Gd/Sm-Pr Co-Doped Ceria: A First Report of the Precipitation Method Effect on Flash Sintering
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
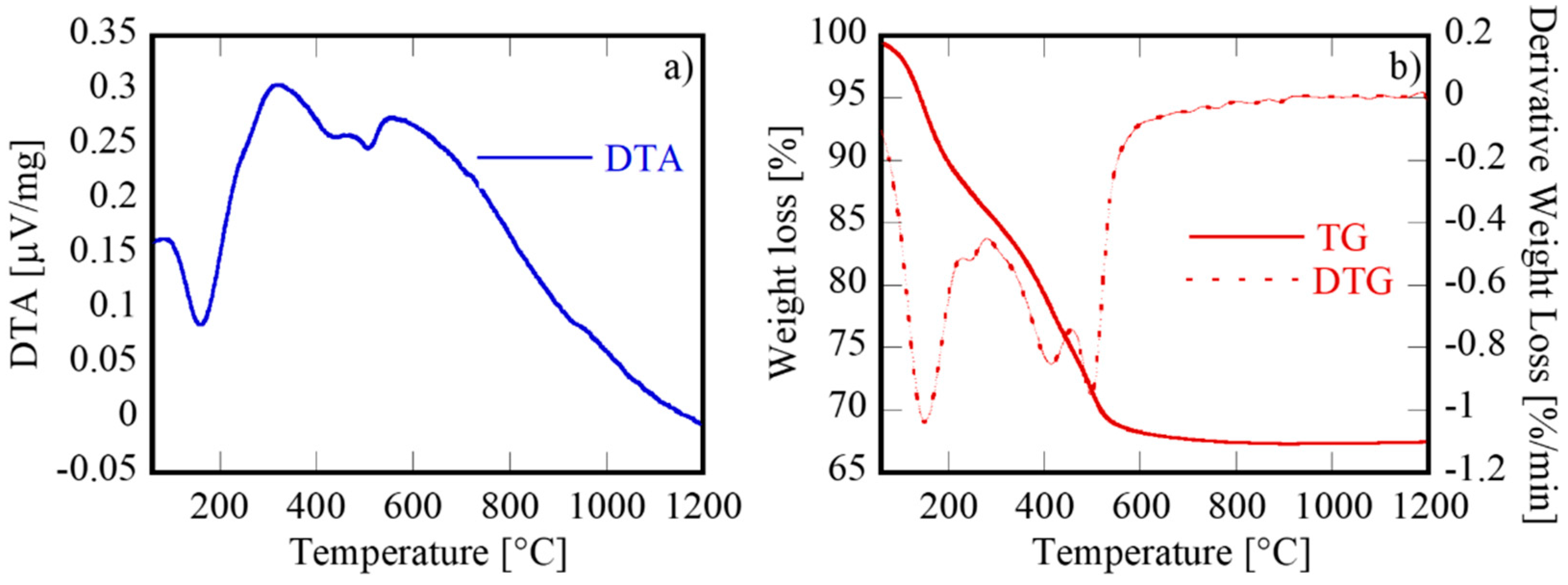
3.1. Characterization of the Samples
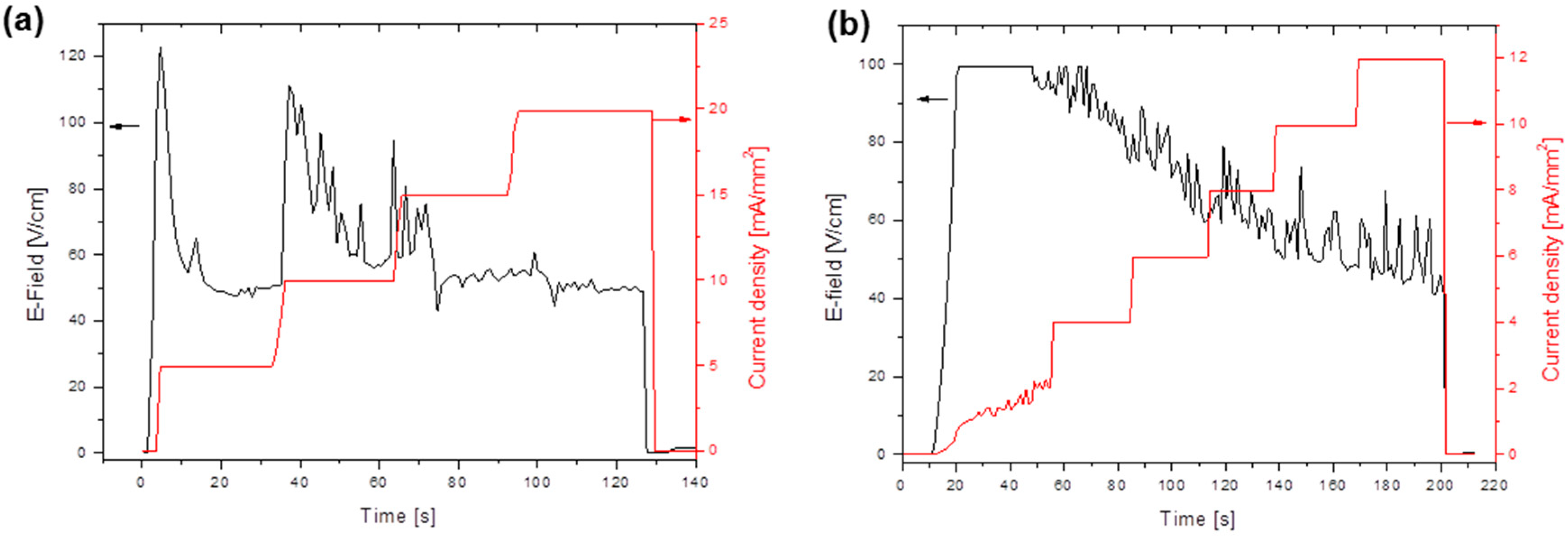
3.2. Flash Sintering
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hao, X.; Liu, Y.; Wang, Z.; Qiao, J.; Sun, K. A novel sintering method to obtain fully dense gadolinia doped ceria by applying a direct current. J. Power Source 2012, 210, 86–91. [Google Scholar] [CrossRef]
- Jaiswala, N.; Tanwarb, K.; Sumana, R.; Uppadhyac, D.K.S.; Parkash, O. A Brief Review on Ceria Based Solid Electrolytes for Solid Oxide Fuel Cells. J. Alloys Compd. 2019, 781, 984–1005. [Google Scholar] [CrossRef]
- Santos, T.H.; Grilo, J.P.F.; Loureiro, F.J.A.; Fagg, D.P.; Fonseca, F.C.; Macedo, D.A. Structure, densification and electrical properties of Gd3+ and Cu2+ co-doped ceria solid electrolytes for SOFC applications: Effects of Gd2O3 content. Ceram. Int. 2018, 44, 2745–2751. [Google Scholar] [CrossRef]
- Anwar, M.; Muhammad Ali, S.A.; Muchtar, A.; Somalu, M.R. Influence of strontium co-doping on the structural, optical, and electrical properties of erbium-doped ceria electrolyte for intermediate temperature solid oxide fuel cells. Ceram. Int. 2019, 45, 5627–5636. [Google Scholar] [CrossRef]
- Liu, Y.; Mushtaq, M.N.; Zhang, W.; Teng, A.; Liu, X. Single-phase electronic-ionic conducting Sm3+/Pr3+/Nd3+ triple-doped ceria for new generation fuel cell technology. Int. J. Hydrog. Energy 2018, 43, 12817–12824. [Google Scholar] [CrossRef]
- Bowman, W.J.; Zhu, J.; Sharma, R.; Crozier, P.A. Electrical conductivity and grain boundary composition of Gd-doped and Gd/Pr co-doped ceria. Solid State Ion. 2015, 272, 9–17. [Google Scholar] [CrossRef]
- Spiridigliozzi, L.; Dell’Agli, G.; Accardo, G.; Yoon, S.P.; Frattini, D. Electro-morphological, structural, thermal and ionic conduction properties of Gd/Pr co-doped ceria electrolytes exhibiting mixed Pr3+/Pr4+ cations. Ceram. Int. 2019, 45, 4570–4580. [Google Scholar] [CrossRef]
