DFT Analysis of NO Adsorption on the Undoped and Ce-Doped LaCoO3 (011) Surface
Abstract
:1. Introduction
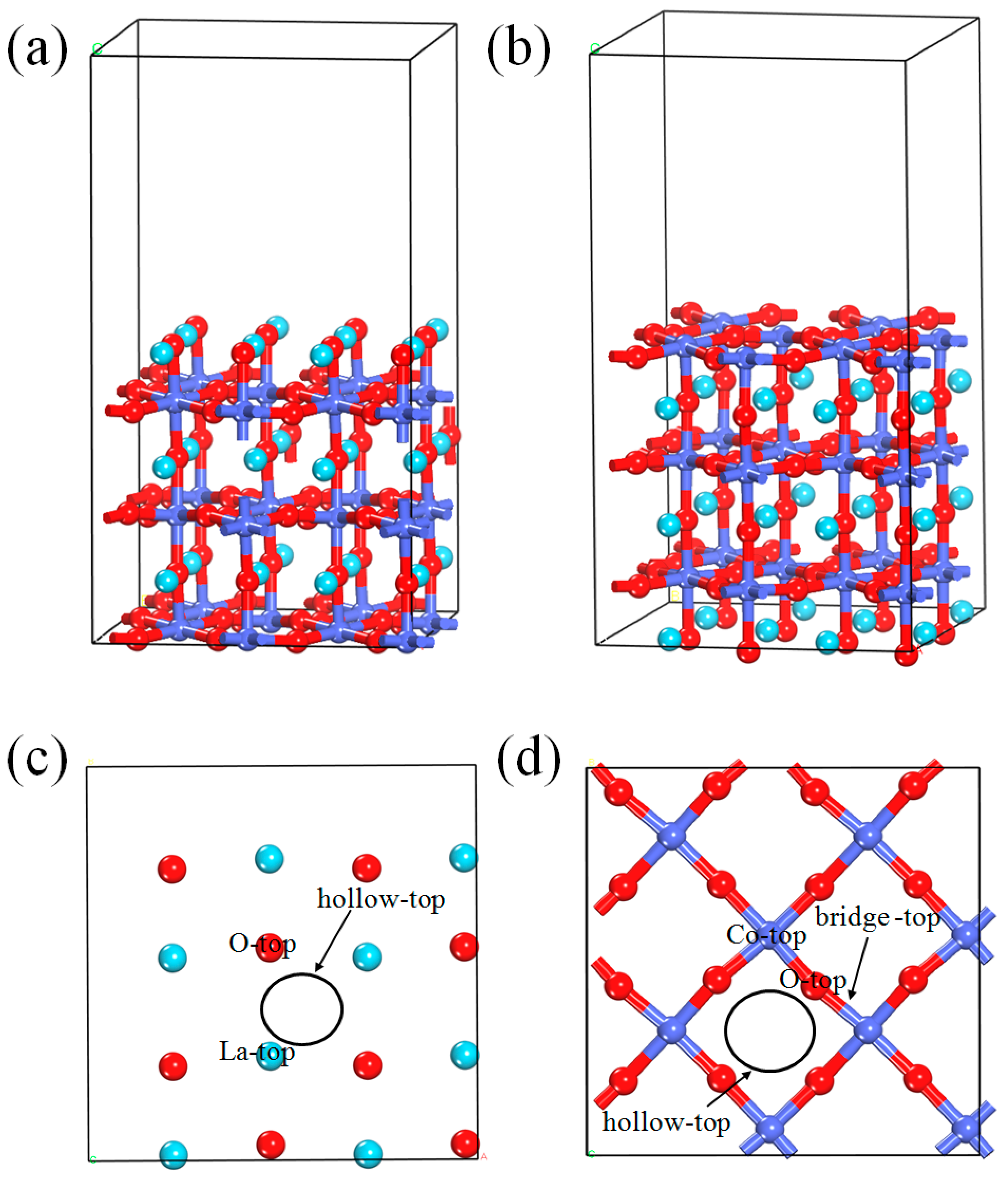
2. Computational Methods and Models
3. Results and Discussion
3.1. NO Adsorption on the (011) Surface of Perfect LaCoO3
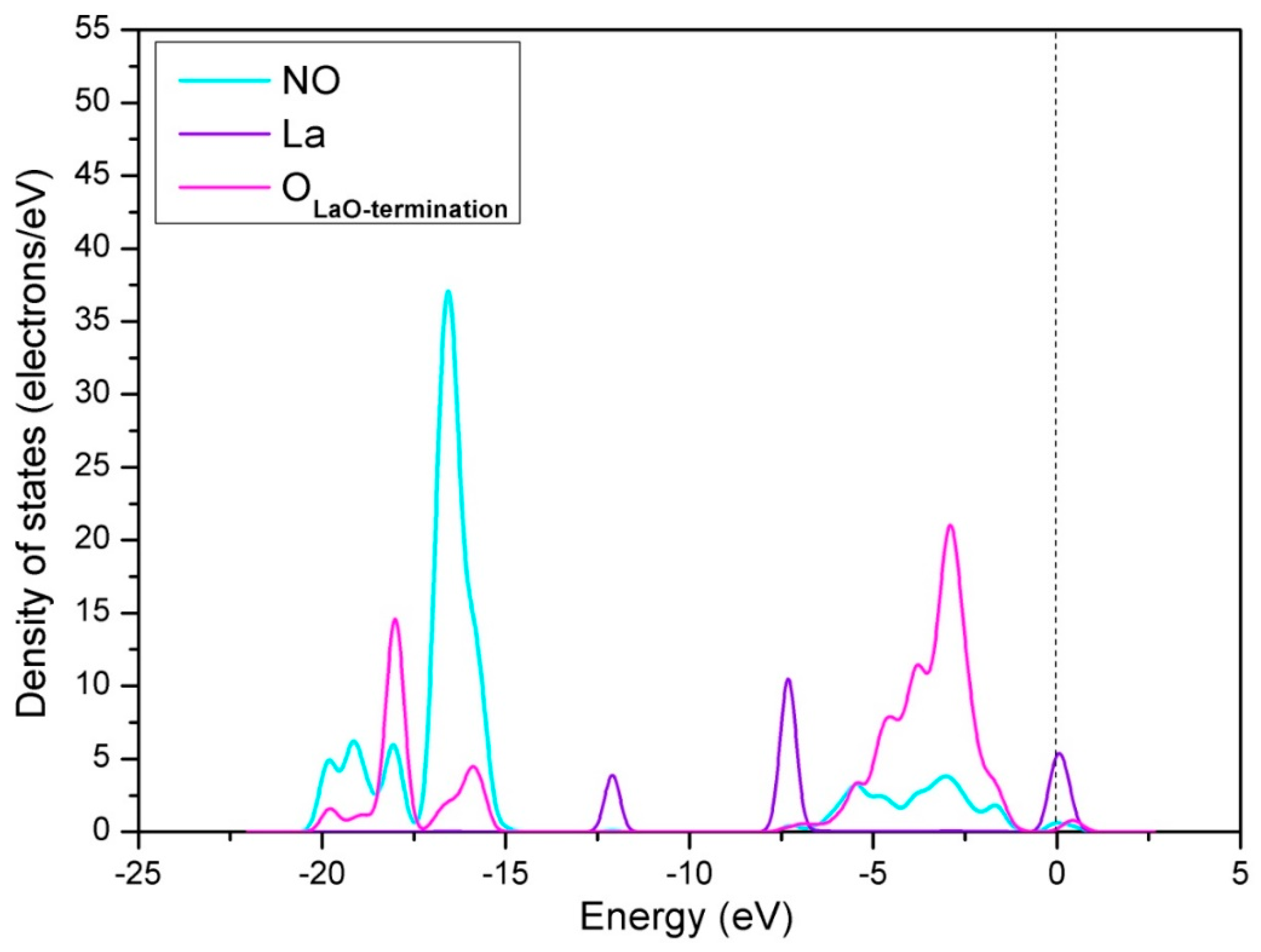
3.1.1. Calculation for the Perfect LaO-Terminated Surface
3.1.2. Calculation for the Perfect CoO2-Terminated Surface
3.2. NO Adsorption on the (011) Surface of Ce-Doped LaCoO3
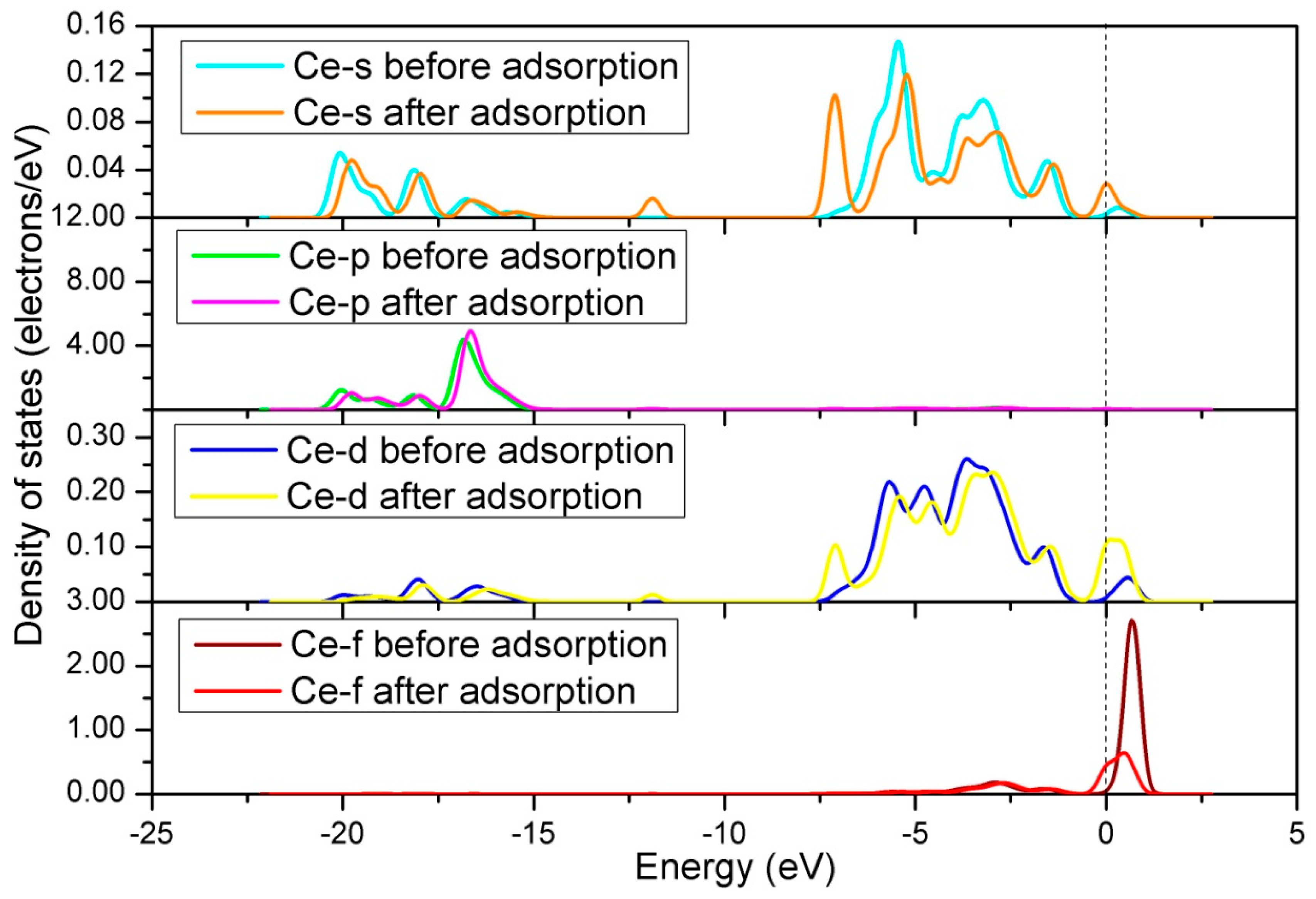
3.2.1. Calculation for the Ce-doped LaO-Terminated Surface
3.2.2. Calculation for the Ce-doped CoO2-Terminated Surface
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Iwamoto, M.; Hamada, H. Removal of nitrogen monoxide from exhaust gases through novel catalytic processes. Catal. Today. 1991, 10, 57–71. [Google Scholar] [CrossRef]
- Ohtsuka, H. The selective catalytic reduction of nitrogen oxides by methane on noble metal-loaded sulfated zirconia. Appl. Catal. B Environ. 2001, 33, 325–333. [Google Scholar] [CrossRef]
- Takahashi, N.; Shinjoh, H.; Iijima, T.; Suzuki, T.; Yamazaki, K.; Yokota, K.; Suzuki, H.; Miyoshi, N.; Matsumoto, S.; Tanizawa, T.; et al. The new concept 3-way catalyst for automotive lean-burn engine: NOx storage and reduction catalyst. Catal. Today. 1996, 27, 63–69. [Google Scholar] [CrossRef]
- Chen, J.; Shen, M.; Wang, X.; Qi, G.; Wang, J.; Li, W. The influence of nonstoichiometry on LaMnO3 perovskite for catalytic NO oxidation. Appl. Catal. B-Environ. 2013, 134, 251–257. [Google Scholar] [CrossRef]
- Chen, J.; Shen, M.; Wang, X.; Wang, J.; Su, Y.; Zhao, Z. Catalytic performance of NO oxidation over LaMeO3 (Me = Mn, Fe, Co) perovskite prepared by the sol-gel method. Catal. Commun. 2013, 37, 105–108. [Google Scholar]
- Li, Z.; Meng, M.; Li, Q.; Xie, Y.; Hu, T.; Zhang, J. Fe-substituted nanometric La0.9K0.1Co1−xFexO3-δ perovskite catalysts used for soot combustion, NOx storage and simultaneous catalytic removal of soot and NOx. Chem. Eng. J. 2010, 164, 98–105. [Google Scholar] [CrossRef]
- Guo, X.; Meng, M.; Dai, F.; Li, Q.; Zhang, Z.; Jiang, Z.; Zhang, S.; Huang, Y. NOx-assisted soot combustion over dually substituted perovskite catalysts La1−xKxCo1−yPdyO3-δ. Appl. Catal. B-Environ. 2013, 142, 278–289. [Google Scholar] [CrossRef]
- Li, X.; Gao, H. Role of ceria in the improvement of NO removal of lanthanum-based perovskite-type catalysts. Rsc Adv. 2018, 8, 11778–11784. [Google Scholar] [CrossRef] [Green Version]
