Effect of LiNO3 on Expansion of Alkali–Silica Reaction in Rock Prisms and Concrete Microbars Prepared by Sandstone
Abstract
1. Introduction
2. Materials and Methods
2.1. Aggregate
2.2. Rock Prism Test
2.3. Concrete Microbar Test
2.4. Expansion Stress Test Apparatus
3. Results and Discussion
3.1. Rock Prism and Concrete Microbar Deformation
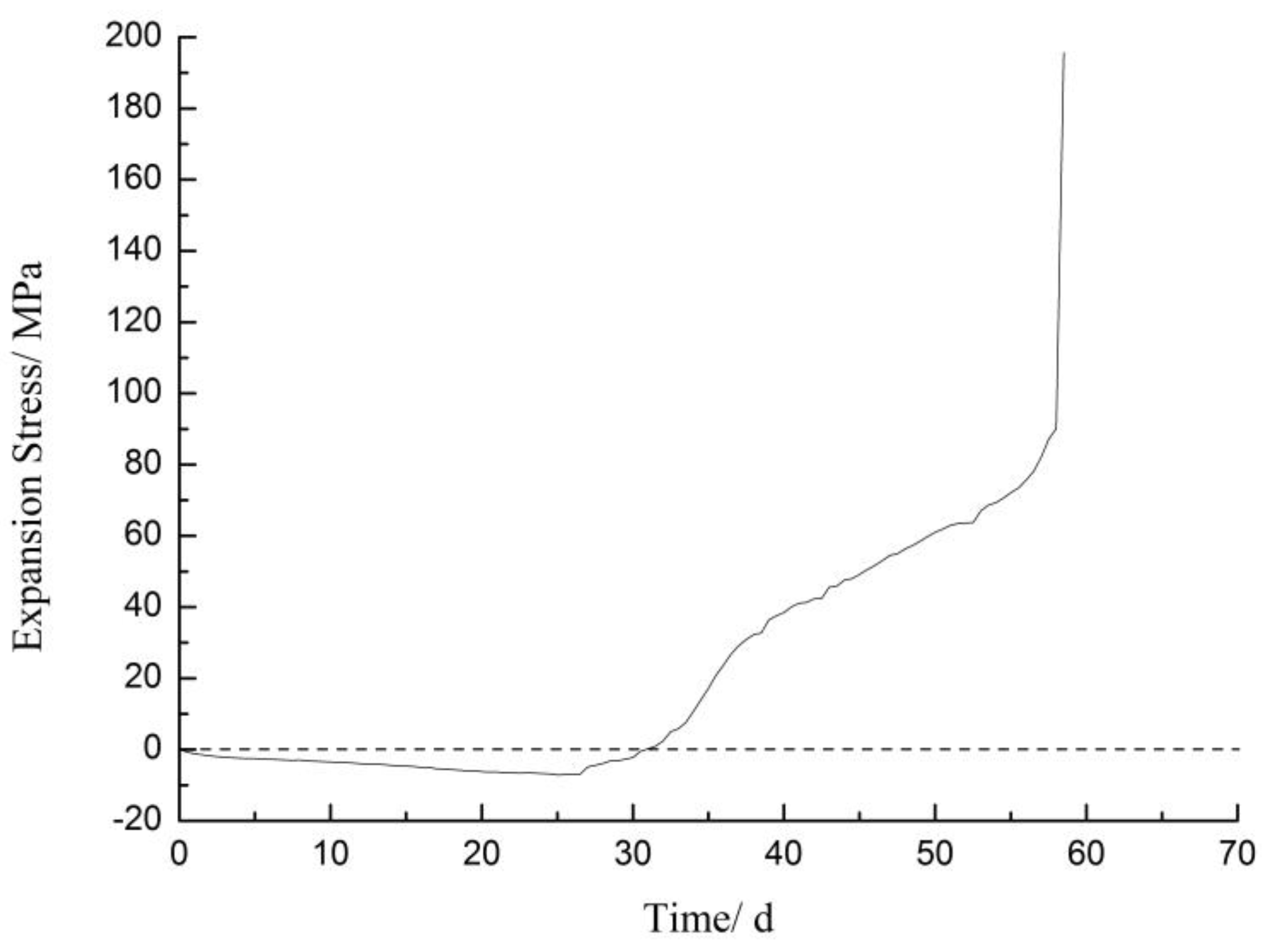
3.2. Expansion Stress
3.3. Microstructure
4. Conclusions
- LiNO3 did not decrease the expansion of rock prisms and concrete microbars with sandstone until the molar ratio of [Li]/[Na + K] exceeded 1.66, and expansion increased when the LiNO3 concentration was below the critical concentration.
