A Multiscale Model of Oxidation Kinetics for Cu-Based Oxygen Carrier in Chemical Looping with Oxygen Uncoupling
Abstract
:1. Introduction
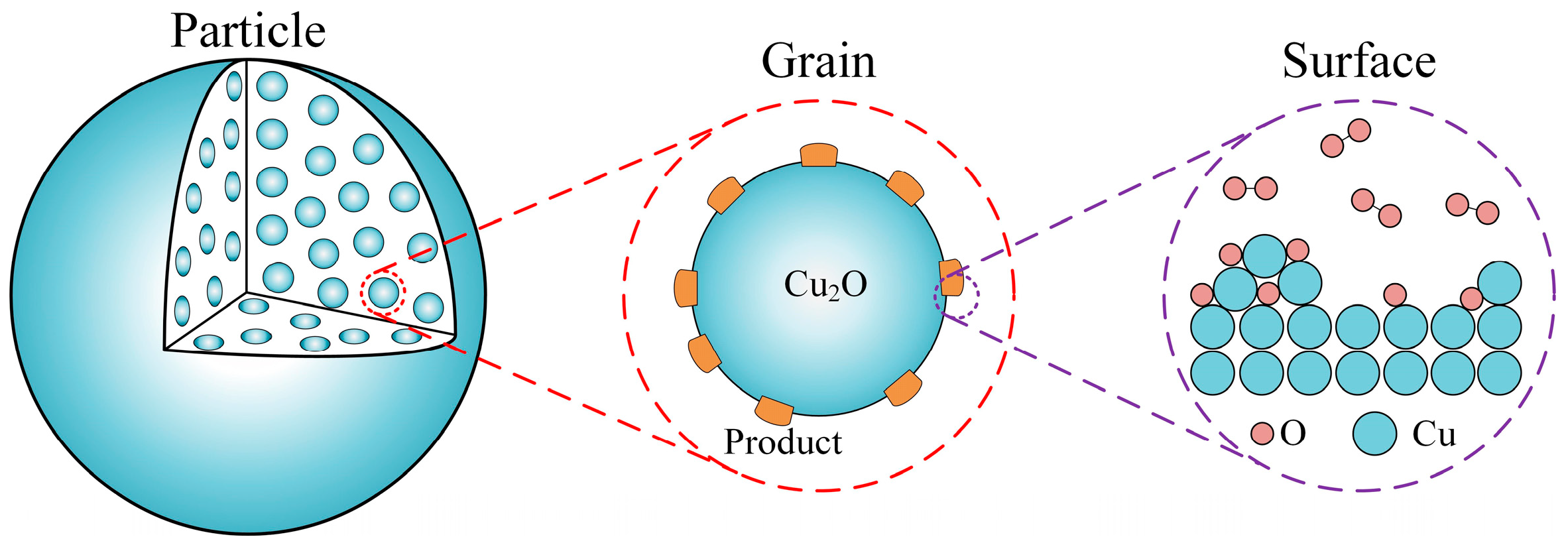
2. Mathematical Model
2.1. Model at Surface Scale
2.2. Model at Grain Scale
2.3. Model at Particle Scale
3. Results
3.1. Effects of O2 Partial Pressure
3.2. Effects of Temperature
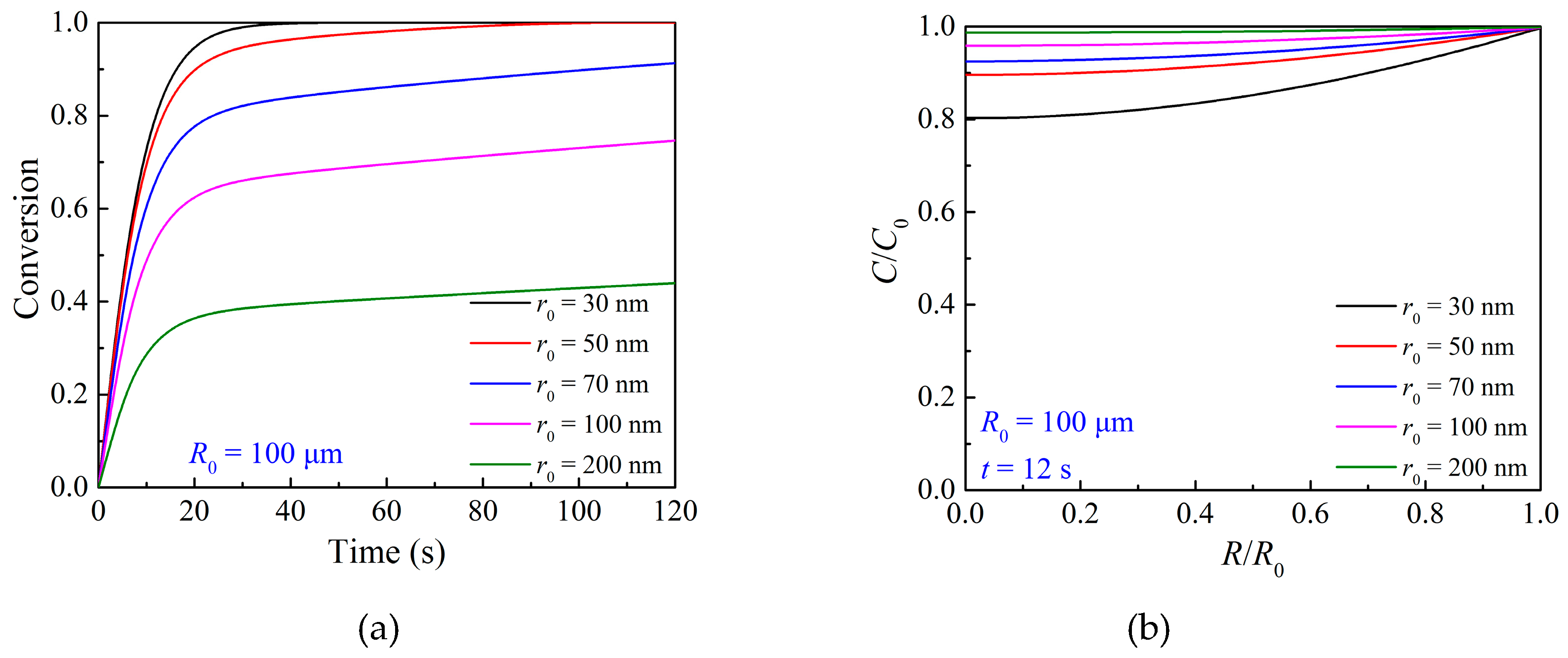
3.3. Effects of Particle Structure
4. Discussion
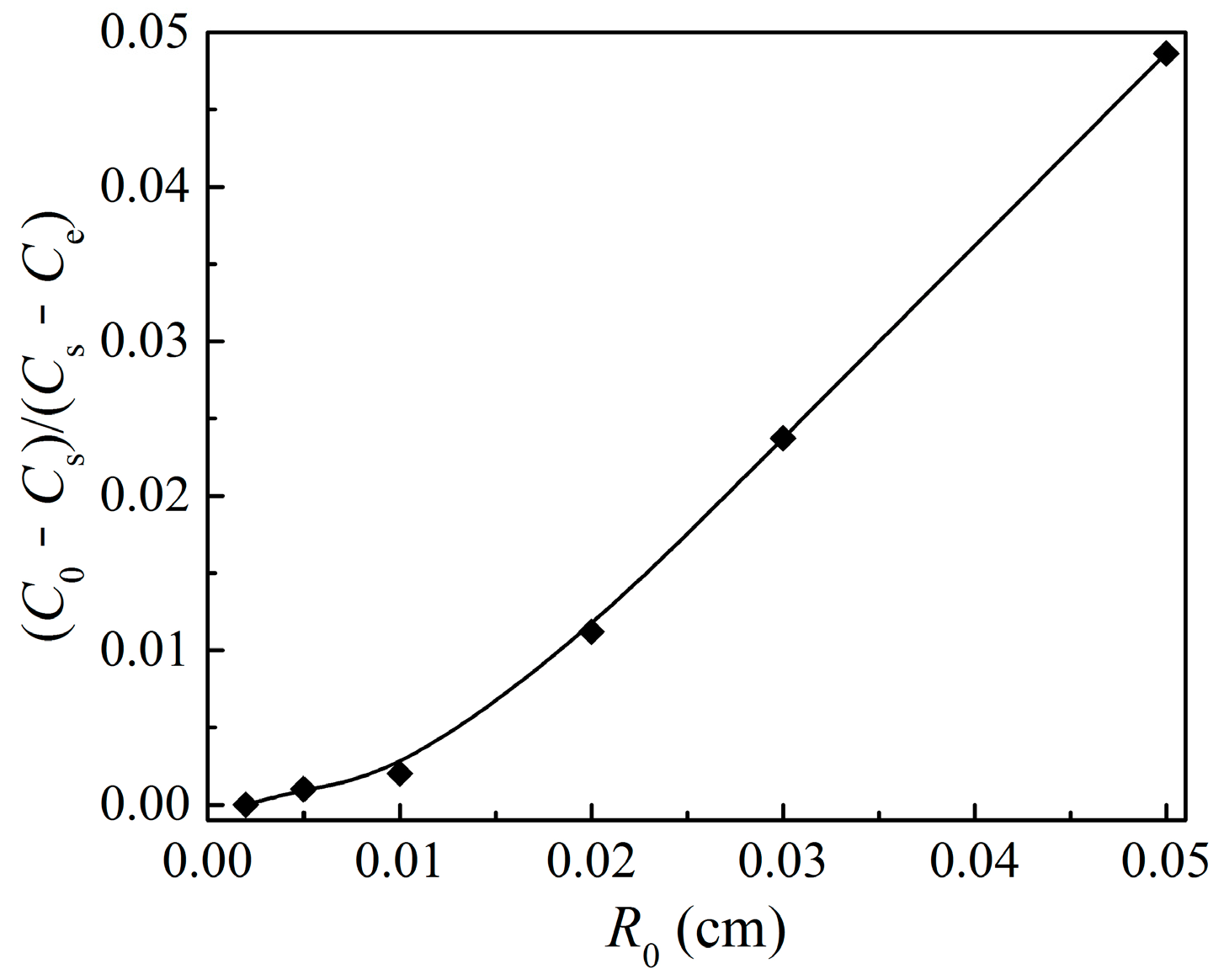
4.1. External Mass Transfer
4.2. Effectiveness Factor
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| C | concentration of O2 inside the particle, mol/cm3 |
| C0 | concentration of O2 in the ambient gas, mol/cm3 |
| Ce | equilibrium concentration of O2, mol/cm3 |
| Cmet | concentration of metal ions on the Cu2O/CuO interface, mol/cm3 |
| Cs | concentration of O2 on the particle external surface, mol/cm3 |
| D1 | diffusivity of gas through the particle pores, cm2/s |
| De | effective diffusivity of gas through the particle pores, cm2/s |
| DK | Knudsen diffusivity, cm2/s |
| O2 molecular diffusivity, cm2/s | |
| Ds | diffusivity of metal ions through the product layer, cm2/s |
| Ek, ED | activation energy, kJ/mol |
| hc | critical solid reactant layer thickness, cm |
| k | chemical reaction rate constant, cm/s |
| kg | external mass transfer coefficient, cm/s |
| P | pressure, atm |
| Pe | equilibrium partial pressure of O2, atm |
| r | grain radius, cm |
| r0 | initial grain radius, cm |
| r1 | grain radius of CuO/O2 interface, cm |
| r1c | critical grain radius of CuO/O2 interface, cm |
| r2 | grain radius of Cu2O/CuO interface, cm |
| r2c | critical grain radius of Cu2O/CuO interface, cm |
| R | particle radius, cm |
| R0 | initial particle radius, cm |
| Rg | the universal gas constant, J/(mol·K) |
| t | reaction time, s |
| T | temperature, K |
