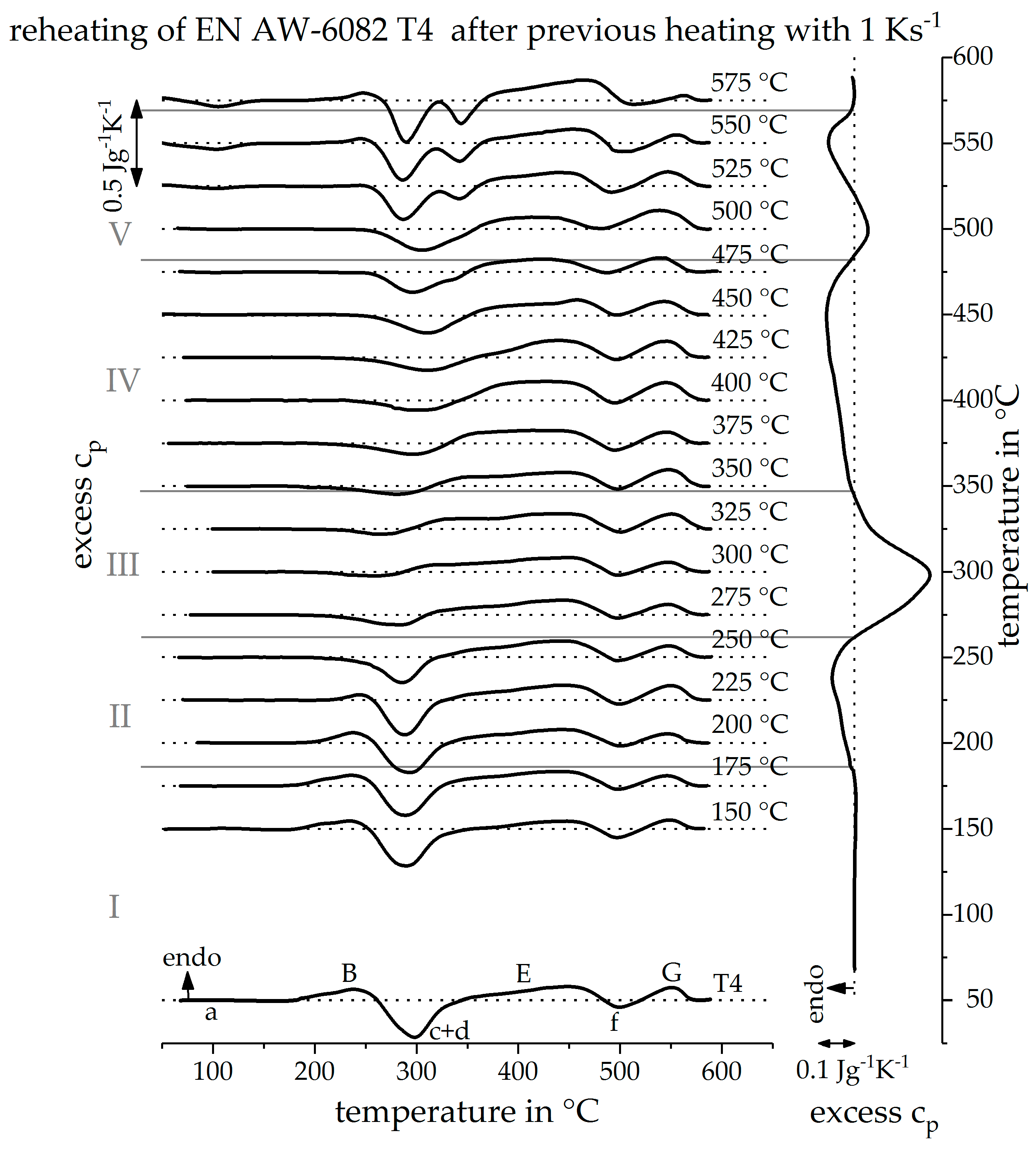
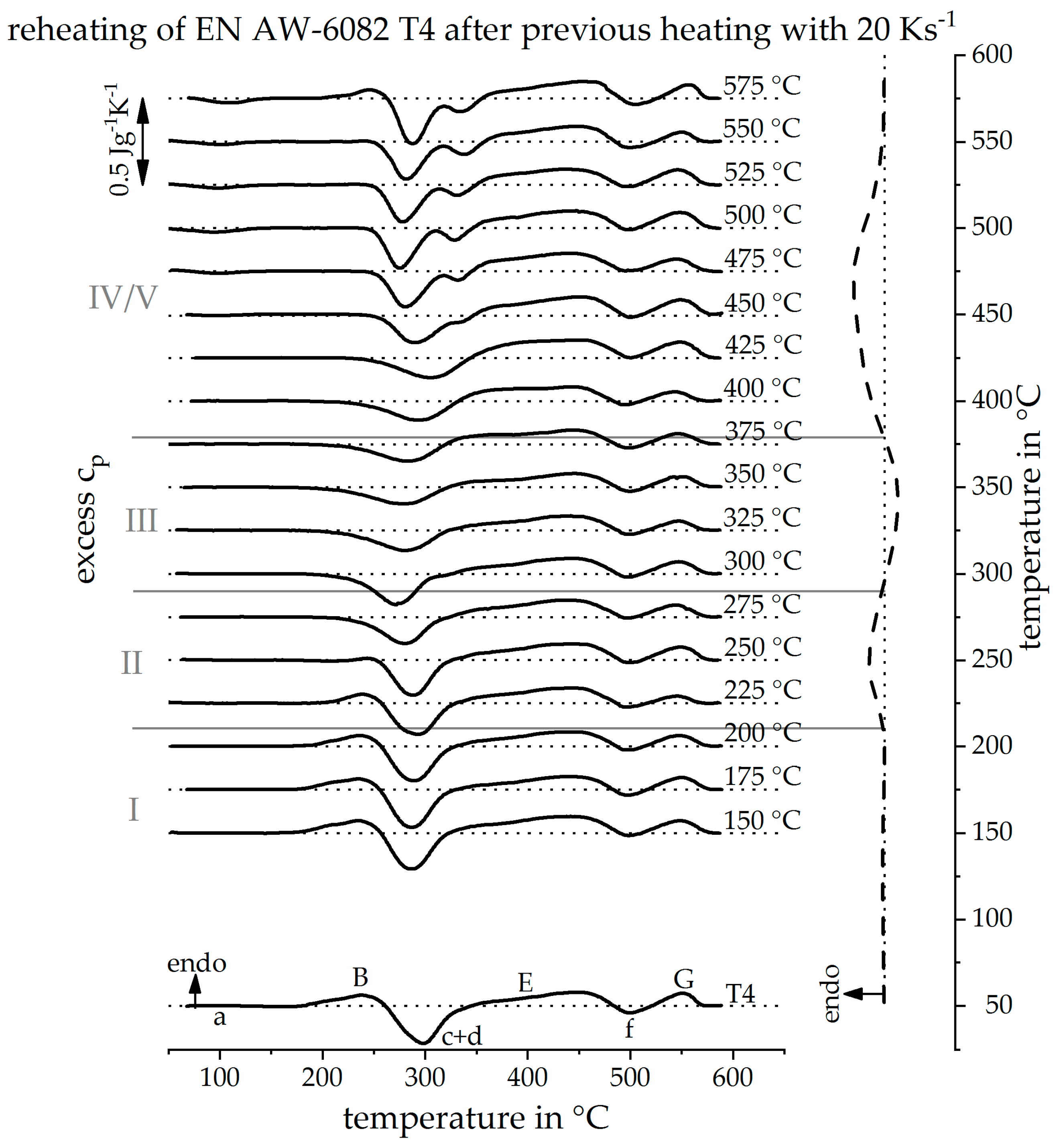
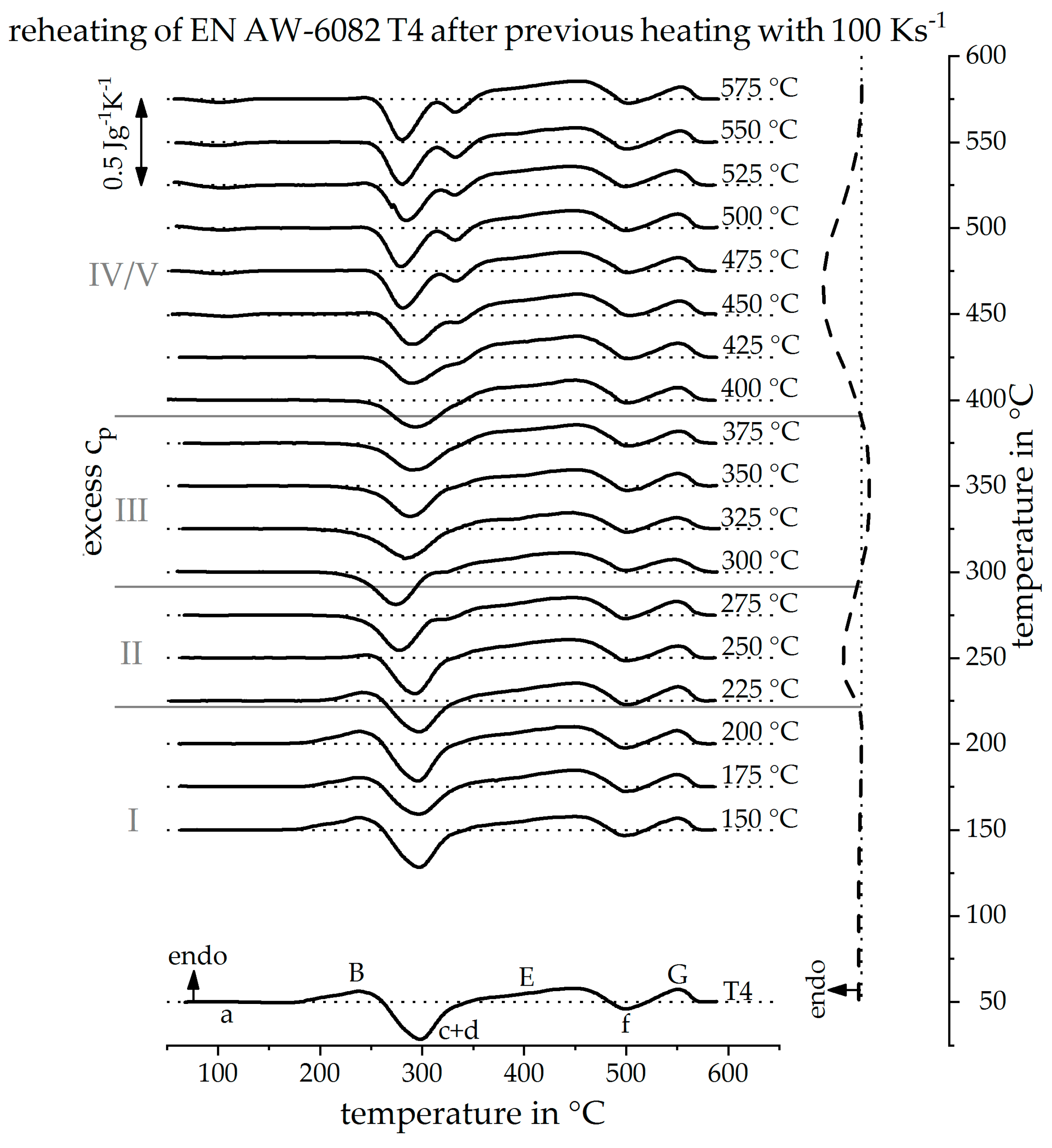
3.2. Validation of Indirect Rehaeting Method Versus the Known Heating Curve of 1 Ks−1
3.3. Reconstruction of DSC Heating Curve for 20 Ks−1 and 100 Ks−1