- The expansion stress test proved that Li2SiO3 is obviously expansive and the expansion stress was more than 195 MPa at the end of the test.
- The XRD and SEM analyses indicated that product of Li2SiO3 caused greater expansion of samples, and reaction consumed the reactants belonging to ASR and inhibited the formation of ASR gel. LiNO3 reacted with the microcrystalline quartz inside the aggregate of sandstone and formed Li2SiO3; the expansion increased the reaction area, accelerated the reaction rate and finally, caused more and larger cracks.
- The long-term effectiveness of excessive LiNO3 at inhibiting ASR was questionable. The high concentration of LiNO3 only inhibited the ASR reaction in the early stages and the formation of Li2SiO3 caused expansion and cracks in the concrete after a long period of time.
Author Contributions
Funding
Conflicts of Interest
References
- McCoy, E.J.; Caldwell, A.G. New approach to inhibiting alkali-aggregate expansion. J. Am. Concr. Inst. 1951, 47, 693–706. [Google Scholar]
- Islam, M.S.; Ghafoori, N. Experimental study and empirical modeling of lithium nitrate for alkali–silica reactivity. Constr. Build. Mater. 2016, 121, 717–726. [Google Scholar] [CrossRef]
- Kurtis, K.E.; Monteiro, P. Chemical additives to control expansion of ASR gel: Proposed mechanisms of control. J. Mater. Sci. 2003, 38, 2027–2036. [Google Scholar] [CrossRef]
- Ohama, Y.; Demura, K.; Kakegawa, M. Inhibiting ASR with chemical admixtures. In Proceedings of the 8th International Conference Alkali–Aggregate Reaction, Kyoto, Japan, 17–20 July 1989. [Google Scholar]
- Tremblay, C.; Bérubé, M.A.; Fournier, B.; Thomas, M.D.A.; Folliard, K.J. Experimental investigation of the mechanisms by which LiNO3 is effective against ASR. Cem. Concr. Res. 2010, 40, 583–597. [Google Scholar] [CrossRef]
- Collins, C.L.; Ideker, J.H.; Willis, G.S.; Kurtis, K.E. Examination of the effects of LiOH, LiCl, and LiNO3 on alkali–silica reaction. Cem. Concr. Res. 2004, 34, 1403–1415. [Google Scholar] [CrossRef]
- Lawrence, M.; Vivian, H.F. The reactions of various alkalis with silica. Aust. J. Appl. Sci. 1961, 12, 96–103. [Google Scholar]
- Wijnen, P.W.J.G.; Beelen, T.P.M.; Haan, J.W.; Rummens, C.P.J.; Ven, L.J.M.; Santen, R.A. Silica gel dissolution in aqueous alkali metal hydroxides studied by 29Si NMR. J. Non Cryst. Solids. 1989, 109, 85–94. [Google Scholar] [CrossRef]
- Diamond, S.; Ong, S. The mechanisms of lithium effects on ASR. In Proceedings of the 9th International Conference Alkali-Aggregate Reaction, London, UK, 27–31 July 1992; pp. 269–278. [Google Scholar]