- Spiridigliozzi, L. Doped Ceria Electrolytes: Synthesis Methods. In Doped-Ceria Electrolytes; SpringerNature: Cham, Switzerland, 2018; pp. 25–55. ISBN 978-3-319-99394-2. [Google Scholar]
- Spiridigliozzi, L.; Dell’Agli, G.; Marocco, A.; Accardo, G.; Pansini, M.; Kwon, Y.; Yoon, S.P.; Frattini, D. Engineered co-precipitation chemistry with ammonium carbonate for scalable synthesis and sintering of improved Sm0.2Ce0.8O1.90 and Gd0.16Pr0.04Ce0.8O1.90 electrolytes for IT-SOFCs. J. Ind. Eng. Chem. 2018, 59, 17–27. [Google Scholar] [CrossRef]
- Accardo, G.; Spiridigliozzi, L.; Cioffi, R.; Ferone, C.; Di Bartolomeo, E.; Yoon, S.; Dell’Agli, G. Gadolinium-doped ceria nanopowders synthesized by Urea-Based Homogeneous co-Precipitation (UBHP). Mater. Chem. Phys. 2017, 187, 149–155. [Google Scholar] [CrossRef]
- Joh, D.W.; Rath, M.K.; Park, W.J.; Park, H.J.; Cho, H.K.; Lee, S.; Yoon, J.K.; Lee, J.H.; Lee, T.K. Sintering behavior and electrochemical performances of nano-sized gadolinium-doped ceria via ammonium carbonate assisted co-precipitation for solid oxide fuel cells. J. Alloys Compd. 2016, 682, 188–195. [Google Scholar] [CrossRef]
- Robert, C.L.; Long, J.W.; Lucas, E.M.; Pettigrew, K.A.; Stroud, R.M.; Doescher, M.S.; Rolison, D.R. Sol-Gel-Derived Ceria Nanoarchitectures: Synthesis, Characterization and Electrical Properties. Chem. Mater. 2006, 18, 50–58. [Google Scholar] [CrossRef]
- Dell’Agli, G.; Spiridigliozzi, L.; Marocco, A.; Accardo, G.; Frattini, D.; Kwon, Y.; Yoon, S.P. Morphological and crystalline evolution of Sm-(20 mol%)–doped ceria nanopowders prepared by a combined co-precipitation/hydrothermal synthesis for solid oxide fuel cell applications. Ceram. Int. 2017, 43, 12799–12808. [Google Scholar] [CrossRef]
- Dell’Agli, G.; Spiridigliozzi, L.; Pansini, M.; Accardo, G.; Yoon, S.P.; Frattini, D. Effect of the carbonate environment on morphology and sintering behaviour of variously co-doped (Ca, Sr, Er, Pr) Samarium-doped Ceria in co-precipitation/hydrothermal synthesis. Ceram. Int. 2018, 44, 17935–17944. [Google Scholar] [CrossRef]