| v | diffusion volume for molecule, cm3 |
| molar volume of CuO, cm3/mol | |
| molar volume of Cu2O, cm3/mol | |
| Z | stoichiometric molar volume ratio of the solid product to the solid reactant |
| α | local conversion |
| overall conversion | |
| δ | ratio of the unoccupied area on the grain surface |
| ε | local porosity |
| ε0 | initial porosity |
| effectiveness factor | |
| Thiele modulus |
References
- Lyngfelt, A. Chemical-Looping Combustion of Solid Fuels—Status of Development. Appl. Energy 2014, 113, 1869–1873. [Google Scholar]
- Lyngfelt, A.; Leckner, B.; Mattisson, T. A fluidized-bed combustion process with inherent CO2 separation application of chemical-looping combustion. Chem. Eng. Sci. 2001, 56, 3101–3113. [Google Scholar]
- Padurean, A.; Cormos, C.C.; Agachi, P.S. Pre-Combustion Carbon Dioxide Capture by Gas–Liquid Absorption for Integrated Gasification Combined Cycle Power Plants. Int. J. Greenh. Gas Con. 2012, 7, 1–11. [Google Scholar] [CrossRef]
- Romeo, L.M.; Bolea, I.; Escosa, J.M. Integration of Power Plant and Amine Scrubbing to Reduce CO2 Capture Costs. Appl. Therm. Eng. 2008, 28, 1039–1046. [Google Scholar]
- Petrakopoulou, F.; Boyano, A.; Cabrera, M.; Tsatsaronis, G. Exergoeconomic and Exergoenvironmental Analyses of a Combined Cycle Power Plant with Chemical Looping Technology. Int. J. Greenh. Gas Con. 2011, 5, 475–482. [Google Scholar]
- Mattisson, T.; Lyngfelt, A.; Leion, H. Chemical-looping with oxygen uncoupling for combustion of solid fuels. Int. J. Greenh. Gas Con. 2009, 3, 11–19. [Google Scholar] [CrossRef]
- Abad, A.; Mattisson, T.; Lyngfelt, A.; Rydén, M. Chemical looping combustion in a 300 Wth continuously operating reactor system using a manganese-based oxygen carrier. Fuel 2006, 85, 1174–1185. [Google Scholar] [CrossRef]