- Cohen, R.E.; Lin, Y. Prediction of a potential high-pressure structure of FeSiO3. Phys. Rev. B. 2014, 90, 140102. [Google Scholar] [CrossRef]
- You, R.; Zhang, Y.; Liu, D.; Meng, M.; Zheng, L.; Zhang, J.; Hu, T. YCeZrO Ternary Oxide Solid Solution Supported Nonplatinic Lean-Burn NOx Trap Catalysts Using LaCoO3 Perovskite as Active Phase. J. Phys. Chem. C 2014, 118, 25403–25420. [Google Scholar] [CrossRef]
- Li, X.-G.; Dong, Y.-H.; Xian, H.; Hernandez, W.Y.; Meng, M.; Zou, H.-H.; Ma, A.-J.; Zhang, T.-Y.; Jiang, Z.; Tsubaki, N.; Vernoux, P. De-NOx in alternative lean/rich atmospheres on La1−xSrxCoO3 perovskites. Energy Environ. Sci. 2011, 4, 3351–3354. [Google Scholar]
- Kim, C.H.; Qi, G.; Dahlberg, K.; Li, W. Strontium-Doped Perovskites Rival Platinum Catalysts for Treating NOx in Simulated Diesel Exhaust. Science 2010, 327, 1624–1627. [Google Scholar] [CrossRef] [PubMed]
- Onrubia, J.A.; Pereda-Ayo, B.; De-La-Torre, U.; Gonzalez-Velasco, J.R. Key factors in Sr-doped LaBO3 (B = Co or Mn) perovskites for NO oxidation in efficient diesel exhaust purification. Appl. Catal. B-Environ. 2017, 213, 198–210. [Google Scholar] [CrossRef]
- Wen, Y.; Zhang, C.; He, H.; Yu, Y.; Teraoka, Y. Catalytic oxidation of nitrogen monoxide over La1−xCexCoO3 perovskites. Catal. Today. 2007, 126, 400–405. [Google Scholar] [CrossRef]
- Forni, L.; Oliva, C.; Vatti, F.P.; Kandala, M.A.; Ezerets, A.M.; Vishniakov, A.V. LaCeCo perovskites as catalysts for exhaust gas depollution. Appl. Catal. B Environ. 1996, 7, 269–284. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, S.; Yang, Y.; Zheng, C.; Zhou, J.; Gao, X.; Tu, X. Enhanced performance for plasma-catalytic oxidation of ethyl acetate over La1−xCexCoO3+δ catalysts. Appl. Catal. B-Environ. 2017, 213, 97–105. [Google Scholar] [CrossRef]
- Zhao, B.; Ran, R.; Sun, L.; Yang, Z.; Wu, X.; Weng, D. A high-surface-area La-Ce-Mn mixed oxide with enhanced activity for CO and C3H8 oxidation. Catal. Commun. 2018, 105, 26–30. [Google Scholar] [CrossRef]
- Guguchia, Z.; Adachi, T.; Shermadini, Z.; Ohgi, T.; Chang, J.; Bozin, E.S.; Von Rohr, F.; Dos Santos, A.M.; Molaison, J.J.; Boehler, R.; et al. Pressure tuning of structure, superconductivity, and novel magnetic order in the Ce-underdoped electron-doped cuprate T′−Pr1.3−xLa0.7CexCuO4 (x = 0.1). Phys. Rev. B 2017, 96, 094515. [Google Scholar] [CrossRef]
