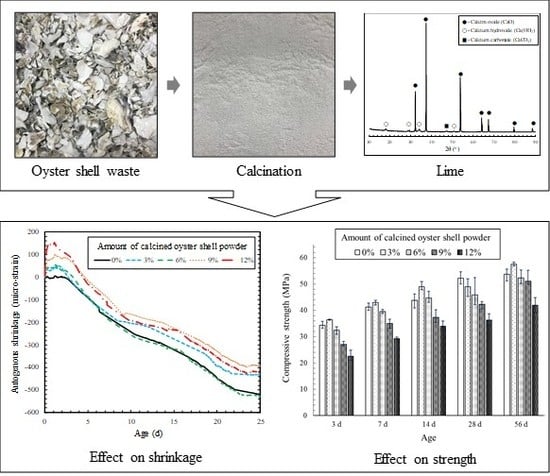
Calcined Oyster Shell Powder as an Expansive Additive in Cement Mortar
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials and Sample Preparation
2.2. Test Methods
3. Results and Discussion
3.1. Heat of Hydration
3.2. Workability of Fresh Mortar
3.3. Compressive Strength
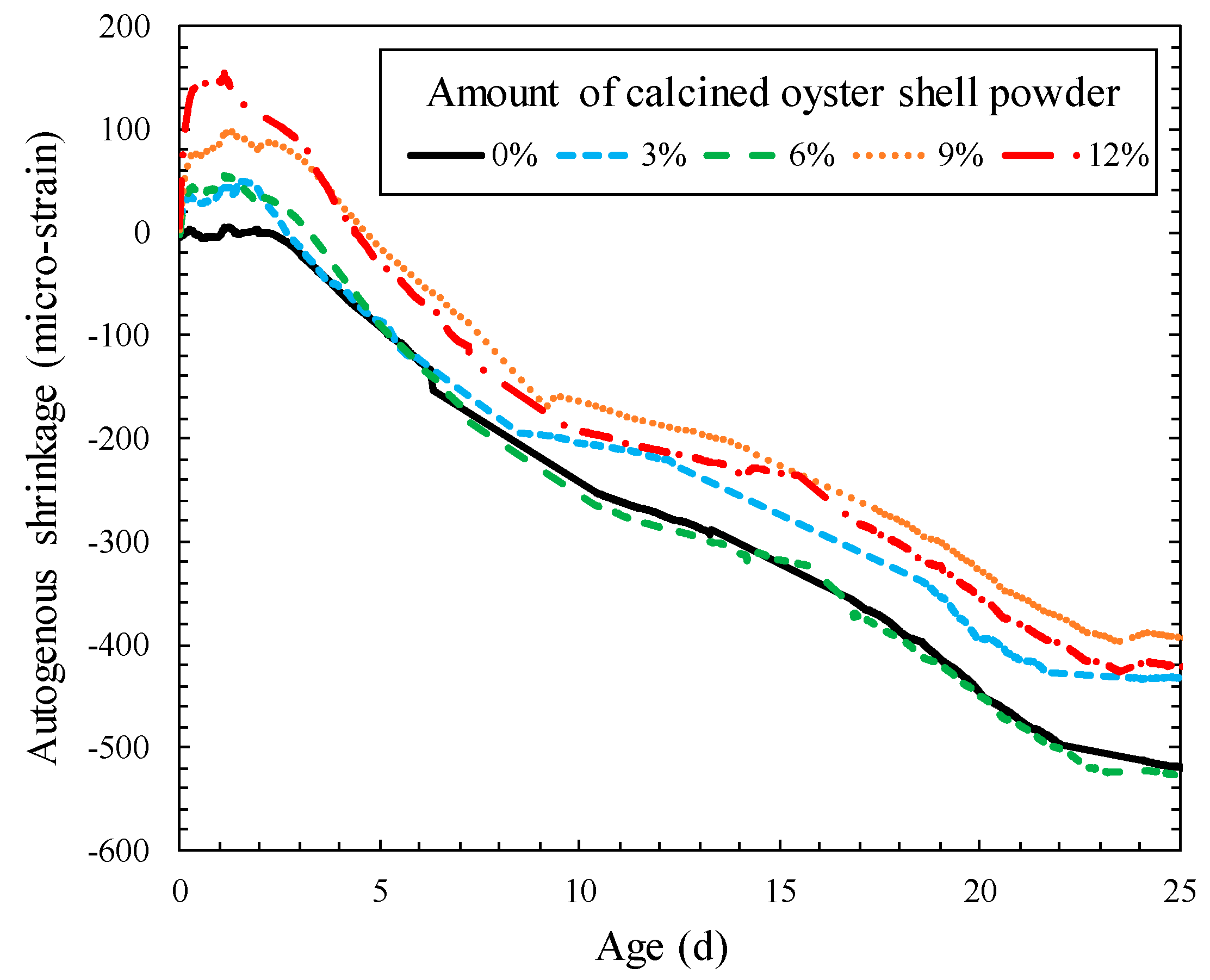
3.4. Autogenous Shrinkage
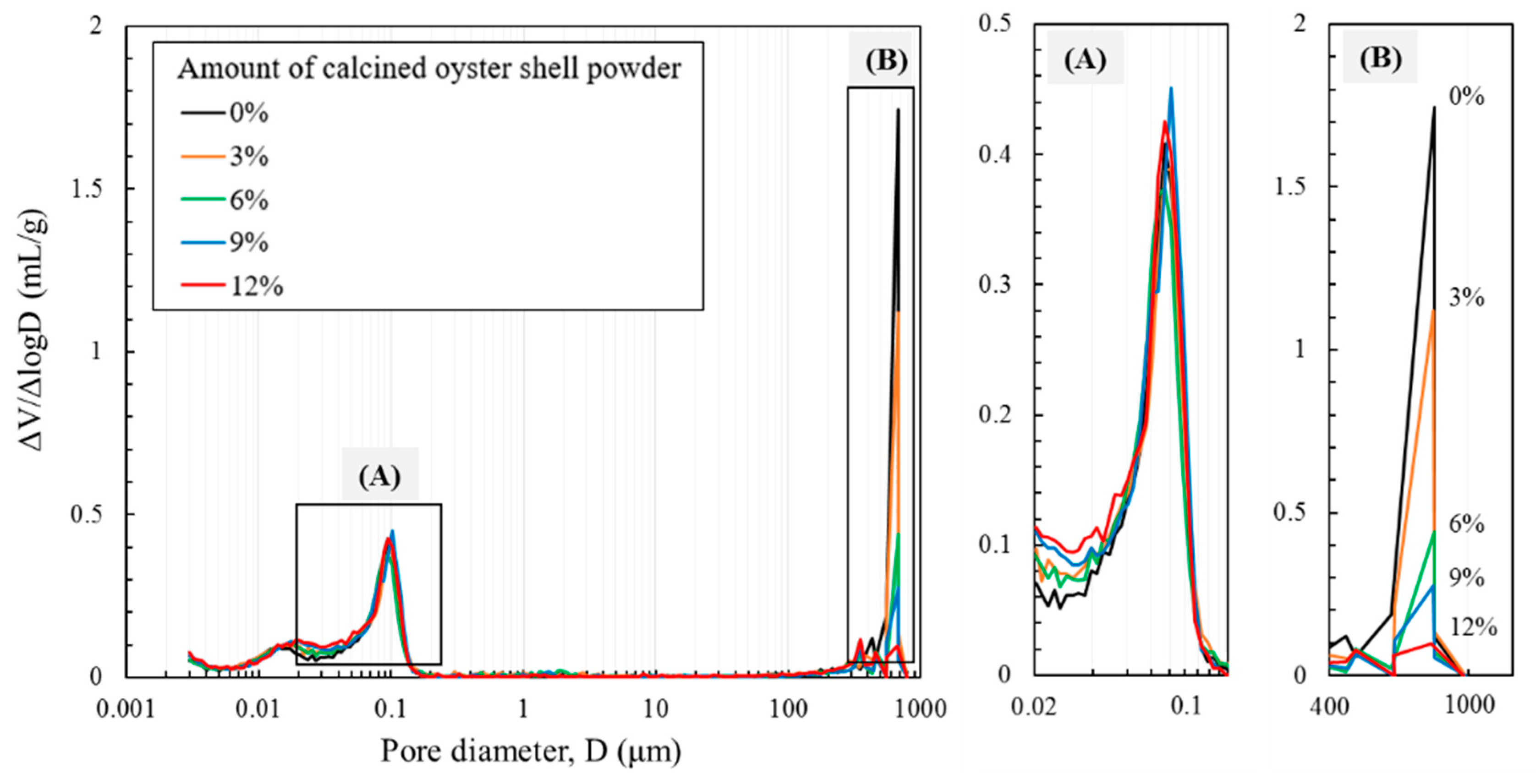
3.5. Microstructural Characterizations
4. Conclusions
- (1)
- In the hydration process of cement, calcined oyster shell powder incorporation increased the heat of hydration at the initial reaction stage due to the dissolution of CaO and formation of Ca(OH)2, demonstrating that the excessive incorporation of calcined oyster shell powder somewhat affect the rate of C–S–H formation in acceleratory period.