- Chuang, S.Y.; Dennis, J.S.; Hayhurst, A.N.; Scott, S.A. Kinetics of the oxidation of a co-precipitated mixture of Cu and Al2O3 by O2 for chemical-looping combustion. Energy Fuel 2010, 24, 3917–3927. [Google Scholar]
- Chuang, S.; Dennis, J.S.; Hayhurst, A.N.; Scott, S.A. Development and performance of Cu-based oxygen carriers for chemical-looping combustion. Combust. Flame 2008, 154, 109–121. [Google Scholar]
- Adanez-Rubio, I.; Abad, A.; Gayán, P.; Diego, L.F.D.; García-Labiano, F.; Adánez, J. Identification of operational regions in the chemical-looping with oxygen uncoupling (CLOU)process with a Cu-based oxygen carrier. Fuel 2012, 102, 634–645. [Google Scholar]
- Diego, L.F.D.; García-Labiano, F.; Adánez, J.; Gayán, P.; Abad, A.; Corbella, B.M.; Palaciosb, J.M. Development of Cu-based oxygen carriers for chemical-looping combustion. Fuel 2004, 83, 1749–1757. [Google Scholar] [Green Version]
- Goldstein, E.A.; Mitchell, R.E. Chemical kinetics of copper oxide reduction with CO. Proc. Combust. Inst. 2011, 33, 2803–2810. [Google Scholar] [CrossRef]
- Gayán, P.; Adánez-Rubio, I.; Abad, A.; Diego, L.F.D.; García-Labiano, F.; Adánez, J. Development of Cu-based oxygen carriers for chemical-looping with oxygen uncoupling (CLOU) process. Fuel 2012, 96, 226–238. [Google Scholar] [CrossRef]
- Adánez-Rubio, I.; Arjmand, M.; Leion, H.; Gayán, P.; Abad, A.; Mattisson, T.; Lyngfelt, A. Investigation of combined supports for Cu-based oxygen carriers for chemical-looping with oxygen uncoupling (CLOU). Energy Fuel 2013, 27, 3918–3927. [Google Scholar] [CrossRef]
- Diego, L.F.D.; Gayán, P.; García-Labiano, F.; Celaya, J.; Abad, A.; Adánez, J. Impregnated CuO/Al2O3 oxygen carriers for chemical-looping combustion, avoiding fluidized bed agglomeration. Energy Fuel 2005, 19, 1850–1856. [Google Scholar] [CrossRef]
