Influence of Polycarboxylate Superplasticizers with Different Functional Units on the Early Hydration of C3A-Gypsum
Abstract
:1. Introduction
- —relative humidity,
- —room temperature,
- —CaO,
- —Al2O3,
- —H2O.
- —SO3.
2. Experimental
2.1. Minerals (C3A/Gypsum)
2.2. PC Superplasticizers (PCs)
2.3. Preparation of C3A-Gypsum Samples
2.4. Hydration Heat
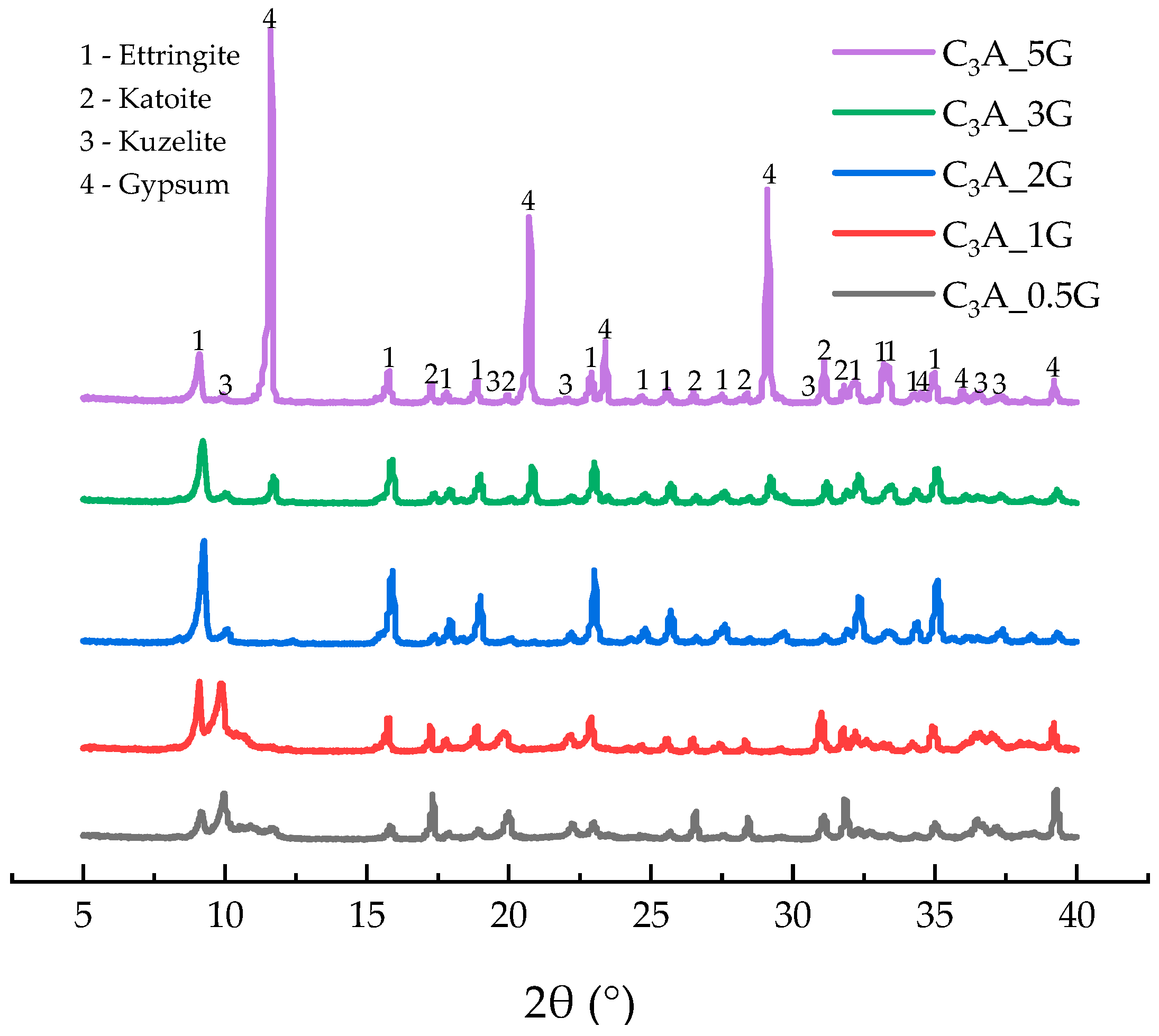
2.5. XRD Analysis