- (2)
- The hydration of calcined oyster shell powder can induce the loss of workability by the increased consistency of fresh mortar due to the formation of Ca(OH)2, however the degree of flow loss was still inconsequential and rapid flow loss was not observed.
- (3)
- The incorporation of calcined oyster shell powder had a significant influence on the compressive strength development of cement mortar. A small dose of calcined oyster shell powder (~3%) positively contributed to the compressive strength development. However, incorporation of the calcined oyster shell powder over 9% had a considerably negative effect on the compressive strength development.
- (4)
- The incorporation of calcined oyster shell resulted in the expansion of matrix of cement mortar at an initial stage of hydration, thereby the compensating the length change by autogenous shrinkage.
- (5)
- Considering the fresh and hardened properties of cement mortar, incorporating a small amount of calcined oyster shell powder at around 3% by weight of cement can contribute to the enhancement of the properties.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yoon, G.L.; Kim, B.T.; Kimm, B.O.; Han, S.H. Chemical-mechanical characteristics of crushed oyster-shell. Waste Manag. 2003, 23, 825–834. [Google Scholar] [CrossRef]
- Wang, H.Y.; Kuo, W.T.; Lin, C.C.; Po-Yo, C. Study of the material properties of fly ash added to oyster cement mortar. Const. Build. Mater. 2013, 41, 532–537. [Google Scholar] [CrossRef]
- Yang, E.I.; Kim, M.Y.; Park, H.G.; Yi, S.T. Effect of partial replacement of sand with dry oyster shell on the long-term performance of concrete. Constr. Build. Mater. 2010, 24, 758–765. [Google Scholar] [CrossRef]
- Kwon, H.B.; Lee, C.W.; Jun, B.S.; Yun, J.D.; Weon, S.Y.; Koopman, B. Recycling waste oyster shells for eutrophication control. Resour. Conserv. Recy. 2004, 41, 75–82. [Google Scholar] [CrossRef]
- Hsu, T.C. Experimental assessment of adsorption of Cu2+ and Ni2+ from aqueous solution by oyster shell powder. J. Hazard. Mater. 2009, 171, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- De Alvarenga, R.A.F.; Galindro, B.M.; de Fátima Helpa, C.; Soares, S.R. The recycling of oyster shells: An environmental analysis using life cycle assessment. J. Environ. Manag. 2012, 106, 102–109. [Google Scholar] [CrossRef]
- Lertwattanaruk, P.; Makul, N.; Siripattarapravat, C. Utilization of ground waste seashells in cement mortars for masonry and plastering. J. Environ. Manag. 2012, 111, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Chiou, I.J.; Chen, C.H.; Li, Y.H. Using oyster-shell foamed bricks to neutralize the acidity of recycled rainwater. Constr. Build. Mater. 2014, 64, 480–487. [Google Scholar] [CrossRef]
- Eo, S.H.; Yi, S.T. Effect of oyster shell as an aggregate replacement on the characteristics of concrete. Mag. Concr. Res. 2015, 67, 833–842. [Google Scholar] [CrossRef]
- Safi, B.; Saidi, M.; Daoui, A.; Bellal, A.; Mechekak, A.; Toumi, K. The use of seashells as a fine aggregate (by sand substitution) in self-compacting mortar (SCM). Constr. Build. Mater. 2015, 78, 430–438. [Google Scholar] [CrossRef]
- Eziefula, U.G.; Ezeh, J.C.; Eziefula, B.I. Properties of seashell aggregate concrete: A review. Constr. Build. Mater. 2018, 192, 287–300. [Google Scholar] [CrossRef]
- Khan, M.D.; Ahn, J.W.; Nam, G. Environmental benign synthesis, characterization and mechanism studies of green calcium hydroxide nano-plates derived from waste oyster shells. J. Environ. Manag. 2018, 223, 947–951. [Google Scholar] [CrossRef]
- Mo, K.H.; Alengaram, U.J.; Jumaat, M.Z.; Lee, S.C.; Goh, W.I.; Yuen, C.W. Recycling of seashell waste in concrete: A review. Constr. Build. Mater. 2018, 162, 751–764. [Google Scholar] [CrossRef]
- Monneron-Gyurits, M.; Joussein, E.; Soubrand, M.; Fondanèche, P.; Rossignol, S. Valorization of mussel and oyster shells toward metakaolin-based alkaline activated material. Appl. Clay Sci. 2018, 162, 15–26. [Google Scholar] [CrossRef]