- Clayton, C.K.; Sohn, H.Y.; Whitty, K.J. Oxidation Kinetics of Cu2O in Oxygen Carriers for Chemical Looping with Oxygen Uncoupling. Ind. Eng. Chem. Res. 2014, 53, 2976–2986. [Google Scholar] [CrossRef]
- García-Labiano, F.; Diego, L.F.D.; Adánez, J.; Abad, A.; Gayán, P. Temperature variations in the oxygen carrier particles during their reduction and oxidation in a chemical-looping combustion system. Chem. Eng. Sci. 2005, 60, 851–862. [Google Scholar] [CrossRef] [Green Version]
- Maya, J.C.; Chejne, F. Modeling of Oxidation and Reduction of a Copper-Based Oxygen Carrier. Energy Fuel 2014, 28, 5434–5444. [Google Scholar] [CrossRef]
- Dennis, J.S.; Pacciani, R. The rate and extent of uptake of CO2 by a synthetic, CaO-containing sorbent. Chem. Eng. Sci. 2009, 64, 2147–2157. [Google Scholar] [CrossRef]
- Liu, W.; Dennis, J.S.; Sultan, D.S.; Redfern, S.A.T.; Scott, S.A. An investigation of the kinetics of CO2 uptake by a synthetic calcium based sorbent. Chem. Eng. Sci. 2012, 69, 644–658. [Google Scholar] [CrossRef]
- Szekely, J.; Evans, J.W.; Sohn, H.Y. Gas−Solid Reactions; Academic Press: New York, NY, USA, 1976. [Google Scholar]
- Gattinoni, C.; Michaelides, A. Atomistic details of oxide surfaces and surface oxidation: The example of copper and its oxides. Surf. Sci. Rep. 2015, 70, 424–447. [Google Scholar]
- Zhang, R.; Liu, H.; Zheng, H.; Ling, L.; Wang, B. Adsorption and dissociation of O2 on the Cu2O(111) surface: Thermochemistry, reaction barrier. Appl. Surf. Sci. 2011, 257, 4787–4794. [Google Scholar]
- Yu, X.; Zhang, X.; Tian, X.; Wang, S.; Feng, G. Density functional theory calculations on oxygen adsorption on the Cu2O surfaces. Appl. Surf. Sci. 2015, 324, 53–60. [Google Scholar] [CrossRef]
- Bao, J.; Li, Z.; Sun, H.; Cai, N. Experiment and rate equation modeling of Fe oxidation kinetics in chemical looping combustion. Combust. Flame 2013, 160, 808–817. [Google Scholar]