- Zhang, X.; Deces-Petit, C.; Yick, S.; Robertson, M.; Kesler, O.; Maric, R.; Ghosh, D. A study on sintering aids for Sm0.2Ce0.8O1.9 electrolyte. J. Power Sources 2006, 162, 480–485. [Google Scholar] [CrossRef]
- Biesuz, M.; Spiridigliozzi, L.; Frasnelli, M.; Dell’Agli, G.; Sglavo, V.M. Rapid densification of Samarium-doped Ceria ceramic with nanometric grain size at 900–1100 °C. Mater. Lett. 2017, 190, 17–19. [Google Scholar] [CrossRef] [Green Version]
- Charoonsuk, T.; Sukkha, U.; Kolodiazhnyi, T.; Vittayakorn, N. Enhancing the densification of ceria ceramic at low temperature via the cold sintering assisted two-step sintering process. Ceram. Int. 2018, 44, S54–S57. [Google Scholar] [CrossRef]
- Cologna, M.; Rashkova, B.; Raj, R. Flash sintering of nanograin zirconia in <5 s at 850 °C. J. Am. Ceram. Soc. 2010, 93, 3556–3559. [Google Scholar] [CrossRef]
- Biesuz, M.; Sglavo, V.M. Flash sintering of ceramics. J. Eur. Ceram. Soc. 2019, 39, 115–143. [Google Scholar] [CrossRef]
- Becker, M.Z.; Shomrat, N.; Tsur, Y. Recent Advances in Mechanism Research and Methods for Electric-Field-Assisted Sintering of Ceramics. Adv. Mater. 2018, 30, 1706369. [Google Scholar] [CrossRef]
- Todd, R.I.; Zapata-Solvas, E.; Bonilla, R.S.; Sneddon, T.; Wilshaw, P.R. Electrical characteristics of flash sintering: Thermal runaway of Joule heating. J. Eur. Ceram. Soc. 2015, 35, 1865–1877. [Google Scholar] [CrossRef]
- da Silva, J.G.P.; Al-Qureshi, H.A.; Keil, F.; Janssen, R. A dynamic bifurcation criterion for thermal runaway during the flash sintering of ceramics. J. Eur. Ceram. Soc. 2016, 36, 1261–1267. [Google Scholar] [CrossRef]
- Dong, Y. On the Hotspot Problem in Flash Sintering; Department of Materials Science and Engineering, University of Pennsylvania: Philadelphia, PA, USA, 2017. [Google Scholar]
- Charalambous, H.; Jha, S.K.; Christian, K.; Lay, R.; Tsakalakos, T. Flash Sintering using Controlled Current Ramp. J. Eur. Ceram. Soc. 2018, 38, 3689–3693. [Google Scholar] [CrossRef]
- Kumar, P.; Yadav, D.; Lebrun, J.; Raj, R. Flash Sintering with Current-Rate: A Different Approach Punith. J. Am. Ceram. Soc. 2019, 102, 823–835. [Google Scholar] [CrossRef]