- Zhang-Steenwinkel, Y.; Beckers, J.; Bliek, A. Surface properties and catalytic performance in CO oxidation of cerium substituted lanthanum–manganese oxides, Appl. Catal. Gen. 2002, 235, 79–92. [Google Scholar] [CrossRef]
- Białobok, B.; Trawczyński, J.; Miśta, W.; Zawadzki, M. Ethanol combustion over strontium- and cerium-doped LaCoO3 catalysts. Appl. Catal. B Environ. 2007, 72, 395–403. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Gao, H. Adsorption properties of NO molecules on the hexagonal LaCoO3 (001) surface: A density functional theory study. RSC Adv. 2017, 7, 34714–34721. [Google Scholar] [CrossRef]
- Penninger, M.W.; Kim, C.H.; Thompson, L.T.; Schneider, W.F. DFT Analysis of NO Oxidation Intermediates on Undoped and Doped LaCoO 3 Perovskite. J. Phys. Chem. C 2015, 119, 20488–20494. [Google Scholar] [CrossRef]
- Chen, Z.; Kim, C.H.; Thompson, L.T.; Schneider, W.F. LDA+U evaluation of the stability of low-index facets of LaCoO3 perovskite. Surf. Sci. 2014, 619, 71–76. [Google Scholar]
- Sun, L.; Hu, J.; Qin, H.; Zhao, M.; Fan, K. Influences of Ca Doping and Oxygen Vacancy upon Adsorption of CO on the LaFeO3 (010) Surface: A First-Principles Study. J. Phys. Chem. C 2011, 115, 5593–5598. [Google Scholar] [CrossRef]
- Sun, L.; Li, G.; Chen, W.; Hu, J. Adsorption of CO on the oxygen defective LaCoO3 (001) surface: A first-principles study. Comput. Mater. Sci. 2016, 115, 154–157. [Google Scholar]
- Carlotto, S.; Natile, M.M.; Glisenti, A.; Paul, J.-F.; Blanck, D.; Vittadini, A. Energetics of CO oxidation on lanthanide-free perovskite systems: the case of Co-doped SrTiO3. Phys. Chem. Chem. Phys. 2016, 18, 33282–33286. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, Z.; Wen, Y.; Chen, R.; Shan, B. Surface stabilities and NO oxidation kinetics on hexagonal-phase LaCoO3 facets: a first-principles study. Catal. Sci. Technol. 2014, 4, 3687–3696. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, X.; Wu, C.; Wen, Y.; Xue, Y.; Chen, R.; Zhang, Z.; Shan, B.; Yin, H.; Wang, W.G. NO oxidation catalysis on copper doped hexagonal phase LaCoO3: a combined experimental and theoretical study. Phys. Chem. Chem. Phys. 2014, 16, 5106–5112. [Google Scholar] [CrossRef]
- Wang, L.; Chen, W. Density functional theory for adsorption of HCHO on the FeO(100) surface. J. Nat. Gas Chem. 2010, 19, 21–24. [Google Scholar] [CrossRef]