- Li, Z.S.; Fang, F.; Cai, N.S. Characteristic of solid product layer of MgSO4 in the reaction of MgO with SO2. Sci. China Technol. Sci. 2010, 53, 1869–1876. [Google Scholar] [CrossRef]
- Fang, F.; Li, Z.S.; Cai, N.S.; Tang, X.Y.; Yang, H.T. AFM investigation of solid product layer of MgSO4 generated on MgO surfaces in the reaction of MgO with SO2 and O2. Chem. Eng. Sci. 2011, 66, 1142–1149. [Google Scholar] [CrossRef]
- Tang, X.Y.; Li, Z.S.; Fang, F.; Cai, N.S.; Yang, H.T. AFM investigation of the morphology of CaSO4 product layer formed during direct sulfation on polished single-crystal CaCO3 surfaces at high CO2 concentrations. Proc. Combust. Inst. 2011, 33, 2683–2689. [Google Scholar]
- Schulz, K.H.; Cox, D.F. Photoemission and low-energy-electron-diffraction study of clean and oxygen-dosed Cu2O (111) and (100) surfaces. Phys. Rev. B 1991, 43, 1610–1621. [Google Scholar]
- Le, D.; Stolbov, S.; Rahman, T.S. Reactivity of the Cu2O (100) surface: Insights from first principles calculations. Surf. Sci. 2009, 603, 1637–1645. [Google Scholar]
- Zhou, L.J.; Zou, Y.C.; Zhao, J.; Wang, P.P.; Feng, L.L.; Sun, L.W.; Wang, D.J.; Li, G.D. Facile synthesis of highly stable and porous Cu2O/CuO cubes with enhanced gas sensing properties. Sens. Actuators B 2013, 188, 533–539. [Google Scholar] [CrossRef]
- Trinh, T.T.; Tu, N.H.; Le, H.H.; Ryu, K.Y.; Le, K.B.; Pillai, K.; Yi, J. Improving the ethanol sensing of ZnO nano-particle thin films—The correlation between the grain size and the sensing mechanism. Sens. Actuators B 2011, 152, 73–81. [Google Scholar]
- Umar, A.; Alshahrani, A.A.; Algarni, H.; Kumar, R. CuO nanosheets as potential scaffolds for gas sensing applications. Sens. Actuators B 2017, 250, 24–31. [Google Scholar] [CrossRef]
- Wang, M.; Liu, J.; Shen, F.; Cheng, H.; Dai, J.; Long, Y. Theoretical study of stability and reaction mechanism of CuO supported on ZrO2 during chemical looping combustion. Appl. Surf. Sci. 2016, 367, 485–492. [Google Scholar]
