Aging of Bioactive Glass-Based Foams: Effects on Structure, Properties, and Bioactivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production of Bioglass®-Based Glass-Ceramic Scaffold
2.2. Preparation and Aging of Bioglass® Foam Samples
2.3. Preparation of Simulated Body Fluid for Bioactivity Evaluation
2.4. Characterization of Bioglass® Foam Samples
2.4.1. Chemical and Physical Characterization
2.4.2. Morphological Characterization
2.4.3. Mechanical Characterization
2.4.4. Bioactivity
3. Results
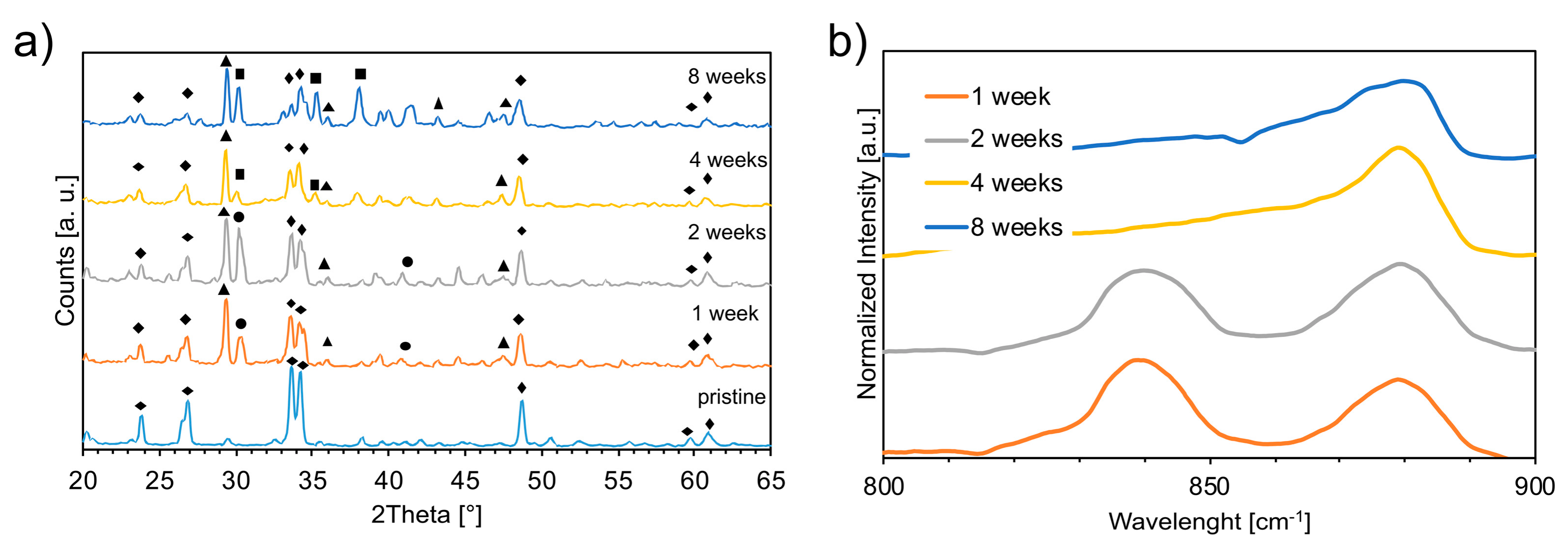