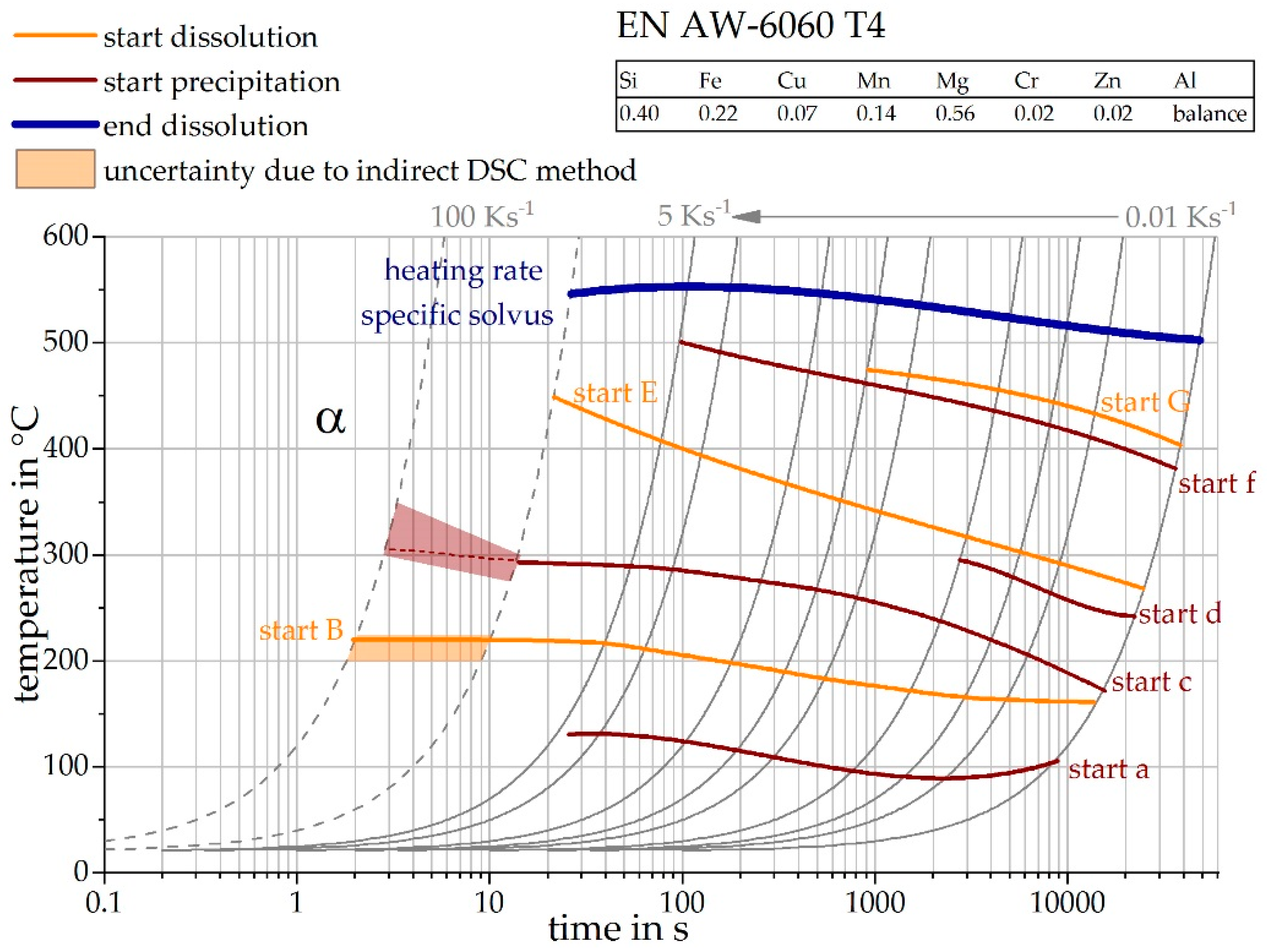
3.4. The Continuous Heating Dissolution Diagram of EN AW-6082 T4
4. Transfer of This Measurement Methodology
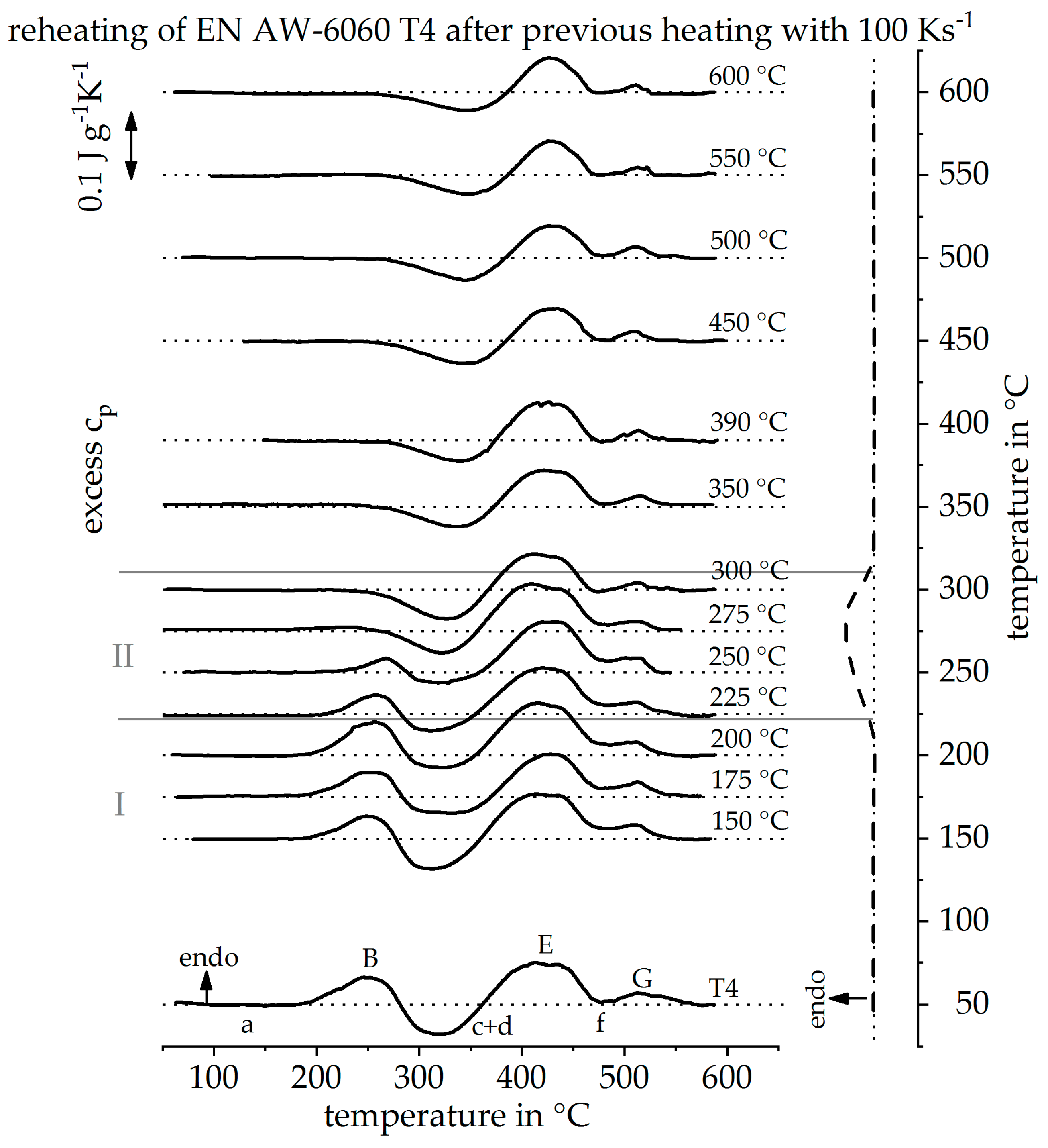
4.1. Transfer to Other Alloys
4.2. Transfer to Non-Linear Heating and Cooling
5. Conclusions
- Reconstruction of DSC curves for fast or non-linear heat treatments, which previously were not assessable, is now possible.
- This method is applicable for linear and non-linear heat treatments, as well as for heating and cooling.
- Temperature ranges of the main reactions can be reconstructed quantitatively.
- Very time-consuming method due to many individual measurements.
- Additional device is necessary for a defined heat treatment, including the necessity of overcritical quenching to allow for process interruption.
- Validation based on a known DSC heating curve is required. This will be necessary for new alloys and new heat treatment processes. As long as alloys and processes are similar, as in in the above examples, one typical validation will be sufficient.