- Alvarez, D.; Abanades, J.C. Determination of the critical product layer thickness in the reaction of CaO with CO2. Ind. Eng. Chem. Res. 2005, 44, 5608–5615. [Google Scholar]
- Marks, L.D. Modified wulff constructions for twinned particles. J. Cryst. Growth 1983, 61, 556–566. [Google Scholar] [CrossRef]
- Li, Z.S.; Sun, H.M.; Cai, N.S. Rate Equation Theory for the Carbonation Reaction of CaO with CO2. Energy Fuel 2012, 26, 4607–4616. [Google Scholar]
- Wang, H.; Li, Z.S.; Fan, X.X.; Cai, N.S. Rate-Equation-Based Grain Model for the Carbonation of CaO with CO2. Energy Fuel 2017, 31, 14018–14032. [Google Scholar] [CrossRef]
- Rapp, R.A. The high temperature oxidation of metals forming cation-diffusing scales. Metall. Mater. Trans. A 1984, 15, 765–782. [Google Scholar] [CrossRef]
- Fuller, E.N.; Ensley, K.; Giddings, J.C. New method for prediction of binary gas-phase diffusion coefficients. Ind. Eng. Chem. Res. 1996, 58, 19–27. [Google Scholar] [CrossRef]
- Khoshandam, B.; Kumar, R.V.; Allahgholi, L. Mathematical modeling of co2removal using carbonation with CaO: The grain model. Korean J. Chem. Eng. 2010, 27, 766–776. [Google Scholar] [CrossRef]
- Adánez-Rubio, I.; Gayán, P.; Abad, A.; García-Labiano, F.; De Diego, L.F.; Adánez, J. Kinetic analysis of a cu-based oxygen carrier: Relevance of temperature and oxygen partial pressure on reduction and oxidation reactions rates in chemical looping with oxygen uncoupling (CLOU). Chem. Eng. J. 2014, 256, 69–84. [Google Scholar]
- Whitty, K.; Clayton, C. Measurement and Modeling of Kinetics for Copper-Based Chemical Looping with Oxygen Uncoupling. In Proceedings of the 2nd International Conference on Chemical Looping, Darmstadt, Germany, 26–28 September 2012. [Google Scholar]