- Campos, J.V.; Lavagnini, I.R.; de Sousa, R.V.; Ferreira, J.A.; de Pallone, E.M. Development of an instrumented and automated flash sintering setup for enhanced process monitoring and parameter control. J. Eur. Ceram. Soc. 2019, 39, 531–538. [Google Scholar] [CrossRef]
- Biesuz, M.; Dell’Agli, G.; Spiridigliozzi, L.; Ferone, C.; Sglavo, V.M. Conventional and Field-Assisted Sintering of Nanosized Gd-doped Ceria Synthesized by Co-precipitation. Ceram. Int. 2016, 42, 11766–11771. [Google Scholar] [CrossRef]
- Spiridigliozzi, L.; Biesuz, M.; Dell’Agli, G.; Di Bartolomeo, E.; Zurlo, F.; Sglavo, V.M. Microstructural and electrical investigation of flash-sintered Gd/Sm-doped ceria. J. Mater. Sci. 2017, 52, 7479–7488. [Google Scholar] [CrossRef]
- Li, J.; Guan, W.; Luo, M.; Song, J.; Song, X.; An, S. Sintering behavior of samarium doped ceria under DC electrical field. Ceram. Int. 2018, 44, 2470–2477. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, Z.; Zhang, J.; Hao, X.; Rooney, D.; Liu, Y.; Sun, W.; Qiao, J.; Sun, K. Understanding the Flash Sintering of Rare-Earth-Doped Ceria for Solid Oxide Fuel Cell. J. Am. Ceram. Soc. 2015, 98, 1717–1723. [Google Scholar] [CrossRef]
- Kim, P.; Anderko, A.; Navrotsky, A.; Riman, R.E. Trends in Structure and Thermodynamic Properties of Normal Rare Earth Carbonates and Rare Earth Hydroxycarbonates. Minerals 2018, 8, 106. [Google Scholar] [CrossRef]
- Accardo, G.; Dell’Agli, G.; Mascolo, M.C.; Spiridigliozzi, L.; Yoon, S.P. Controlled Coprecipitation of Amorphous Cerium-Based Carbonates with Suitable Morphology as Precursors of Ceramic Electrolytes for IT-SOFCs. Materials 2019, 12, 702. [Google Scholar] [CrossRef]
- Li, J.-G.; Ikegami, T.; Wang, Y.; Mori, T. Reactive Ceria Nanopowders via carbonate precipitation. J. Am. Ceram. Soc. 2002, 85, 2376–2378. [Google Scholar] [CrossRef]
- Spiridigliozzi, L.; Dell’Agli, G.; Biesuz, M.; Sglavo, V.M.; Pansini, M. Effect of the Precipitating Agent on the Synthesis and Sintering Behaviour of 20 mol% Sm-doped Ceria. Adv. Mater. Sci. Eng. 2016, 6096123. [Google Scholar] [CrossRef]