- Wei, Y.L.; Kuo, P.J.; Yin, Y.Z.; Huang, Y.T.; Chung, T.H.; Xie, X.Q. Co-sintering oyster shell with hazardous steel fly ash and harbor sediment into construction materials. Constr. Build. Mater. 2018, 172, 224–232. [Google Scholar] [CrossRef]
- Silva, T.H.; Mesquita-Guimarães, J.; Henriques, B.; Silva, F.S.; Fredel, M.C. The potential use of oyster shell waste in new value-added by-product. Resources 2019, 8, 13. [Google Scholar] [CrossRef]
- Yang, E.; Yi, S.; Leem, Y. Effect of oyster shell substituted for fine aggregate on concrete characteristics: Part I. Fundamental properties. Cem. Concr. Res. 2005, 35, 2175–2182. [Google Scholar] [CrossRef]
- Kuo, W.; Wang, H.; Shu, C.; Su, D. Engineering properties of controlled low strength materials containing waste oyster shells. Constr. Build. Mater. 2013, 46, 128–133. [Google Scholar] [CrossRef]
- Djobo, Y.J.N.; Elimbi, A.; Manga, J.D.; Ndjock, I.B.D.L. Partial replacement of volcanic ash by bauxite and calcined oyster shell in the synthesis of volcanic ash-based geopolymers. Constr. Build. Mater. 2016, 113, 673–681. [Google Scholar] [CrossRef]
- Li, G.; Xu, X.; Chen, E.; Fan, J.; Xiong, G. Properties of cement-based bricks with oyster shells ash. J. Clean. Prod. 2015, 91, 279–287. [Google Scholar] [CrossRef]
- Chatterji, S. Mechanism of expansion of concrete due to the presence of dead-burnt CaO and MgO. Cem. Concr. Res. 1995, 25, 51–56. [Google Scholar] [CrossRef]
- Konik, Z.; Malolepszy, J.; Roszczynialski, W.; Stok, A. Production of expansive additive to Portland cement. J. Eur. Ceram. Soc. 2007, 27, 605–609. [Google Scholar] [CrossRef]
- ASTM C845-96, Standard Specification for Expansive Hydraulic Cement; ASTM Standards: West Conshohocken, PA, USA, 2004.
- ASTM C1437-15, Standard Test Method for Flow of Hydraulic Cement Mortar; ASTM International: West Conshohocken, PA, USA, 2015.
- Richardson, I.; Taylor, H.F.W. Cement Chemistry; ICE Publishing: London, UK, 2017. [Google Scholar]
- Oates, T. Lime and Limestone, Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2007. [Google Scholar]
- Chatveera, B.; Lertwattanaruk, P. Durability of conventional concretes containing black rice husk ash. J. Environ. Manag. 2011, 92, 59–66. [Google Scholar] [CrossRef]
- Jang, J.G.; Kim, G.M.; Kim, H.J.; Lee, H.K. Review on recent advances in CO2 utilization and sequestration technologies in cement based materials. Constr. Build. Mater. 2016, 127, 762–773. [Google Scholar] [CrossRef]
- Jang, J.G.; Lee, H.K. Microstructural densification of CO2 uptake promoted by the carbonation curing of belite-rich Portland cement. Cem. Concr. Res. 2016, 82, 50–57. [Google Scholar] [CrossRef]







| wt.% | CaO | Na2O | MgO | Al2O3 | SiO2 | P2O5 | SO3 | Cl | K2O | MnO | Fe2O3 | SrO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcined oyster shell powder | 98.00 | 0.25 | 0.35 | 0.08 | 0.20 | 0.13 | 0.41 | 0.11 | 0.02 | 0.04 | 0.10 | 0.31 |
| Substitution Ratio of Calcined Oyster Shell (wt.%) | Cement | Calcined Oyster Shell Powder | Sand | Water |
|---|---|---|---|---|
| 0% | 2 | 0 | 3 | 1 |
| 3% | 2 | 0.06 | 3 | 1 |
| 6% | 2 | 0.12 | 3 | 1 |
| 9% | 2 | 0.18 | 3 | 1 |
| 12% | 2 | 0.24 | 3 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, J.H.; Park, S.M.; Yang, B.J.; Jang, J.G. Calcined Oyster Shell Powder as an Expansive Additive in Cement Mortar. Materials 2019, 12, 1322. https://doi.org/10.3390/ma12081322
Seo JH, Park SM, Yang BJ, Jang JG. Calcined Oyster Shell Powder as an Expansive Additive in Cement Mortar. Materials. 2019; 12(8):1322. https://doi.org/10.3390/ma12081322
Chicago/Turabian StyleSeo, Joon Ho, Sol Moi Park, Beom Joo Yang, and Jeong Gook Jang. 2019. "Calcined Oyster Shell Powder as an Expansive Additive in Cement Mortar" Materials 12, no. 8: 1322. https://doi.org/10.3390/ma12081322