- Sahir, A.H.; Lighty, J.A.S.; Sohn, H.Y. Kinetics of Copper Oxidation in the Air Reactor of a Chemical Looping Combustion System using the Law of Additive Reaction Times. Ind. Eng. Chem. Res. 2011, 50, 566–580. [Google Scholar] [CrossRef]
- Alonso, M.; Criado, Y.A.; Abanades, J.C.; Grasa, G. Undesired effects in the determination of CO2 carrying capacities of CaO during TG testing. Fuel 2014, 127, 52–61. [Google Scholar]
- Sedghkerdar, M.H.; Mostafavi, E.; Mahinpey, N. Investigation of the Kinetics of Carbonation Reaction with Cao-Based Sorbents Using Experiments and Aspen Plus Simulation. Chem. Eng. Commun. 2015, 202, 746–755. [Google Scholar]
- Yao, J.G.; Zhang, Z.; Sceats, M.; Maitland, G.C.; Fennell, P.S. Two-phase fluidized bed model for pressurized carbonation kinetics of calcium oxide. Energy Fuel 2017, 31, 11181–11193. [Google Scholar] [CrossRef]





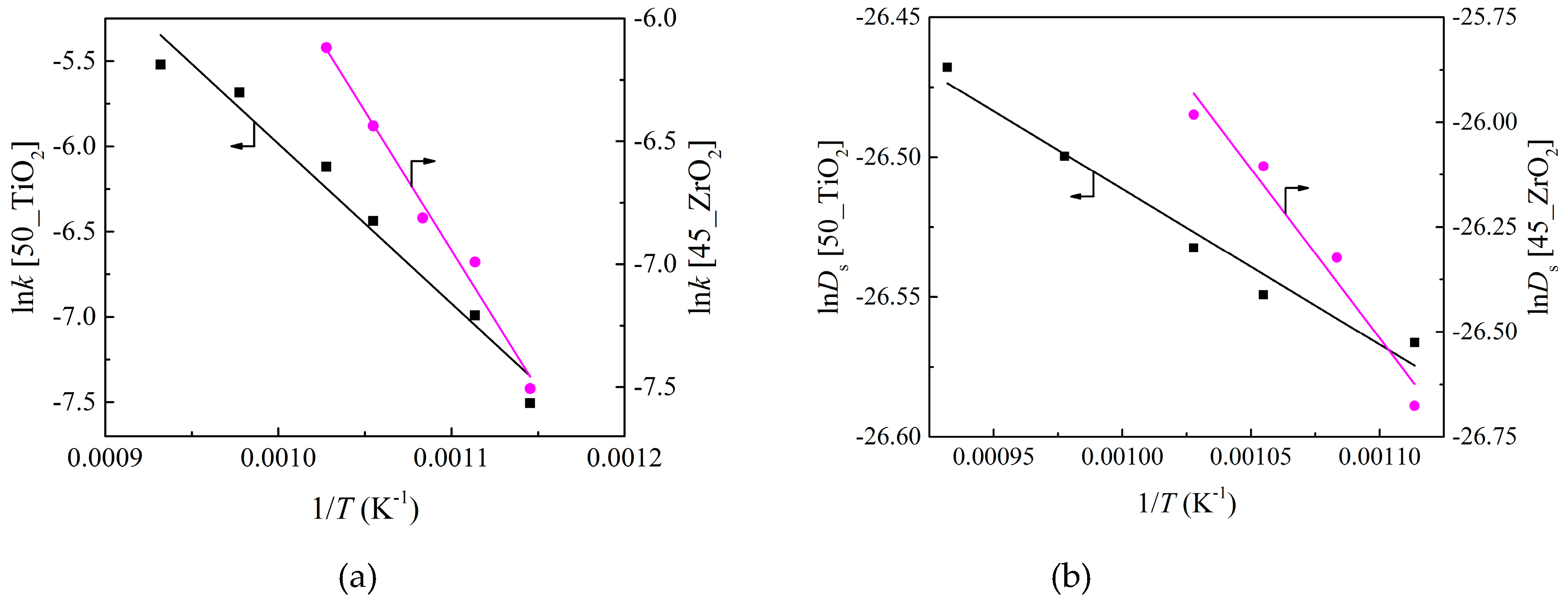





| Parameter | k0 (cm/s) | Ek (kJ/mol) | D0 (cm2/s) | ED (kJ/mol) | a (cm/K) | b (cm) |
|---|---|---|---|---|---|---|
| 50_TiO2 material | 29 | 78 | 5.3 × 10−12 | 4.6 | 9.4 × 10−9 | −7.1 × 10−6 |
| 45_ZrO2 material | 245 | 94 | 2.2 × 10−8 | 67 | 5.7 × 10−9 | −4.4 × 10−6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Li, Z.; Cai, N. A Multiscale Model of Oxidation Kinetics for Cu-Based Oxygen Carrier in Chemical Looping with Oxygen Uncoupling. Materials 2019, 12, 1170. https://doi.org/10.3390/ma12071170
Wang H, Li Z, Cai N. A Multiscale Model of Oxidation Kinetics for Cu-Based Oxygen Carrier in Chemical Looping with Oxygen Uncoupling. Materials. 2019; 12(7):1170. https://doi.org/10.3390/ma12071170
Chicago/Turabian StyleWang, Hui, Zhenshan Li, and Ningsheng Cai. 2019. "A Multiscale Model of Oxidation Kinetics for Cu-Based Oxygen Carrier in Chemical Looping with Oxygen Uncoupling" Materials 12, no. 7: 1170. https://doi.org/10.3390/ma12071170