3. Results and Discussion
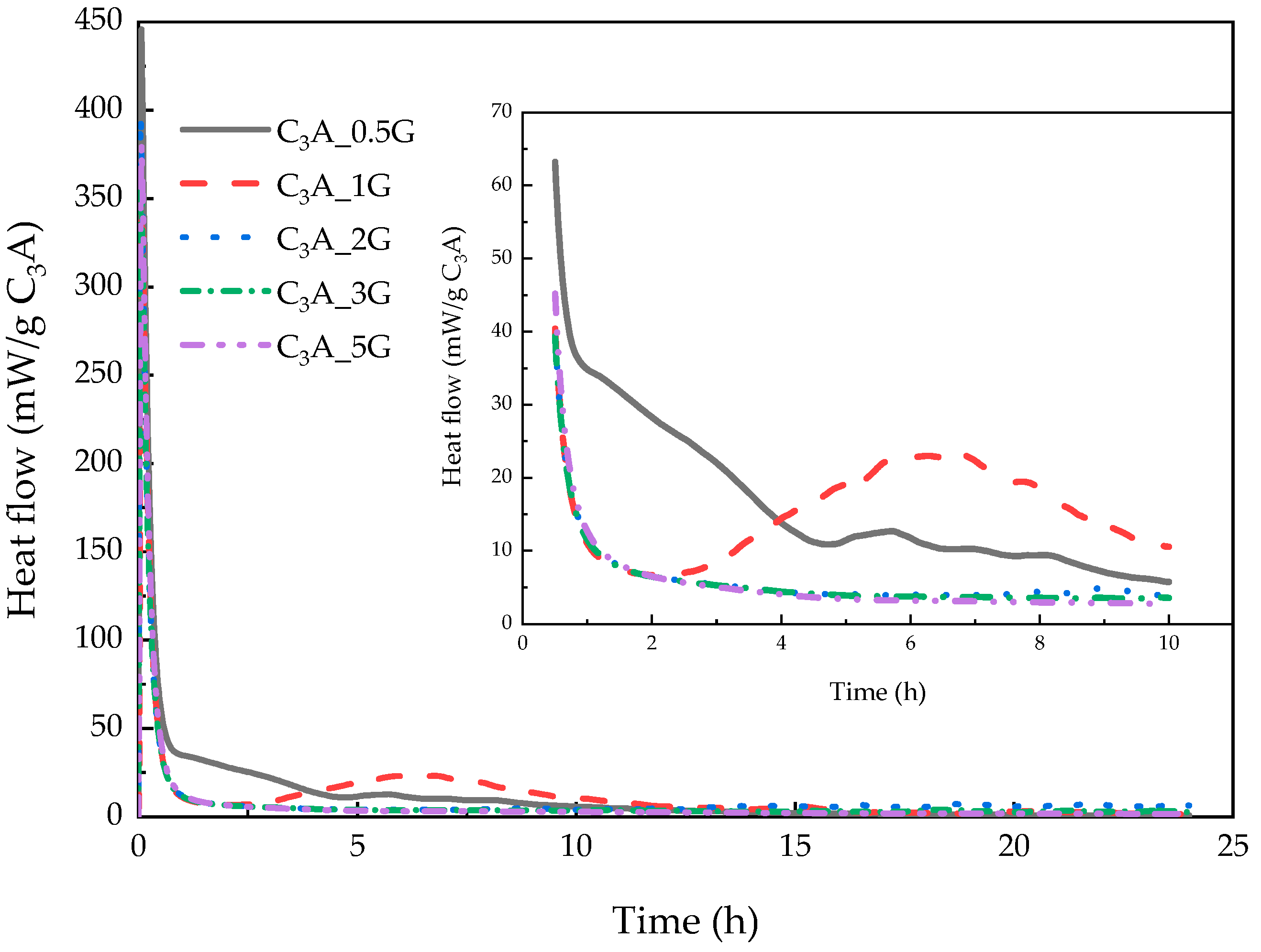
3.1. Early Hydration of C3A-Gypsum System
3.1.1. Dissolution–Crystallization Stage
3.1.2. Induction Stage
3.1.3. Transformation Stage
3.2. Hydration of C3A-Gypsum in the Presence of PCs
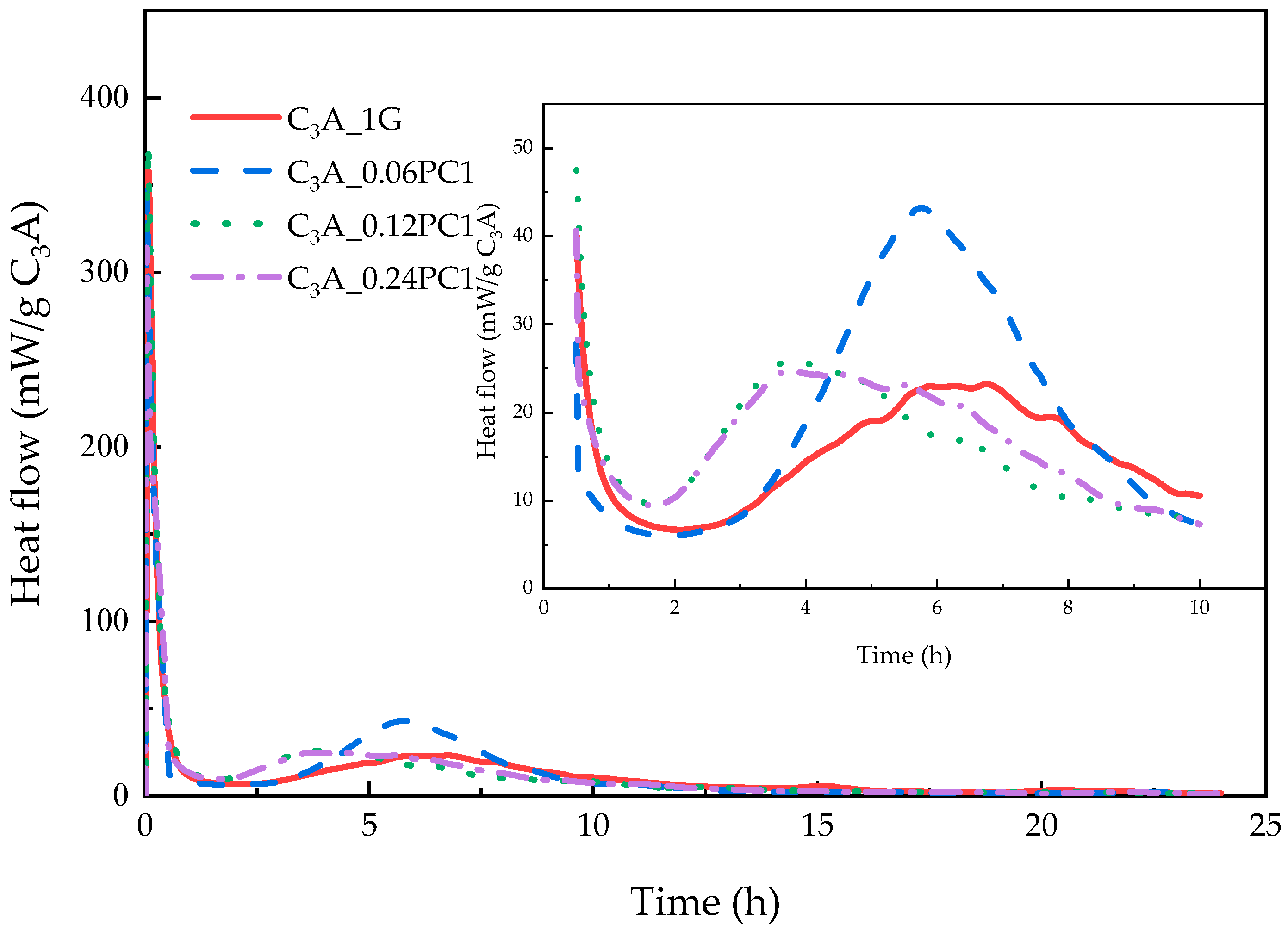
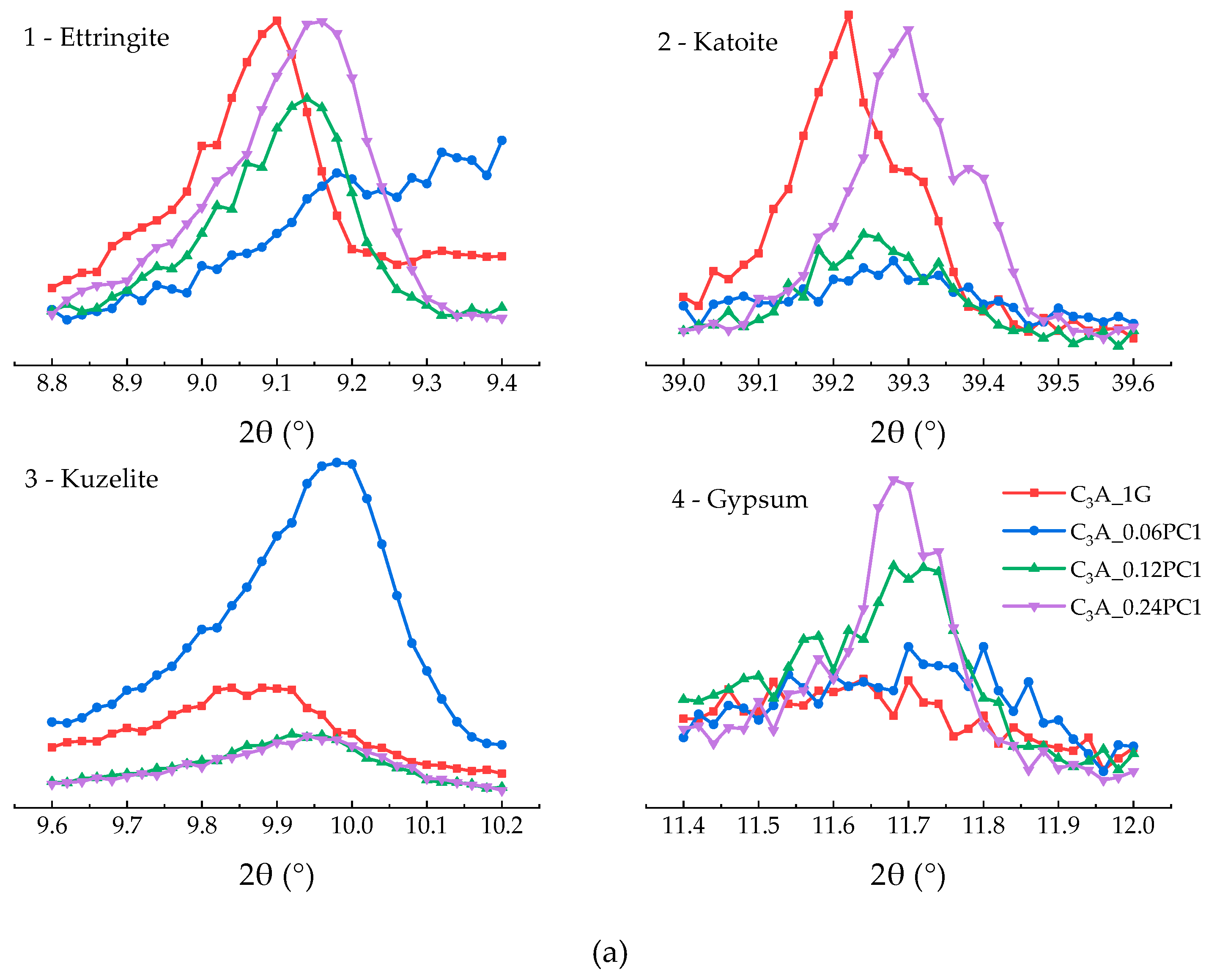
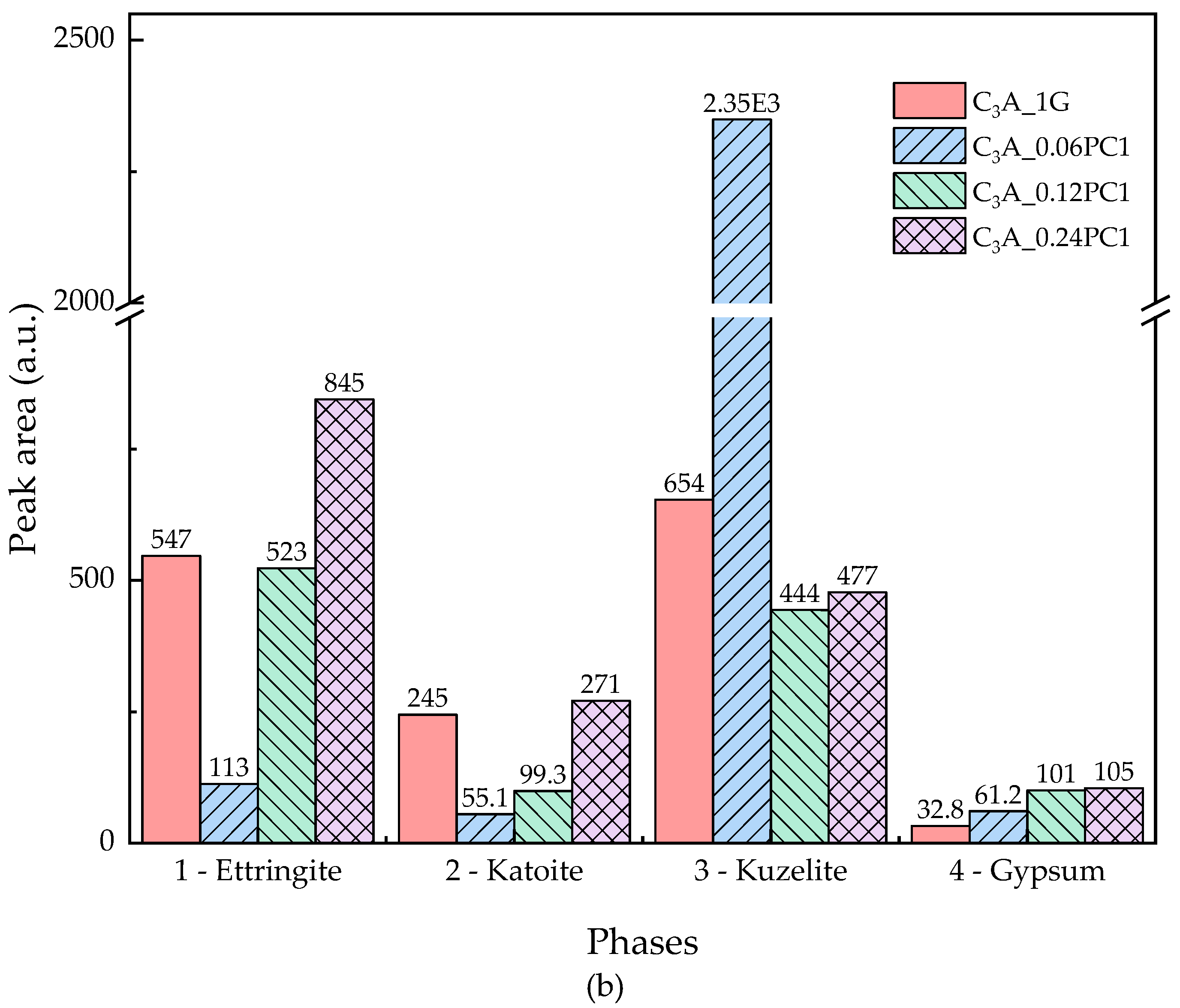
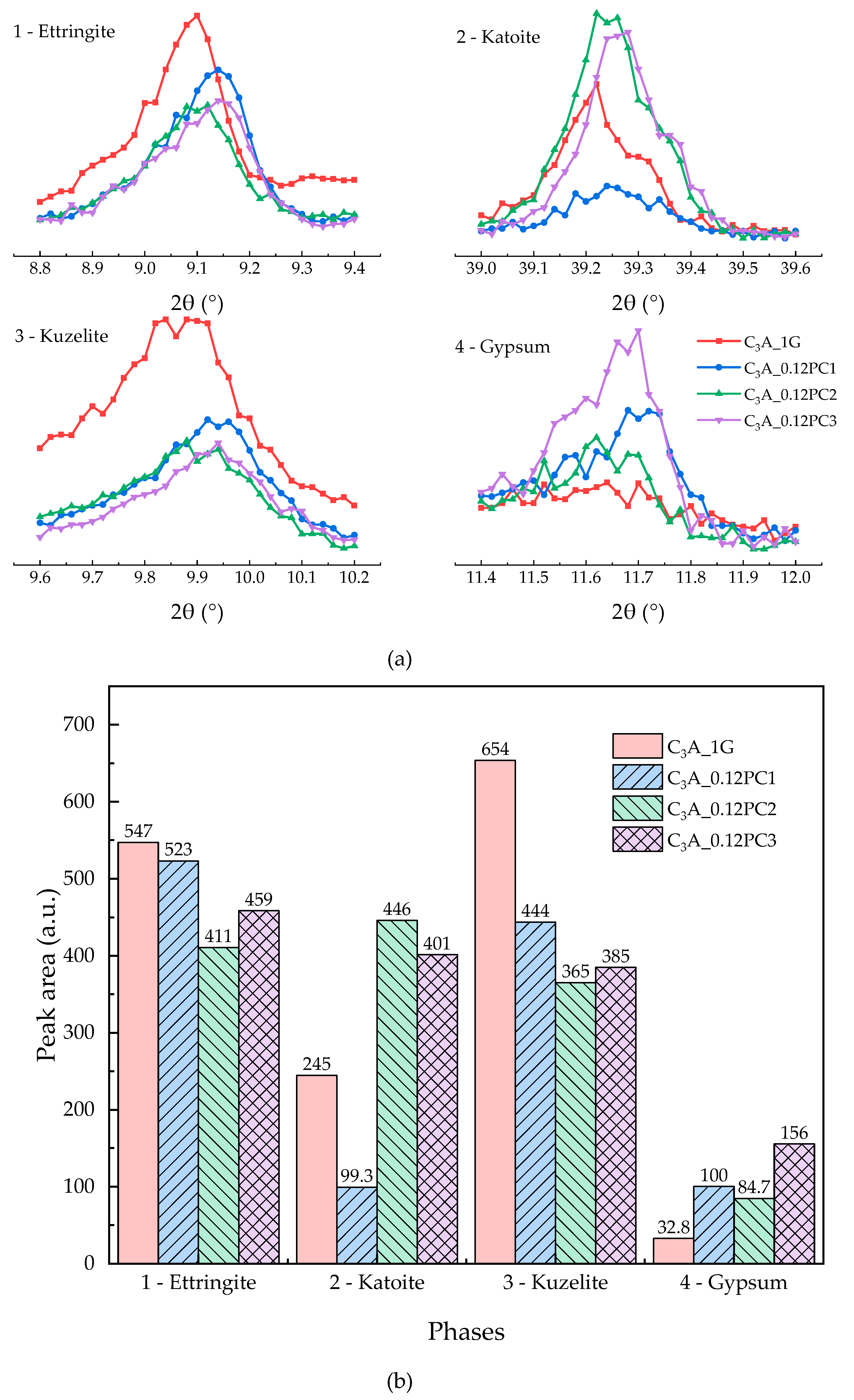