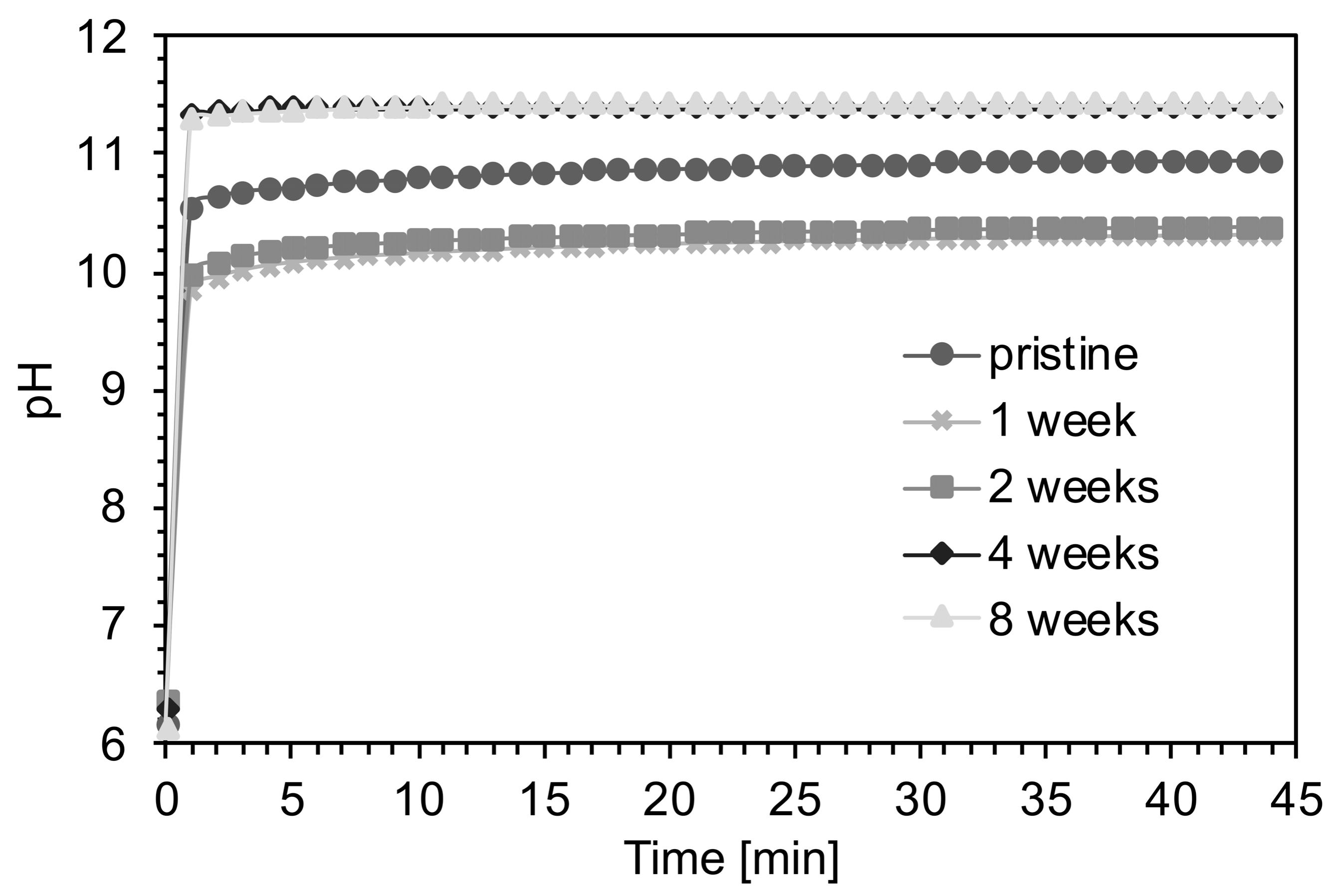
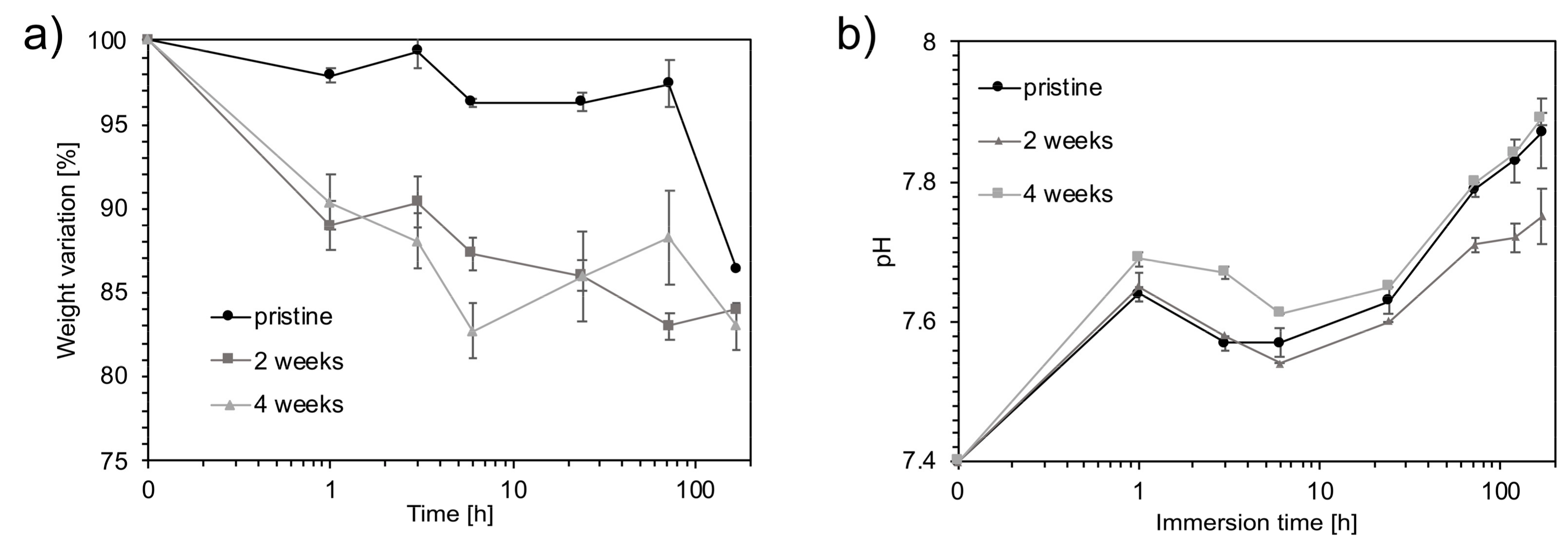
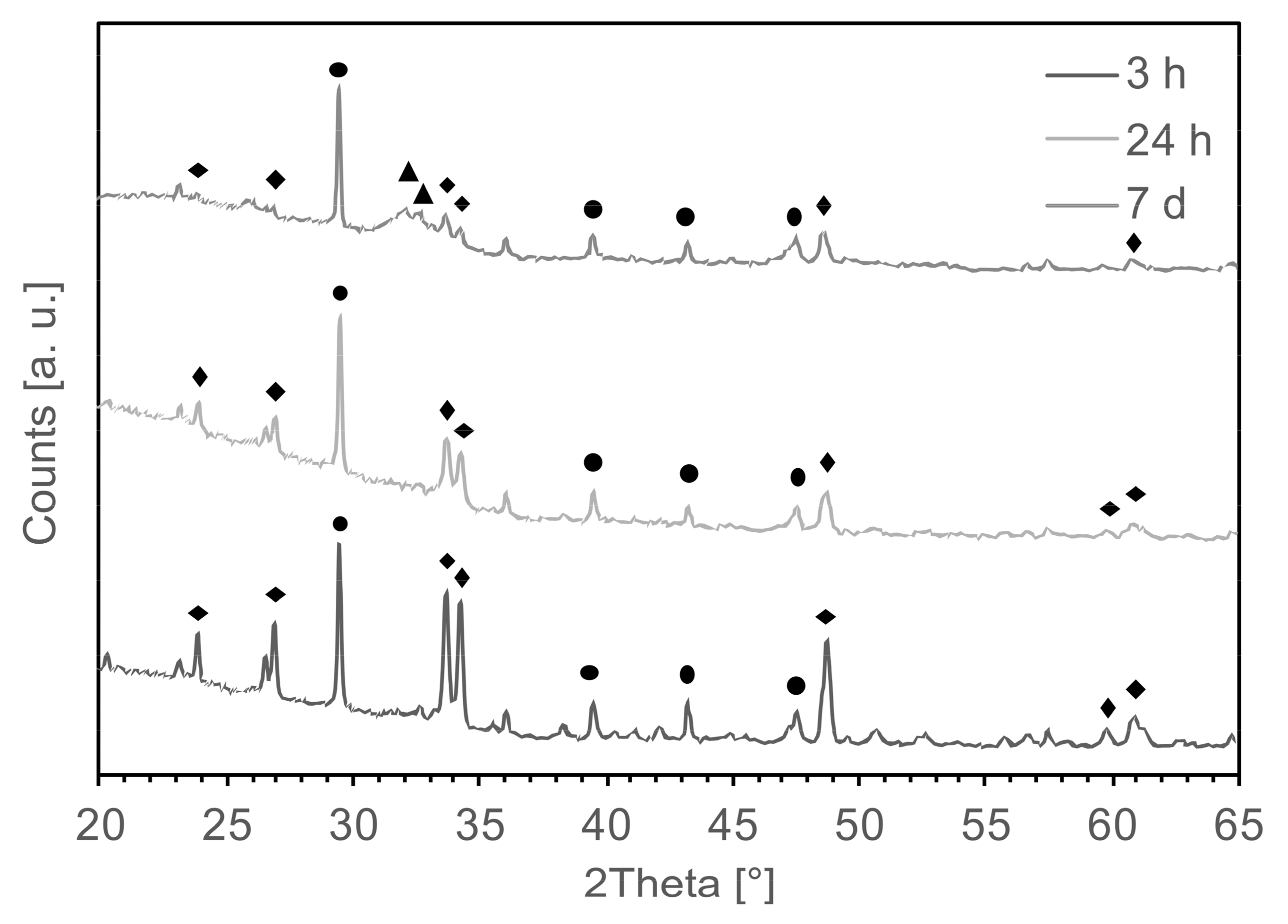
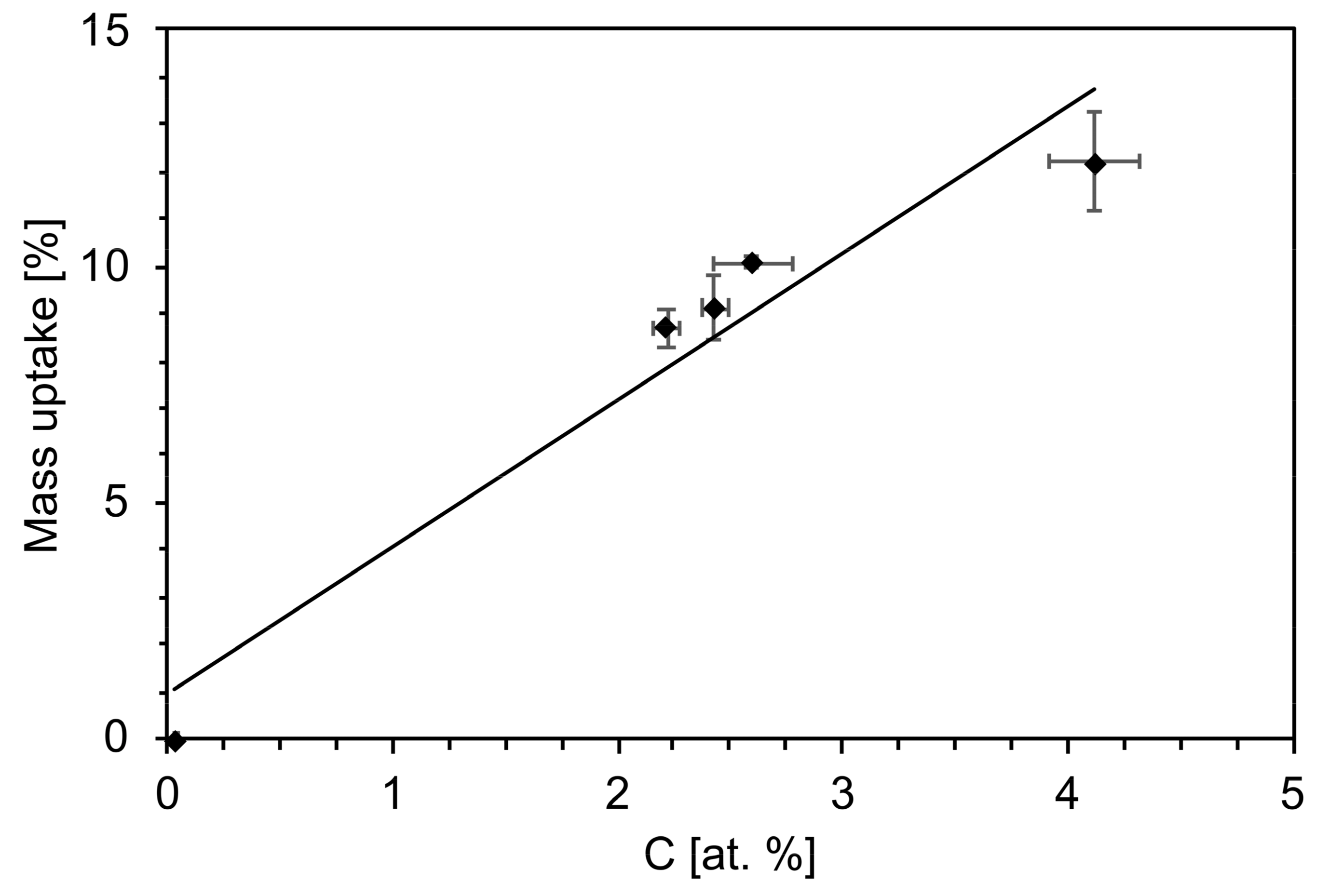