- Lutterotti, L.; Bortolotti, M.; Ischia, G.; Lonardelli, I.; Wenk, H.R. Rietveld texture analysis from diffraction images. Z. Kristallogr. 2007, 26, 125–130. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Nicholas, J.D.; De Jonghe, L.C. Prediction and Evaluation of Sintering Aids for Cerium Gadolinium Oxide. Solid State Ion. 2007, 178, 1187–1194. [Google Scholar] [CrossRef]







| Composition | Precipitating Agent | Flash Cycle | Electric Data | Relative Density [%] |
|---|---|---|---|---|
| 6PrSDC20 | (NH4)2CO3 | Traditional | 50V/cm, 20 mA/mm2 | 81 |
| 6PrSDC20 | (NH4)2CO3 | Traditional | 50V/cm, 40 mA/mm2 | 61 |
| 6PrSDC20 | (NH4)2CO3 | Traditional | 50V/cm, 80 mA/mm2 | 74 |
| 6PrGDC20 | (NH4)2CO3 | Traditional | 50V/cm, 20 mA/mm2 | 88 |
| 6PrGDC20 | (NH4)2CO3 | Traditional | 50V/cm, 40 mA/mm2 | 77 |
| 6PrGDC20 | (NH4)2CO3 | Traditional | 50V/cm, 80 mA/mm2 | 60 |
| GDC20 | (NH4)2CO3 | Traditional | 50V/cm, 15 mA/mm2 | 66 |
| GDC20 | (NH4)2CO3 | Traditional | 50V/cm, 10 mA/mm2 | 68 |
| SDC20 | (NH4)2CO3 | Traditional | 50V/cm, 15 mA/mm2 | 84 |
| 6PrSDC20 | (NH4)2CO3 | Ramp | 5 mA/mm2 steps up to 20 mA/mm2 | 91 |
| 6PrSDC20 | (NH4)2CO3 | Ramp | 2 mA/mm2 steps up to 12 mA/mm2 | 88 |
| 6PrGDC20 | (NH4)2CO3 | Ramp | 2 mA/mm2 steps up to 12 mA/mm2 | 93 |
| 6PrSDC20 | NH3 | Traditional | 50V/cm, 20 mA/mm2 | 94 |
| 6PrSDC20 | NH3 | Traditional | 50V/cm, 40 mA/mm2 | 93 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spiridigliozzi, L.; Pinter, L.; Biesuz, M.; Dell’Agli, G.; Accardo, G.; Sglavo, V.M. Gd/Sm-Pr Co-Doped Ceria: A First Report of the Precipitation Method Effect on Flash Sintering. Materials 2019, 12, 1218. https://doi.org/10.3390/ma12081218
Spiridigliozzi L, Pinter L, Biesuz M, Dell’Agli G, Accardo G, Sglavo VM. Gd/Sm-Pr Co-Doped Ceria: A First Report of the Precipitation Method Effect on Flash Sintering. Materials. 2019; 12(8):1218. https://doi.org/10.3390/ma12081218
Chicago/Turabian StyleSpiridigliozzi, Luca, Lorenzo Pinter, Mattia Biesuz, Gianfranco Dell’Agli, Grazia Accardo, and Vincenzo M. Sglavo. 2019. "Gd/Sm-Pr Co-Doped Ceria: A First Report of the Precipitation Method Effect on Flash Sintering" Materials 12, no. 8: 1218. https://doi.org/10.3390/ma12081218