Author Contributions
Funding
Conflicts of Interest
References
- Osten, J.; Milkereit, B.; Schick, C.; Kessler, O.; Je, J.H. Dissolution and Precipitation Behaviour during Continuous Heating of Al-Mg-Si Alloys in a Wide Range of Heating Rates. Materials 2015, 8, 2830–2848. [Google Scholar] [CrossRef]
- Fröck, H.; Graser, M.; Reich, M.; Lechner, M.; Merklein, M.; Kessler, O. Influence of short-term heat treatment on the microstructure and mechanical properties of EN AW-6060 T4 extrusion profiles: Part A. Prod. Eng. 2016, 10, 383–389. [Google Scholar] [CrossRef]
- Milkereit, B.; Kessler, O.; Schick, C. Continuous Cooling Precipitation Diagrams of Aluminium-Magnesium-Silicon Alloys. In Proceedings of the 11th International Conference on Aluminium Alloys, Aachen, Germany, 22–26 September 2008; pp. 1232–1237. [Google Scholar]
- Milkereit, B.; Fröck, H.; Schick, C.; Kessler, O. Continuous cooling precipitation diagram of cast aluminium alloy Al-7Si-0.3Mg. Trans. Nonferrous Met. Soc. 2014, 24, 2025–2033. [Google Scholar] [CrossRef]
- Zohrabyan, D.; Milkereit, B.; Schick, C.; Kessler, O. Continuous cooling precipitation diagram of high alloyed Al-Zn-Mg-Cu 7049A alloy. Trans. Nonferrous Met. Soc. 2014, 24, 2018–2024. [Google Scholar] [CrossRef]
- Fröck, H.; Milkereit, B.; Wiechmann, P.; Springer, A.; Sander, M.; Kessler, O.; Reich, M. Influence of Solution-Annealing Parameters on the Continuous Cooling Precipitation of Aluminum Alloy 6082. Metals 2018, 8, 265. [Google Scholar] [CrossRef]
- Schick, C.; Mathot, V. Fast Scanning Calorimetry; Springer: Basel, Switzerland, 2016. [Google Scholar]
- Merklein, M.; Böhm, W.; Lechner, M. Tailoring Material Properties of Aluminum by Local Laser Heat Treatment. Phys. Procedia 2012, 39, 232–239. [Google Scholar] [CrossRef] [Green Version]
- Wiechmann, P.; Panwitt, H.; Heyer, H.; Reich, M.; Sander, M.; Kessler, O. Combined Calorimetry, Thermo-Mechanical Analysis and Tensile Test on Welded EN AW-6082 Joints. Materials 2018, 11, 1396. [Google Scholar] [CrossRef]
- Milkereit, B.; Beck, M.; Reich, M.; Kessler, O.; Schick, C. Precipitation kinetics of an aluminium alloy during Newtonian cooling simulated in a differential scanning calorimeter. Thermochim. Acta 2011, 522, 86–95. [Google Scholar] [CrossRef]
- Zohrabyan, D.; Milkereit, B.; Kessler, O.; Schick, C. Precipitation enthalpy during cooling of aluminum alloys obtained from calorimetric reheating experiments. Thermochim. Acta 2012, 529, 51–58. [Google Scholar] [CrossRef]