- Kawamura, M.; Lwahori, K. ASR gel composition and expansive pressure in mortars under restrain. Cem. Concr. Compos. 2004, 26, 47–56. [Google Scholar] [CrossRef]
- Leemann, A.; Lortscher, L.; Bernard, L.; Le Saout, G.; Lothenbach, B.; Espinosa-Marzal, R.M. Mitigation of ASR by the use of LiNO3—Characterization of the reaction products. Cem. Concr. Res. 2014, 59, 73–86. [Google Scholar] [CrossRef]
- Schneider, J.F.; Hasparyk, N.P.; Silva, D.A.; Monteiro, P.J.M. Effect of lithium nitrate on the alkali–silica reaction gel. J. Am. Ceram. Soc. 2008, 91, 3370–3374. [Google Scholar] [CrossRef]
- Feng, X.; Thomas, M.D.A.; Bremner, T.W.; Folliard, K.J.; Fournier, B. New observations on the mechanism of lithium nitrate against alkali silica reaction (ASR). Cem. Concr. Res. 2010, 40, 94–101. [Google Scholar] [CrossRef]
- Mitchell, L.D.; Beaudoin, J.J.; Grattan-Bellew, P. The effects of lithium hydroxide solution on alkali silica reaction gels created with opal. Cem. Concr. Res. 2004, 34, 641–649. [Google Scholar] [CrossRef]
- Diamond, S. Unique response of LiNO3 as an alkali silica reaction-preventive admixture. Cem. Concr. Res. 1999, 29, 1271–1275. [Google Scholar] [CrossRef]