- Giordano, L.; Del Vitto, A.; Pacchioni, G.; Maria Ferrari, A. CO adsorption on Rh, Pd and Ag atoms deposited on the MgO surface: a comparative ab initio study. Surf. Sci. 2003, 540, 63–75. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhao, Y.-P.; Li, L.; Wang, T. Ab Initio Study of ZnO-Based Gas-Sensing Mechanisms: Surface Reconstruction and Charge Transfer. J. Phys. Chem. C 2009, 113, 6107–6113. [Google Scholar] [CrossRef] [Green Version]
- Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol 3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, E.; Sun, L.; Hu, J. Adsorption of NO on the SrFeO3 (001) surface: A DFT study, Comput. Mater. Sci. 2015, 102, 135–139. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G.; Li, Z. First principle study of SrTiO3 (001) surface and adsorption of NO on SrTiO3 (001). Appl. Surf. Sci. 2007, 253, 8345–8351. [Google Scholar] [CrossRef]
- Thornton, G.; Tofield, B.; Hewat, A. A neutron diffraction study of LaCoO3 in the temperature range 4.2 < T < 1248 K. J. Solid State Chem. 1986, 61, 301–307. [Google Scholar]
- Boateng, I.W.; Tia, R.; Adei, E.; Dzade, N.Y.; Catlow, C.R.A.; De Leeuw, N.H. A DFT plus U investigation of hydrogen adsorption on the LaFeO3(010) surface. Phys. Chem. Chem. Phys. 2017, 19, 7399–7409. [Google Scholar] [CrossRef]
- Kizaki, H.; Kusakabe, K. DFT-GGA study of NO adsorption on the LaO (001) surface of LaFeO3. Surf. Sci. 2012, 606, 337–343. [Google Scholar]
- Zhou, Y.; Lu, Z.; Guo, P.; Tian, Y.; Huang, X.; Su, W. First-principles study on the catalytic role of Ag in the oxygen adsorption of LaMnO3 (0 0 1) surface. Appl. Surf. Sci. 2012, 258, 2602–2606. [Google Scholar] [CrossRef]
- Carlotto, S.; Natile, M.M.; Glisenti, A.; Vittadini, A. Adsorption of CO and formation of carbonates at steps of pure and Co-doped SrTiO 3 surfaces by DFT calculations. Appl. Surf. Sci. 2016, 364, 522–527. [Google Scholar] [CrossRef]
- Asthagiri, A.; Sholl, D. DFT study of Pt adsorption on low index SrTiO surfaces: SrTiO (100), SrTiO (111) and SrTiO (110). Surf. Sci. 2005, 581, 66–87. [Google Scholar] [CrossRef]
- Read, M.S.D.; Islam, M.S.; Watson, G.W.; King, F.; Hancock, F.E. Defect chemistry and surface properties of LaCoO3. J. Mater. Chem. 2000, 10, 2298–2305. [Google Scholar] [CrossRef]
- Johnson, R. Computational Chemistry Comparison and Benchmark Database, NIST Standard Reference Database 101. Available online: http://cccbdb.nist.gov/ (accessed on 19 April 2018).