- Schumacher, P.; Pogatscher, S.; Starink, M.J.; Schick, C.; Mohles, V.; Milkereit, B. Quench-induced precipitates in Al-Si alloys: Calorimetric determination of solute content and characterisation of microstructure. Thermochim. Acta 2015, 602, 63–73. [Google Scholar] [CrossRef]
- Yang, B.; Milkereit, B.; Zhang, Y.; Rometsch, P.A.; Kessler, O.; Schick, C. Continuous cooling precipitation diagram of aluminium alloy AA7150 based on a new fast scanning calorimetry and interrupted quenching method. Mater. Charact. 2016, 120, 30–37. [Google Scholar] [CrossRef]
- Milkereit, B.; Wanderka, N.; Schick, C.; Kessler, O. Continuous cooling precipitation diagrams of Al-Mg-Si alloys. Mater. Sci. Eng. A 2012, 550, 87–96. [Google Scholar] [CrossRef]
- Graser, M.; Fröck, H.; Lechner, M.; Reich, M.; Kessler, O.; Merklein, M. Influence of short-term heat treatment on the microstructure and mechanical properties of EN AW-6060 T4 extrusion profiles—Part B. Prod. Eng. 2016, 10, 391–398. [Google Scholar] [CrossRef]
- Sarmast, A.; Kokabi, A.H.; Serajzadeh, S. A study on thermal responses, microstructural issues, and natural aging in gas tungsten arc welding of AA2024-T4. Proc. Inst. Mech. Eng. B J. Eng. Manuf. 2013, 228, 413–421. [Google Scholar] [CrossRef]
- Höhne, G.W.H.; Hemminger, W.F.; Flammersheim, H.J. Differential Scanning Calorimetry, 2nd ed.; Springer: Berlin, Germany, 2003. [Google Scholar]
- Sarge, S.M.; Hemminger, W.; Gmelin, E.; Höhne, G.W.H.; Cammenga, H.K.; Eysel, W. Metrologically based procedures for the temperature, heat and heat flow rate calibration of DSC. J. Therm. Anal. 1997, 49, 1125–1134. [Google Scholar] [CrossRef]
- Höhne, G.; Cammenga, H.; Eysel, W.; Gmelin, E.; Hemminger, W. The temperature calibration of scanning calorimeters. Thermochim. Acta 1990, 160, 1–12. [Google Scholar] [CrossRef]
- Birol, Y. The effect of sample preparation on the DSC analysis of 6061 alloy. J. Mater. Sci. 2005, 40, 6357–6361. [Google Scholar] [CrossRef]
- Birol, Y. DSC Analysis of the precipitation reactions in the alloy AA6082. J. Therm. Anal. 2006, 83, 219–222. [Google Scholar] [CrossRef]
- Birol, Y. DSC analysis of the precipitation reaction in AA6005 alloy. J. Therm. Anal. 2008, 93, 977–981. [Google Scholar] [CrossRef]
- Birol, Y. A calorimetric analysis of the precipitation reactions in AlSi1MgMn alloy with Cu additions. Thermochim. Acta 2017, 650, 39–43. [Google Scholar] [CrossRef]
- Kim, S.N.; Kim, J.H.; Tezuka, H.; Kobayashi, E.; Sato, T. Formation Behavior of Nanoclusters in Al-Mg-Si Alloys with Different Mg and Si Concentration. Mater. Trans. 2013, 54, 297–303. [Google Scholar] [CrossRef]