- Stokes, D.B.; Wang, H.H.; Diamond, S. A lithium-based admixture for ASR control that does not increase the pore solution pH. In Proceedings of the 5th CANMET/ACI International Conference on Superplasticizers and Other Chemical Admixtures in Concrete, SP-173, Rome, Italy, 7–10 October 1997; pp. 855–867. [Google Scholar]
- Zapała-Sławeta, J.; Owsiak, Z. The role of lithium compounds in mitigating alkali-gravel aggregate reaction. Constr. Build. Mater. 2016, 115, 299–303. [Google Scholar] [CrossRef]
- Kawamura, M.; Fuwa, H. Effects of lithium salts on ASR gel composition and expansion of mortars. Cem. Concr. Res. 2003, 33, 913–919. [Google Scholar] [CrossRef]
- Berra, M.; Mangialardi, T.; Paolini, A.E. Use of lithium compounds to prevent expansive alkali–silica reactivity in concrete. Adv. Cem. Based. Mater. 2003, 15, 145–154. [Google Scholar] [CrossRef]
- Feng, X.; Thomas, M.D.A.; Bremner, T.W.; Folliard, K.J.; Fournier, B. Summary of research on the effect of LiNO3 on alkali–silica reaction in new concrete. Cem. Concr. Res. 2010, 40, 636–642. [Google Scholar] [CrossRef]
- Kim, T.; Olek, J. The effects of lithium ions on chemical sequence of alkali–silica reaction. Cem. Concr. Res. 2016, 79, 159–168. [Google Scholar] [CrossRef]
- Zhou, B.F.; Mao, Z.Y.; Deng, M. Reaction of Quartz Glass in Lithium-Containing Alkaline Solutions with or without Ca; Royal Society Open Science: London, UK, 2018; Volume 5. [Google Scholar]