3.2.1. Effects of PC with Only Carboxylate Monomers
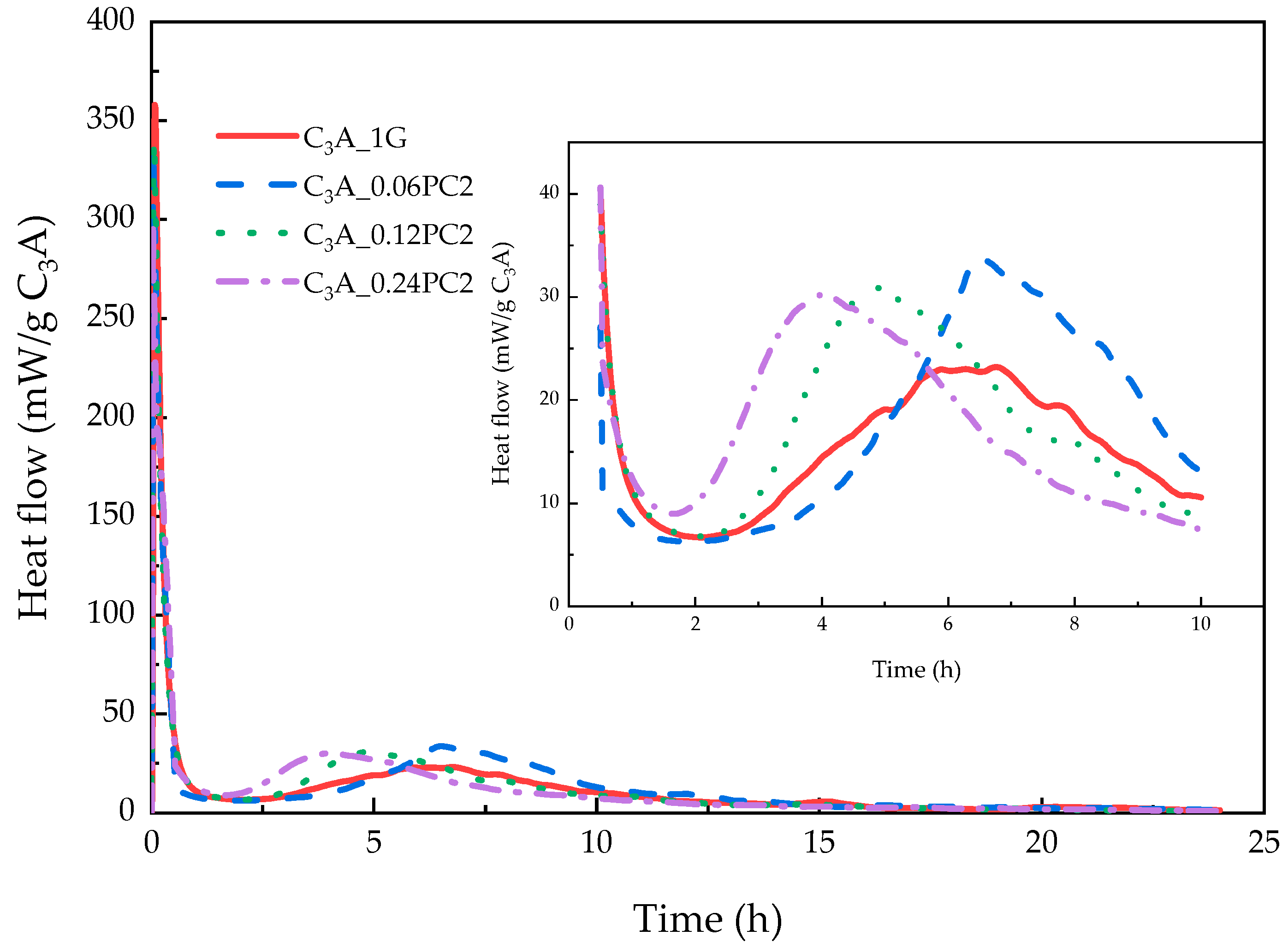
3.2.2. Effects of PC with AM Monomers
3.2.3. Effects of PC with AMPS Monomers
3.2.4. Effect of the PCs with Different Functional Units
4. Conclusions
- In the dissolution–crystallization stage, the precipitation of hydroxy-AFm and AFt coexisted, although there may be a time interval between them. The dosage of gypsum could not have a significant impact on the duration of this stage.
- Unhydrated C3A remained after the sulfate depletion was the precondition of the induction stage, and the specific G/C ratio allowing the induction stage could exist was hard to obtain because of the uncertain consumption of C3A or gypsum during the dissolution–crystallization. The duration of this stage depended on the number of soluble sulfate ions.
- The transformation stage started after the sulfate exhaustion when AFt was converted into AFm. Remaining C3A after the induction stage determined the duration of this stage.
- A low concentration of PC, which copolymerized only with carboxylate unit in solution, could promote the transformation stage of C3A-gypsum hydration significantly. With the increased concentration of PC1, the appearance of second heat flow peak advanced, the amount of remained gypsum increased, and the amount of remained AFt increased.
- The concentration of PC copolymerized with an amide unit influenced the duration of the induction stage. A low concentration of PC2 could prolong it. PC2 may have accelerated the formation of hydrogarnet. Although the amount of AFm created was less than the blank sample, a higher transformation heat flow peak could be found during the hydration of samples with PC2.