- Murayama, M.; Hono, K. Pre-precipitate clusters and precipitation processes in Al-Mg-Si alloys. Acta Mater. 1999, 47, 1537–1548. [Google Scholar] [CrossRef]
- Tsao, C.-S.; Chen, C.-Y.; Jeng, U.-S.; Kuo, T.-Y. Precipitation kinetics and transformation of metastable phases in Al-Mg-Si alloys. Acta Mater. 2006, 54, 4621–4631. [Google Scholar] [CrossRef]
- Edwards, G.; Stiller, K.; Dunlop, G.; Couper, M. The precipitation sequence in Al-Mg-Si alloys. Acta Mater. 1998, 46, 3893–3904. [Google Scholar] [CrossRef]
- Doan, L.C.; Ohmori, Y.; Nakai, K. Precipitation and Dissolution Reactions in a 6061 Aluminum Alloy. Mater. Trans. JIM 2000, 41, 300–305. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.K.; Lloyd, D.J. Study of precipitation kinetics in a super purity Al-0.8 pct Mg-0.9 pct Si alloy using differential scanning calorimetry. Metall. Mater. Trans. A 1999, 30, 879–884. [Google Scholar] [CrossRef]

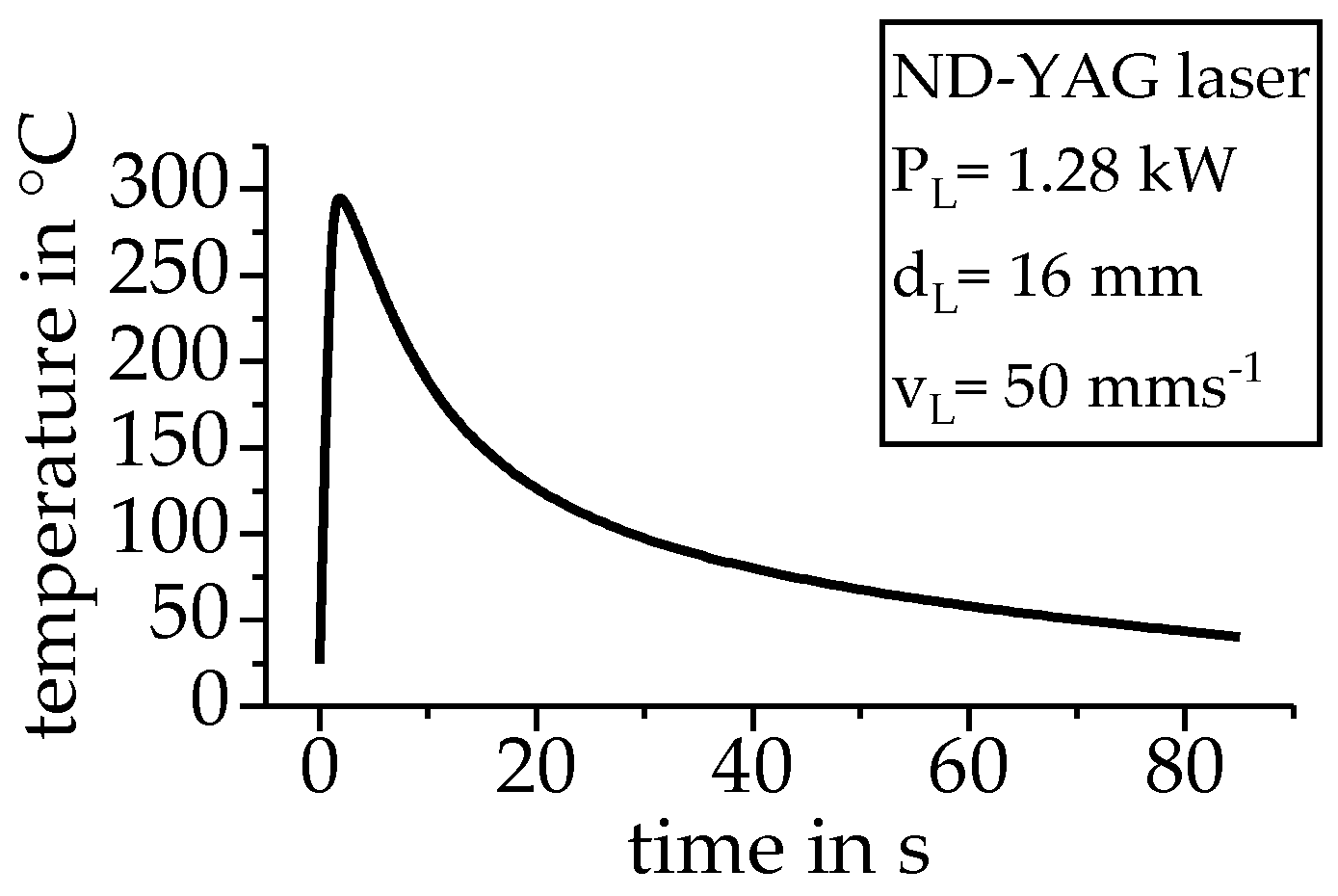






| Previous Heating in Quenching Dilatometer | Reheating in DSC | ||||
|---|---|---|---|---|---|
| Heating-Rate | Investigated Temperature Range | Temperature Step Size | Quenching Rate | Reheating-Rate | Max. Reheating Temperature |
| 1 Ks−1 | 150–575 °C | 25 K | >100 Ks−1 | 1 Ks−1 | 600 °C |
| 20 Ks−1 | |||||
| 100 Ks−1 | |||||
| Alloy | Mass Fraction in % | |||||||
|---|---|---|---|---|---|---|---|---|
| Si | Fe | Cu | Mn | Mg | Cr | Zn | Al | |
| OES EN AW-6082 T4 | 0.94 | 0.19 | 0.05 | 0.58 | 0.76 | 0.08 | 0.20 | balance |
| OES EN AW-6082 T651 | 0.83 | 0.38 | 0.06 | 0.48 | 0.92 | 0.03 | 0.01 | balance |
| DIN EN 573-3 (6082) | 0.7–1.3 | ≤0.5 | ≤0.1 | 0.4–1.0 | 0.6–1.2 | ≤0.25 | ≤0.2 | balance |
| OES EN AW-6060 T4 | 0.40 | 0.22 | 0.07 | 0.14 | 0.56 | 0.02 | 0.02 | Balance |
| DIN EN 573-3 (6060) | 0.5–0.9 | ≤0.35 | ≤0.3 | ≤0.5 | 0.4–0.7 | ≤0.3 | ≤0.2 | balance |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fröck, H.; Reich, M.; Milkereit, B.; Kessler, O. Scanning Rate Extension of Conventional DSCs through Indirect Measurements. Materials 2019, 12, 1085. https://doi.org/10.3390/ma12071085
Fröck H, Reich M, Milkereit B, Kessler O. Scanning Rate Extension of Conventional DSCs through Indirect Measurements. Materials. 2019; 12(7):1085. https://doi.org/10.3390/ma12071085
Chicago/Turabian StyleFröck, Hannes, Michael Reich, Benjamin Milkereit, and Olaf Kessler. 2019. "Scanning Rate Extension of Conventional DSCs through Indirect Measurements" Materials 12, no. 7: 1085. https://doi.org/10.3390/ma12071085