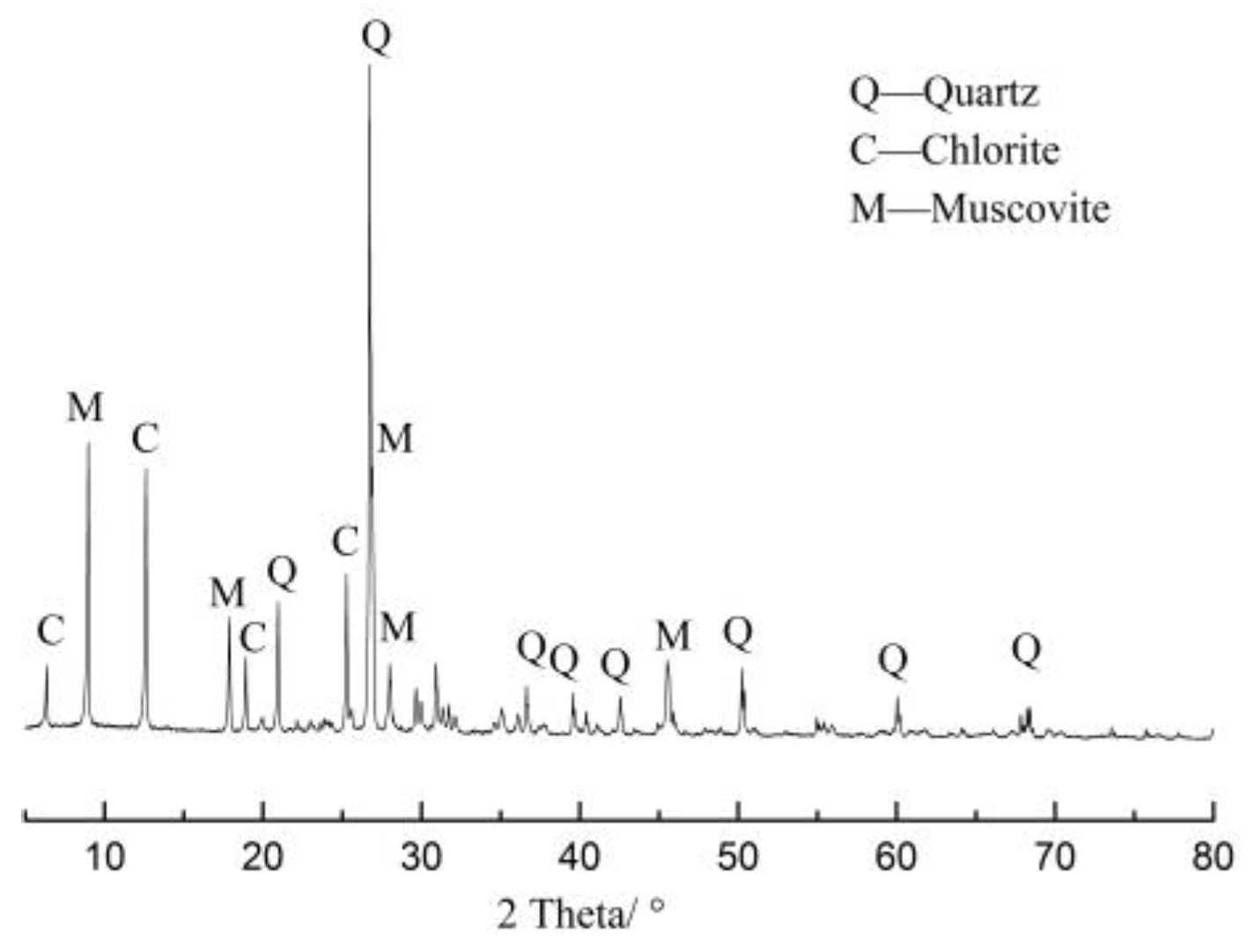
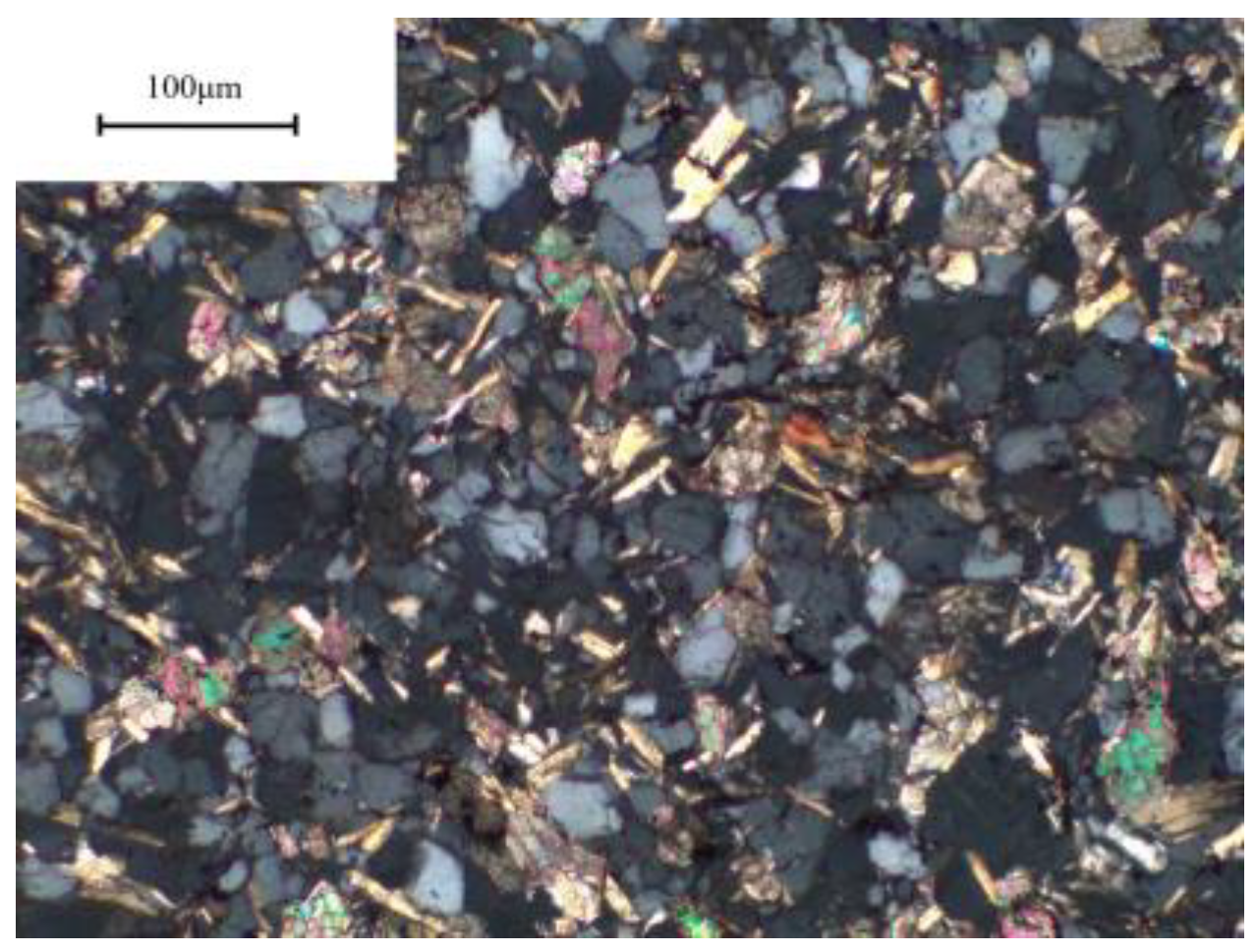
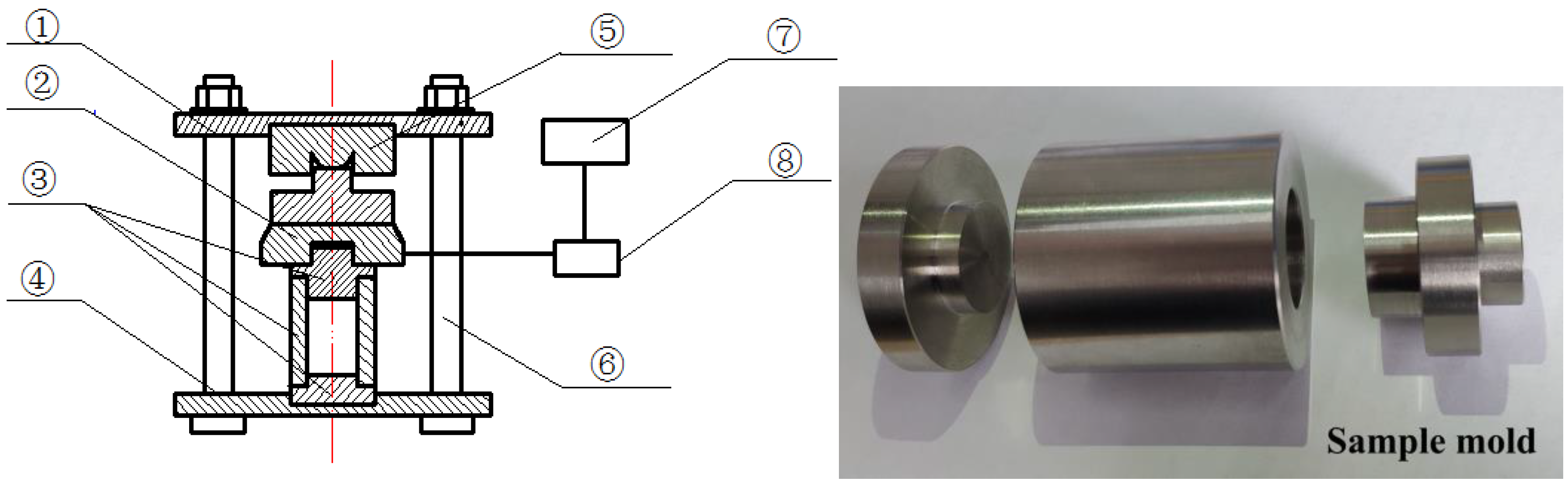


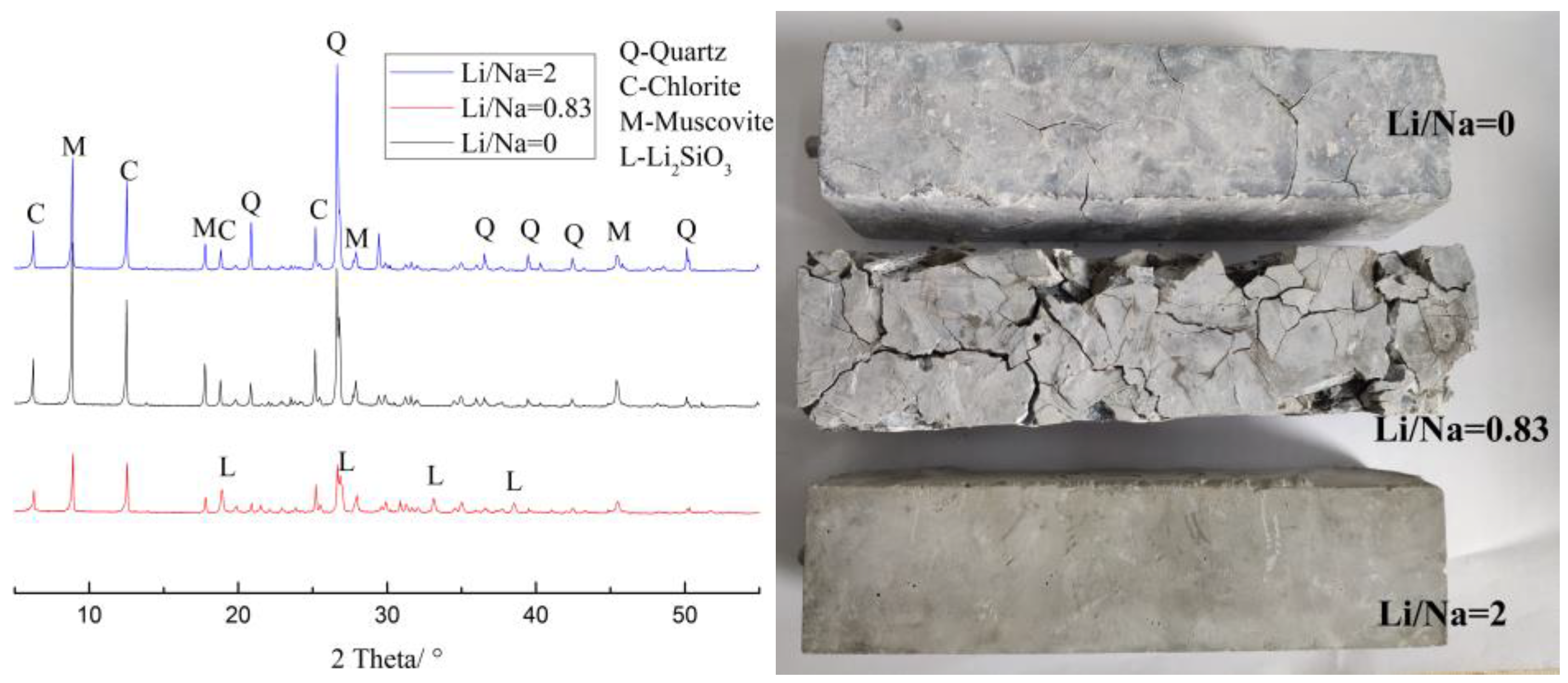


| Oxide Component | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | LOI. |
|---|---|---|---|---|---|---|---|---|
| Sandstone (wt%) | 53.12 | 17.47 | 5.53 | 5.26 | 3.89 | 4.04 | 0.715 | 9.15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Yu, L.; Deng, M. Effect of LiNO3 on Expansion of Alkali–Silica Reaction in Rock Prisms and Concrete Microbars Prepared by Sandstone. Materials 2019, 12, 1150. https://doi.org/10.3390/ma12071150
Liu J, Yu L, Deng M. Effect of LiNO3 on Expansion of Alkali–Silica Reaction in Rock Prisms and Concrete Microbars Prepared by Sandstone. Materials. 2019; 12(7):1150. https://doi.org/10.3390/ma12071150
Chicago/Turabian StyleLiu, Jinxin, Lanqing Yu, and Min Deng. 2019. "Effect of LiNO3 on Expansion of Alkali–Silica Reaction in Rock Prisms and Concrete Microbars Prepared by Sandstone" Materials 12, no. 7: 1150. https://doi.org/10.3390/ma12071150
APA StyleLiu, J., Yu, L., & Deng, M. (2019). Effect of LiNO3 on Expansion of Alkali–Silica Reaction in Rock Prisms and Concrete Microbars Prepared by Sandstone. Materials, 12(7), 1150. https://doi.org/10.3390/ma12071150