- As the concentration of PC that copolymerized with AMPS monomers increased, the duration of the induction stage was shortened first, and then prolonged. A low concentration of PC3 had a minor influence on the hydration of C3A-gypsum, except for the exotherm during the transformation stage. Also, the dosage of PC3 could enhance the hydration of C3A such that the production of hydrogarnet was more than the blank sample.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Quennoz, A.; Scrivener, K.L. Hydration of C3A-gypsum systems. Cem. Concr. Res. 2012, 42, 1032–1041. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford: London, UK, 1997. [Google Scholar]
- Collepardi, M.; Baldini, G.; Pauri, M.; Corradi, M. Tricalcium aluminate hydration in presence of lime, gypsum or sodium-sulfate. Cem. Concr. Res. 1978, 8, 571–580. [Google Scholar] [CrossRef]
- Brown, P.W.; Liberman, L.O.; Frohnsdorff, G. Kinetics of the early hydration of tricalcium aluminate in solutions containing calcium-sulfate. J. Am. Ceram. Soc. 1984, 67, 793–795. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Pratt, P.L. Microstructural studies of the hydration of C3A and C4AF independently and in cement paste. Br. Ceram. Proc. 1984, 35, 207–219. [Google Scholar]
- Minard, H.; Garrault, S.; Regnaud, L.; Nonat, A. Mechanisms and parameters controlling the tricalcium aluminate reactivity in the presence of gypsum. Cem. Concr. Res. 2007, 37, 1418–1426. [Google Scholar] [CrossRef]
- Pourchet, S.; Regnaud, L.; Perez, J.P.; Nonat, A. Early C3A hydration in the presence of different kinds of calcium sulfate. Cem. Concr. Res. 2009, 39, 989–996. [Google Scholar] [CrossRef]
- Hu, K.; Sun, Z.; Yang, H. Effects of polycarboxylate superplasticizers with different functional units on the early hydration behavior of cement paste. J. Mater. Civ. Eng. 2019, in press. [Google Scholar] [CrossRef]
- Winnefeld, F.; Becker, S.; Pakusch, J.; Götz, T. Effects of the molecular architecture of comb-shaped superplasticizers on their performance in cementitious systems. Cem. Concr. Res. 2007, 29, 251–262. [Google Scholar] [CrossRef]
- Zingg, A.; Winnefeld, F.; Holzer, L.; Pakusch, J.; Becker, S.; Gauckler, L. Adsorption of polyelectrolytes and its influence on the rheology, zeta potential, and microstructure of various cement and hydrate phases. J. Colloid Interface Sci. 2008, 323, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.R.; Pan, L.S.; Wang, C.M.; Xu, N. Effects of polycarboxylate superplasticizers with different molecular structure on the hydration behavior of cement paste. Constr. Build. Mater. 2016, 105, 545–553. [Google Scholar] [CrossRef]
- Liu, X.; Guan, J.; Lai, G.; Wang, Z.; Zhu, J.; Cui, S.; Lan, M.; Li, H. Performances and working mechanism of a novel polycarboxylate superplasticizer synthesized through changing molecular topological structure. J. Colloid Interface Sci. 2017, 504, 12–24. [Google Scholar] [CrossRef]
- Merlini, M.; Artioli, G.; Cerulli, T.; Cella, F.; Bravo, A. Tricalcium aluminate hydration in additivated systems. A crystallographic study by SR-XRPD. Cem. Concr. Res. 2008, 38, 477–486. [Google Scholar] [CrossRef]
- Plank, J.; Zhimin, D.; Keller, H.; Hössle, F.V.; Seidl, W. Fundamental mechanisms for polycarboxylate intercalation into C3A hydrate phases and the role of sulfate present in cement. Cem. Concr. Res. 2010, 40, 45–57. [Google Scholar] [CrossRef]
- Dalas, F.; Pourchet, S.; Rinaldi, D.; Nonat, A.; Sabio, S.; Mosquet, M. Modification of the rate of formation and surface area of ettringite by polycarboxylate ether superplasticizers during early C3A–CaSO4 hydration. Cem. Concr. Res. 2015, 69, 105–113. [Google Scholar] [CrossRef]
- Alonso, M.M.; Puertas, F. Adsorption of PCE and PNS superplasticisers on cubic and orthorhombic C3A. Effect of sulfate. Constr. Build. Mater. 2015, 78, 324–332. [Google Scholar] [CrossRef]