- Sun, L.; Hu, J.; Gao, F.; Zhang, Y.; Qin, H. First-principle study of NO adsorption on the LaFeO3 (010) surface. Phys. B-Condens. Matter. 2011, 406, 4105–4108. [Google Scholar] [CrossRef]
- Sorescu, D.C.; Rusu, C.N.; Yates, J.T. Adsorption of NO on the TiO2 (110) Surface: An Experimental and Theoretical Study. J. Phys. Chem. B. 2000, 104, 4408–4417. [Google Scholar] [CrossRef]
- Sun, L.; Li, G.; Chen, W.; Luo, F.; Hu, J.; Qin, H. Adsorption of CO on the LaCoO3 (001) surface by density functional theory calculation. Appl. Surf. Sci. 2014, 309, 128–132. [Google Scholar] [CrossRef]
- Liu, X.; Hu, J.; Cheng, B.; Qin, H.; Zhao, M.; Yang, C. First-principles study of O2 adsorption on the LaFeO3 (010) surface. Sens. Actuators B Chem. 2009, 139, 520–526. [Google Scholar] [CrossRef]
- Hoffmann, R. A chemical and theoretical way to look at bonding on surfaces. Rev. Mod. Phys. 1988, 60, 601–628. [Google Scholar] [CrossRef]
- Ding, K.; Li, J.; Zhang, Y. Cu and NO coadsorption on TiO2(110) surface: A density functional theory study. J. Mol. Struct. THEOCHEM 2005, 728, 123–127. [Google Scholar] [CrossRef]
- Fan, C.; Xiao, W.-D. Origin of site preference of CO and NO adsorption on Pd(111) at different coverages: A density functional theory study. Comput. Theor. Chem. 2013, 1004, 22–30. [Google Scholar] [CrossRef]
- Zeng, Z.-H.; Da Silva, J.L.F.; Li, W.-X. Theory of nitride oxide adsorption on transition metal (111) surfaces: A first-principles investigation. Phys. Chem. Chem. Phys. 2010, 12, 2459. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, F.; Tang, T.C.; He, J.P.; Wang, L. Theoretical analysis and comparation study of multiple-scattering cluster method: For CO/NiO(100) and NO/NiO(100) adsorption systems. Acta Phys. Sin. 2000, 49, 570–576. [Google Scholar]

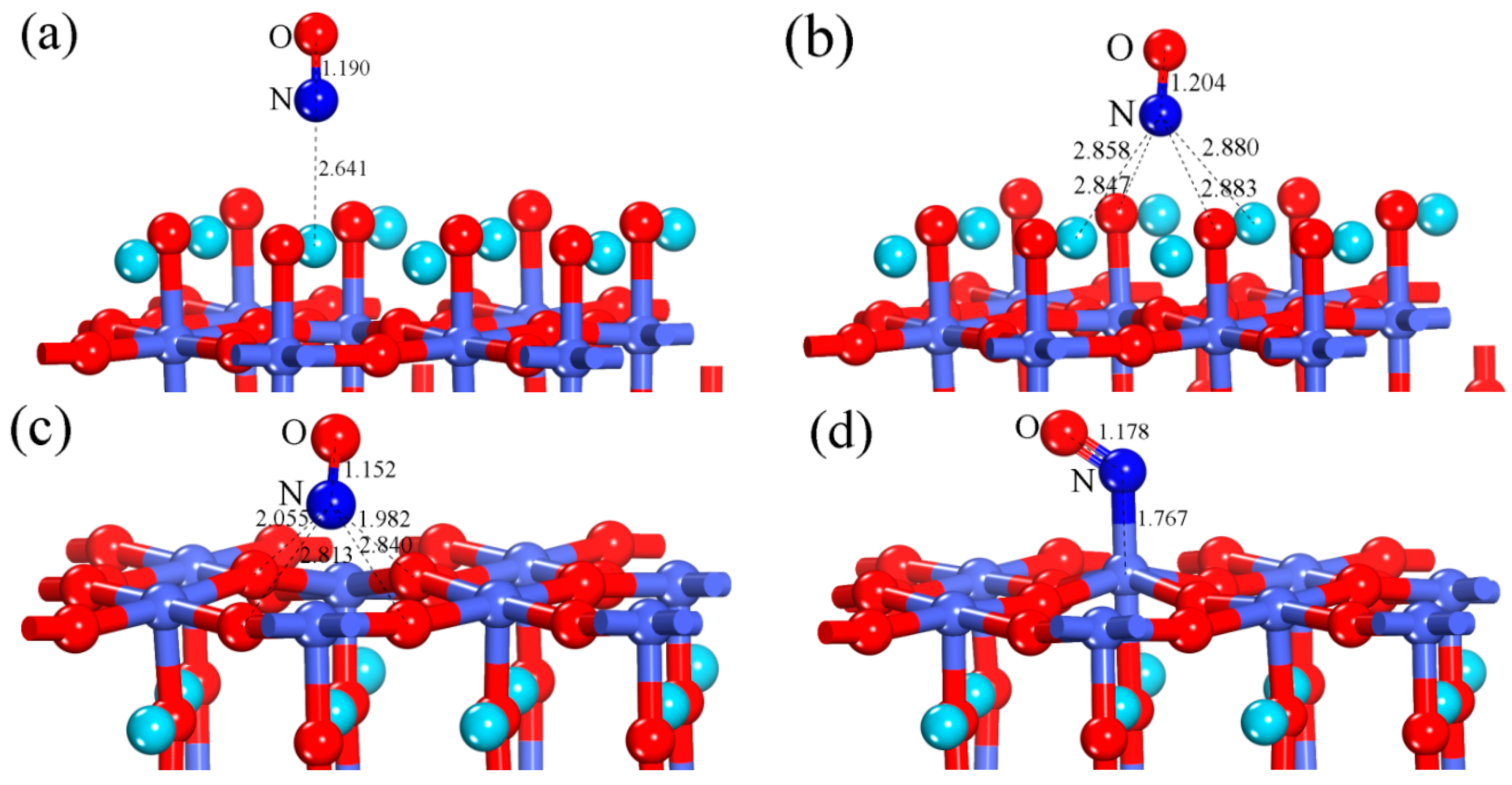

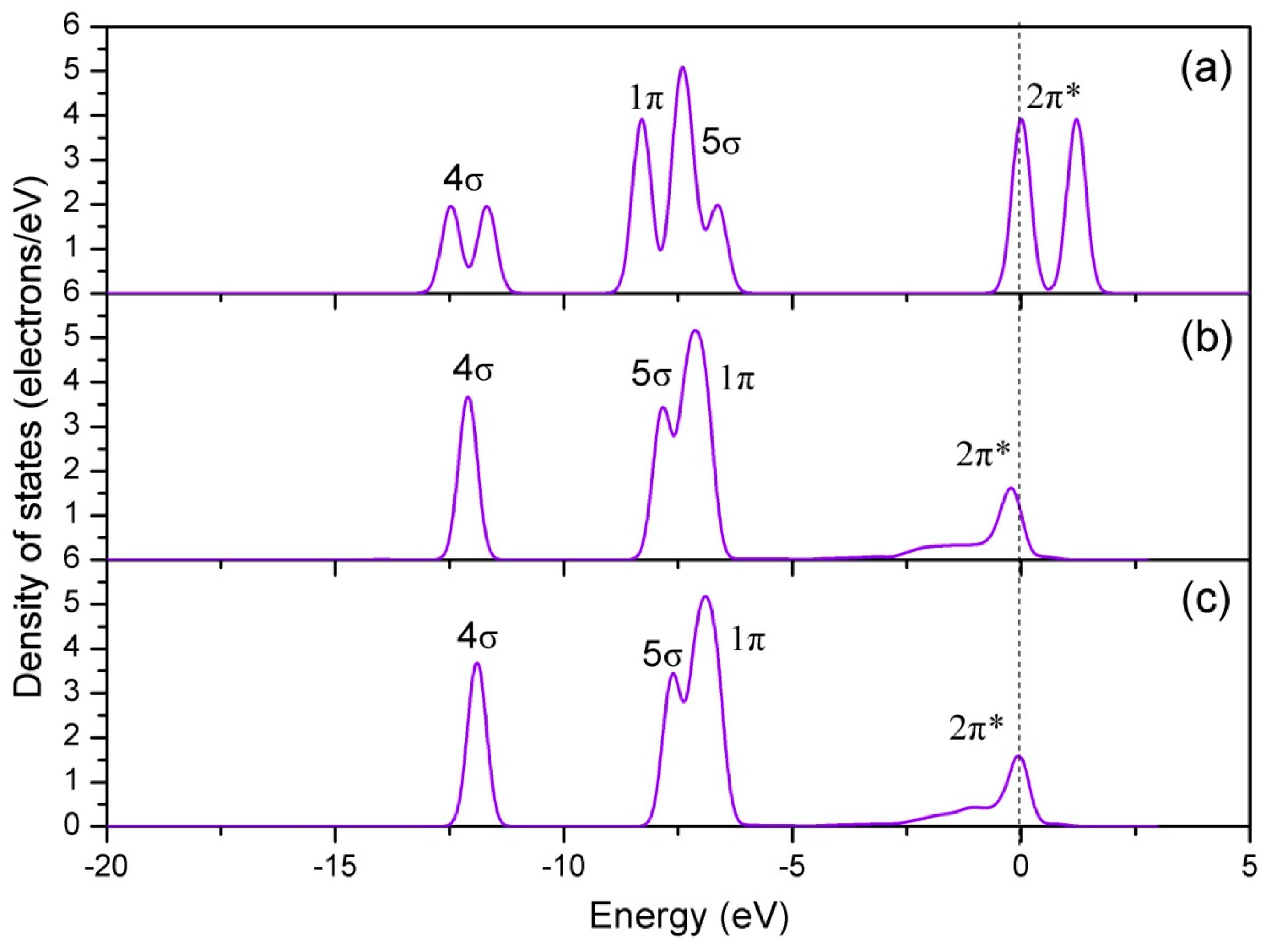
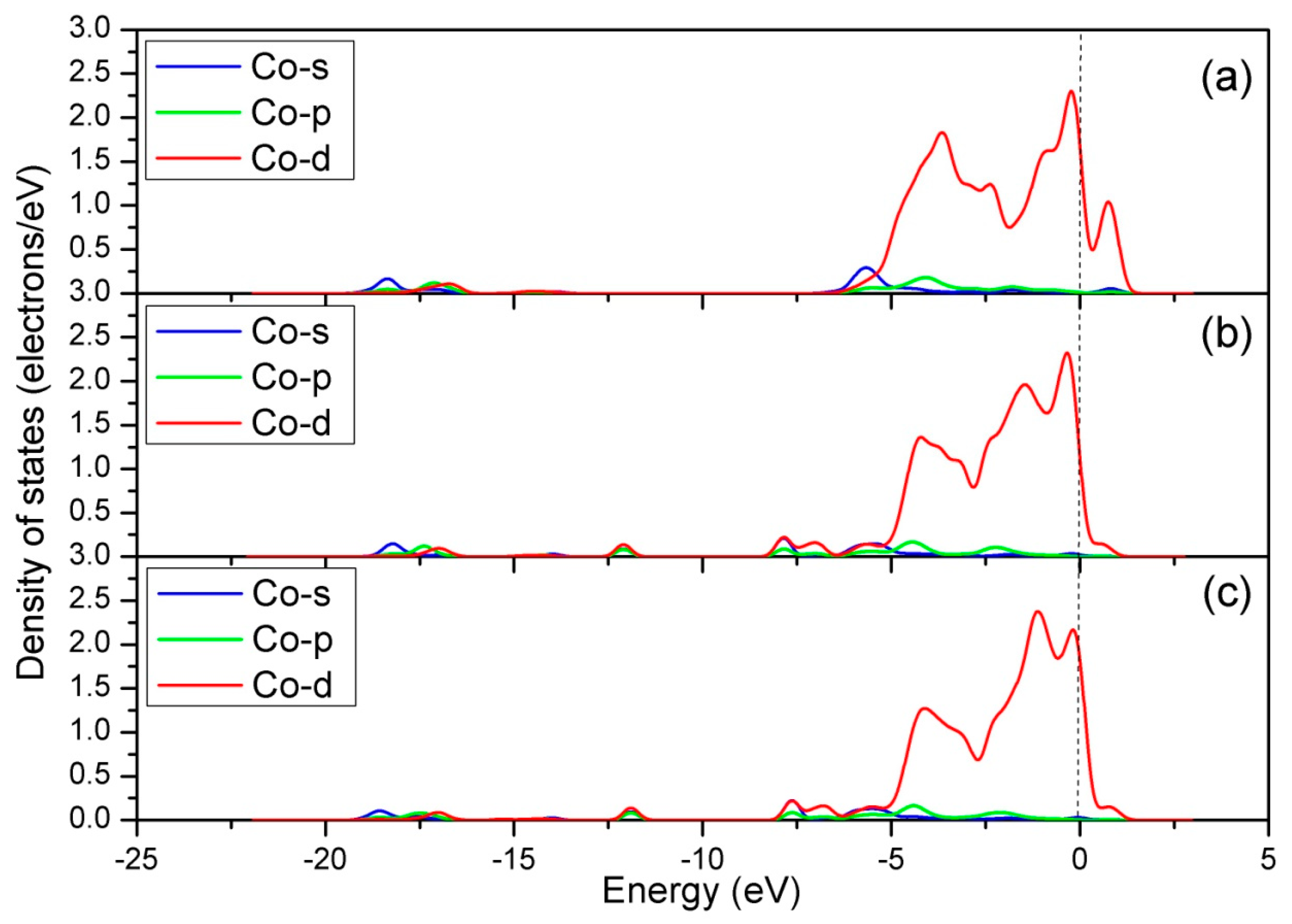
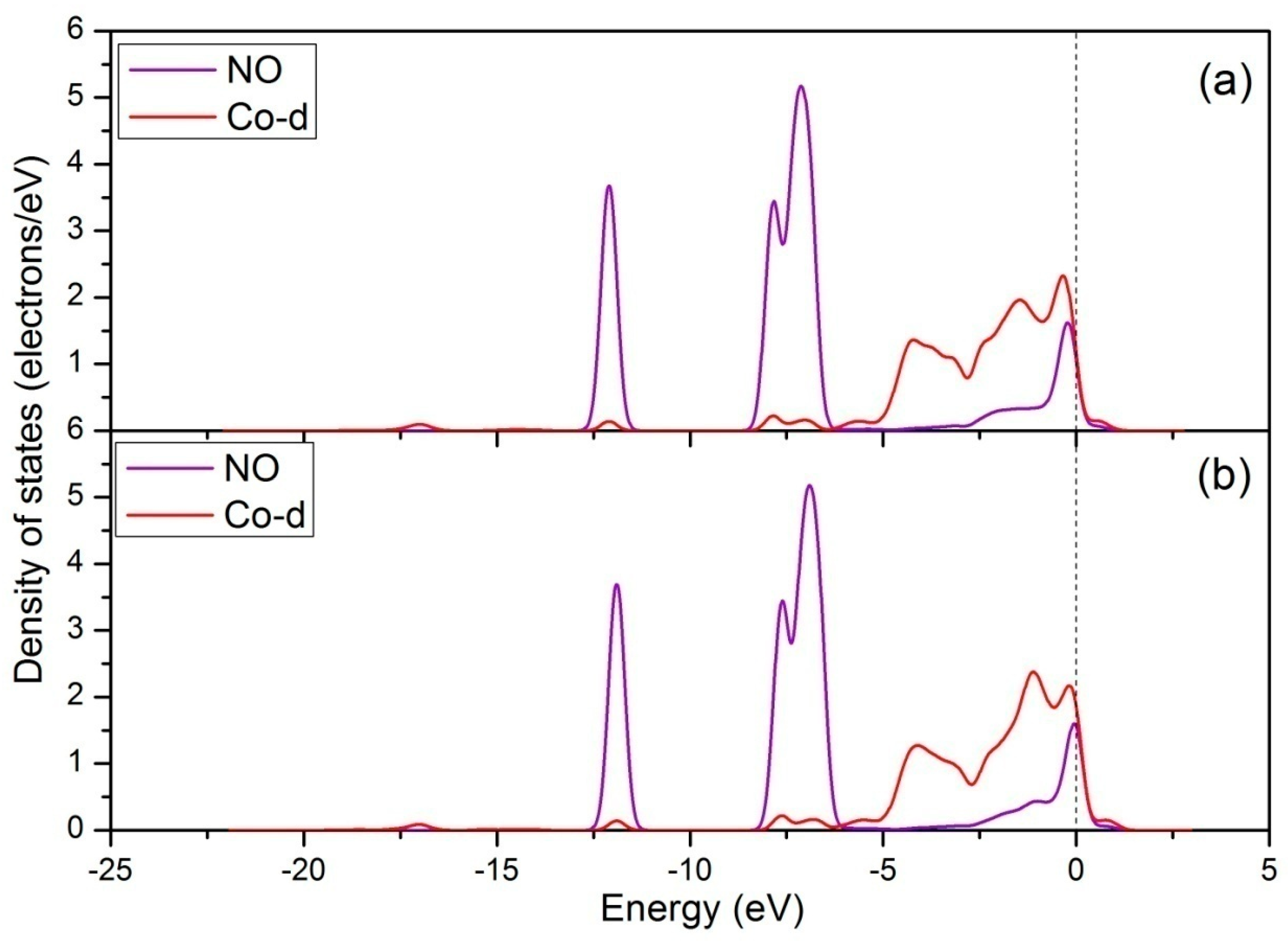
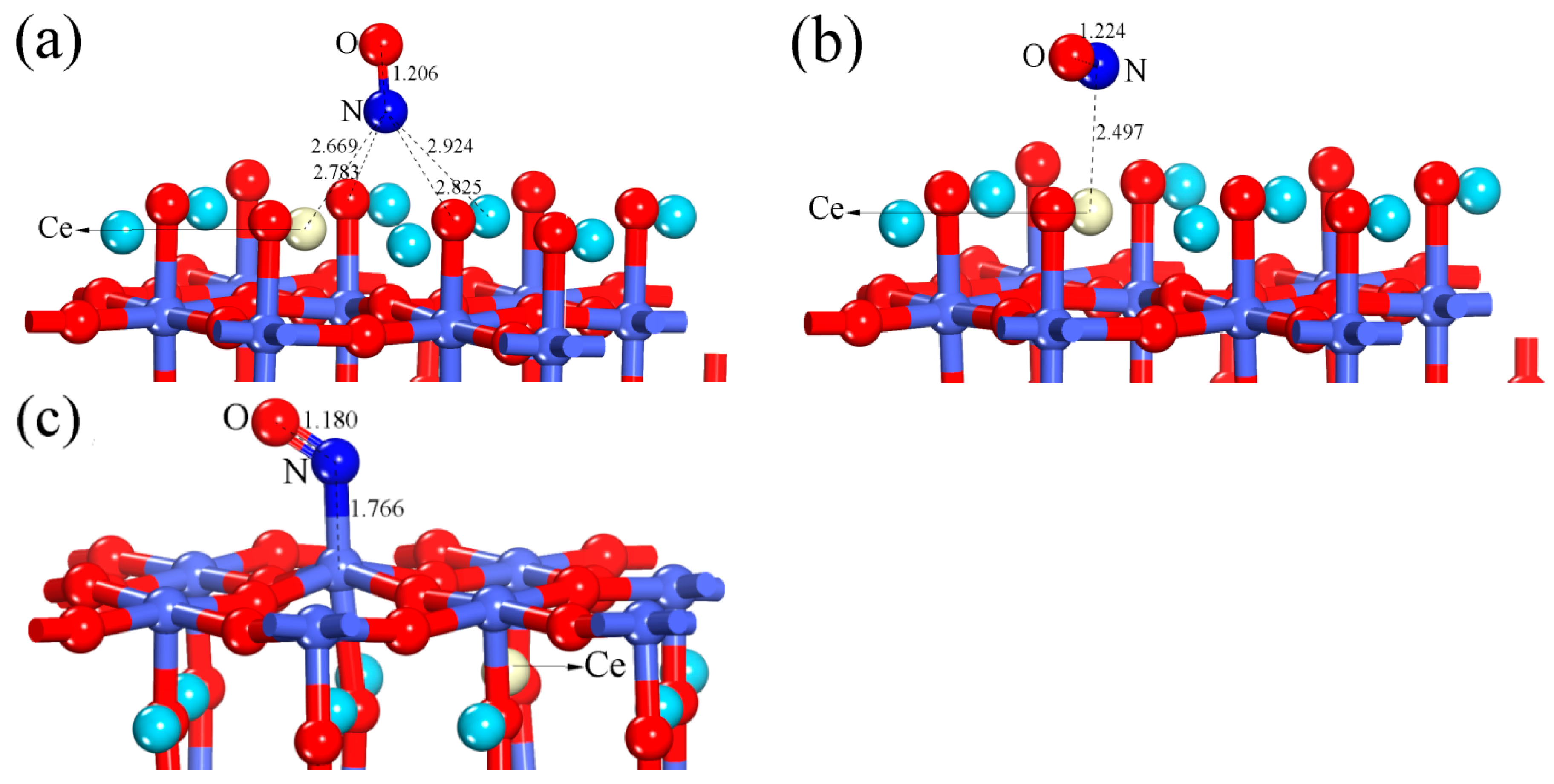

| Configuration | dN-O | ΔdN-O (Å) | d (Å) | qNO (e) | Eads (eV) |
|---|---|---|---|---|---|
| Perfect LaO-terminated surface (hollow-NO) | 1.204 | 0.041 | 2.847 | −0.295 | 0.593 |
| Perfect CoO2-terminated surface (Co-NO) | 1.178 | 0.015 | 1.767 | 0.126 | 1.302 |
| Ce-doped LaO-terminated surface (hollow-NO) | 1.206 | 0.043 | 2.669 | −0.317 | 0.849 |
| Ce-doped LaO-terminated surface (Ce-NO) | 1.224 | 0.061 | 2.497 | −0.371 | 0.797 |
| Ce-doped CoO2-terminated surface (Co-NO) | 1.18 | 0.017 | 1.766 | 0.104 | 1.380 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Gao, H. DFT Analysis of NO Adsorption on the Undoped and Ce-Doped LaCoO3 (011) Surface. Materials 2019, 12, 1379. https://doi.org/10.3390/ma12091379
Li X, Gao H. DFT Analysis of NO Adsorption on the Undoped and Ce-Doped LaCoO3 (011) Surface. Materials. 2019; 12(9):1379. https://doi.org/10.3390/ma12091379
Chicago/Turabian StyleLi, Xiaochen, and Hongwei Gao. 2019. "DFT Analysis of NO Adsorption on the Undoped and Ce-Doped LaCoO3 (011) Surface" Materials 12, no. 9: 1379. https://doi.org/10.3390/ma12091379