- Myers, R.J.; Geng, G.; Li, J.; Rodríguez, E.D.; Ha, J.; Kidkhunthod, P.; Sposito, G.; Lammers, L.N.; Kirchheim, A.P.; Monteiro, P.J. Role of adsorption phenomena in cubic tricalcium aluminate dissolution. Langmuir 2016, 33, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yang, H.; Shui, L.; Liu, Y.; Yang, X.; Ji, Y.; Hu, K.; Luo, Q. Preparation of polycarboxylate-based grinding aid and its influence on cement properties under laboratory condition. Constr. Build. Mater. 2016, 127, 363–368. [Google Scholar] [CrossRef]
- Kirchheim, A.P.; Rodriguez, E.D.; Myers, R.J.; Gobbo, L.A.; Monteiro, P.J.M.; Dal Molin, D.C.C.; de Souza, R.B.; Cincotto, M.A. Effect of Gypsum on the Early Hydration of Cubic and Na-Doped Orthorhombic Tricalcium Aluminate. Materials 2018, 11, 568. [Google Scholar] [CrossRef]
- Zingg, A.; Winnefeld, F.; Holzer, L.; Pakusch, J.; Becker, S.; Figi, R.; Gauckler, L. Interaction of polycarboxylate-based superplasticizers with cements containing different C3A amounts. Cem. Concr. Compos. 2009, 31, 153–162. [Google Scholar] [CrossRef]
- Liu, Y. Research on Synthesis, Properties and Mechanism of Different Carboxyl Density and Functionallizing Polycarboxylate Superplasticizer. Ph.D. Thesis, China University of Mining and Technology, Xuzhou, China, June 2013. [Google Scholar]
- Yoshioka, K.; Tazawa, E.; Kawai, K.; Enohata, T. Adsorption characteristics of superplasticizers on cement component minerals. Cem. Concr. Res. 2002, 32, 1507–1513. [Google Scholar] [CrossRef]
- Zingg, A.; Holzer, L.; Kaech, A.; Winnefeld, F.; Pakusch, J.; Becker, S.; Gauckler, L. The microstructure of dispersed and non-dispersed fresh cement pastes—New insight by cryo-microscopy. Cem. Concr. Res. 2008, 38, 522–529. [Google Scholar] [CrossRef]
- Holzer, L.; Gasser, P.; Kaech, A.; Wegmann, M.; Zingg, A.; Wepf, R.; Muench, B. Cryo-FIB-nanotomography for quantitative analysis of particle structures in cement suspensions. J. Microsc.-Oxf. 2007, 227, 216–228. [Google Scholar] [CrossRef] [Green Version]





| Minerals | Diameter (μm) | |||
|---|---|---|---|---|
| d10 | d50 | d90 | dm | |
| C3A | 3.09 | 6.88 | 15.92 | 8.83 |
| Gypsum | 8.10 | 20.16 | 38.00 | 21.79 |
| Sample | The Molar Ratio of Monomers | Molecular Characteristics | |||||
|---|---|---|---|---|---|---|---|
| AA | AM | AMPS | TPEG2400 | Mn (Da) | Mw (Da) | PDI | |
| PC1 | 5 | 0 | 0 | 1 | 48,600 | 107,400 | 2.21 |
| PC2 | 1 | 4 | 0 | 1 | 35,740 | 67,840 | 1.90 |
| PC3 | 1 | 0 | 4 | 1 | 36,030 | 83,110 | 2.31 |
| Samples | C3A (g) | Gypsum (g) | Deionized Water/PC Solutions |
|---|---|---|---|
| C3A_0.5G | 1 (3.7 mmol) | 0.385 (1.9 mmol) | 10 g deionized water |
| C3A_1G | 1 (3.7 mmol) | 0.770 (3.7 mmol) | 10 g deionized water |
| C3A_2G | 1 (3.7 mmol) | 1.540 (7.4 mmol) | 10 g deionized water |
| C3A_3G | 1 (3.7 mmol) | 2.310 (11.1 mmol) | 10 g deionized water |
| C3A_5G | 1 (3.7 mmol) | 2.310 (18.5 mmol) | 10 g deionized water |
| C3A_0.06PC1 | 1 (3.7 mmol) | 0.770 (3.7 mmol) | 10 g 0.06% PC1 solution |
| C3A_0.12PC1 | 1 (3.7 mmol) | 0.770 (3.7 mmol) | 10 g 0.12% PC1 solution |
| C3A_0.24PC1 | 1 (3.7 mmol) | 0.770 (3.7 mmol) | 10 g 0.24% PC1 solution |
| C3A_0.06PC2 | 1 (3.7 mmol) | 0.770 (3.7 mmol) | 10 g 0.06% PC2 solution |
| C3A_0.12PC2 | 1 (3.7 mmol) | 0.770 (3.7 mmol) | 10 g 0.12% PC2 solution |
| C3A_0.24PC2 | 1 (3.7 mmol) | 0.770 (3.7 mmol) | 10 g 0.24% PC2 solution |
| C3A_0.06PC3 | 1 (3.7 mmol) | 0.770 (3.7 mmol) | 10 g 0.06% PC3 solution |
| C3A_0.12PC3 | 1 (3.7 mmol) | 0.770 (3.7 mmol) | 10 g 0.12% PC3 solution |
| C3A_0.24PC3 | 1 (3.7 mmol) | 0.770 (3.7 mmol) | 10 g 0.24% PC3 solution |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, K.; Sun, Z. Influence of Polycarboxylate Superplasticizers with Different Functional Units on the Early Hydration of C3A-Gypsum. Materials 2019, 12, 1132. https://doi.org/10.3390/ma12071132
Hu K, Sun Z. Influence of Polycarboxylate Superplasticizers with Different Functional Units on the Early Hydration of C3A-Gypsum. Materials. 2019; 12(7):1132. https://doi.org/10.3390/ma12071132
Chicago/Turabian StyleHu, Kuangyi, and Zhenping Sun. 2019. "Influence of Polycarboxylate Superplasticizers with Different Functional Units on the Early Hydration of C3A-Gypsum" Materials 12, no. 7: 1132. https://doi.org/10.3390/ma12071132