3.1. Chemical and Physical Characterization
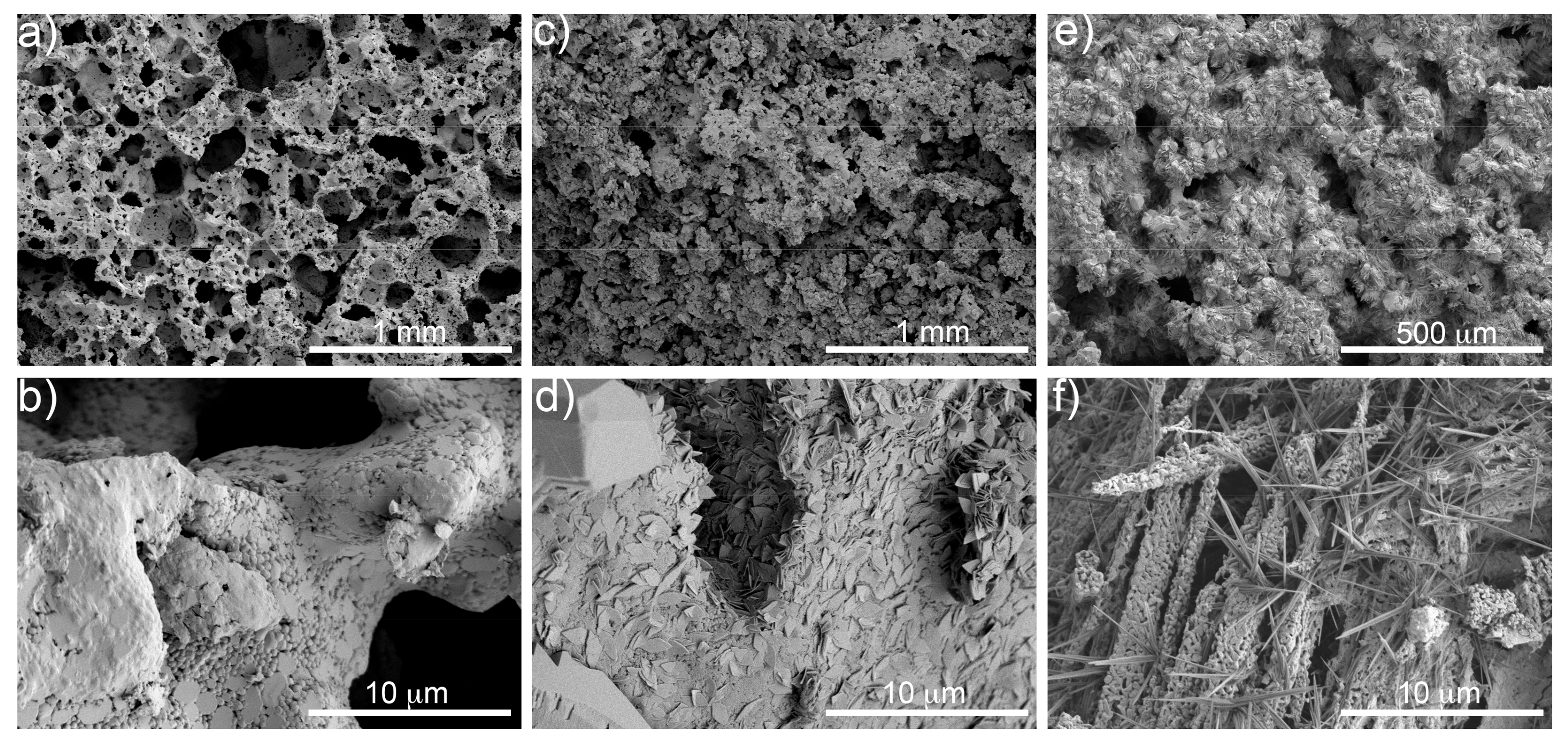
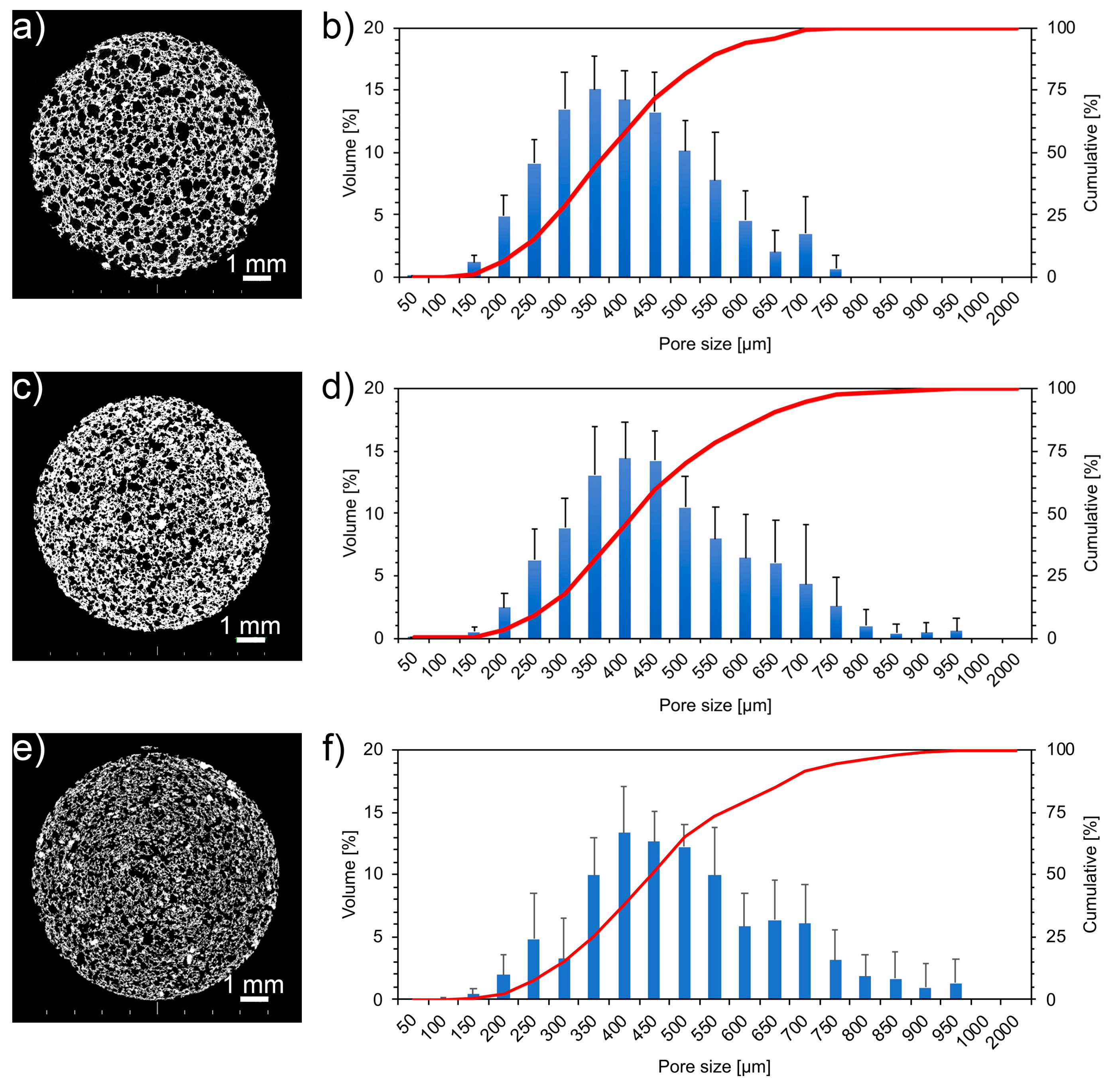
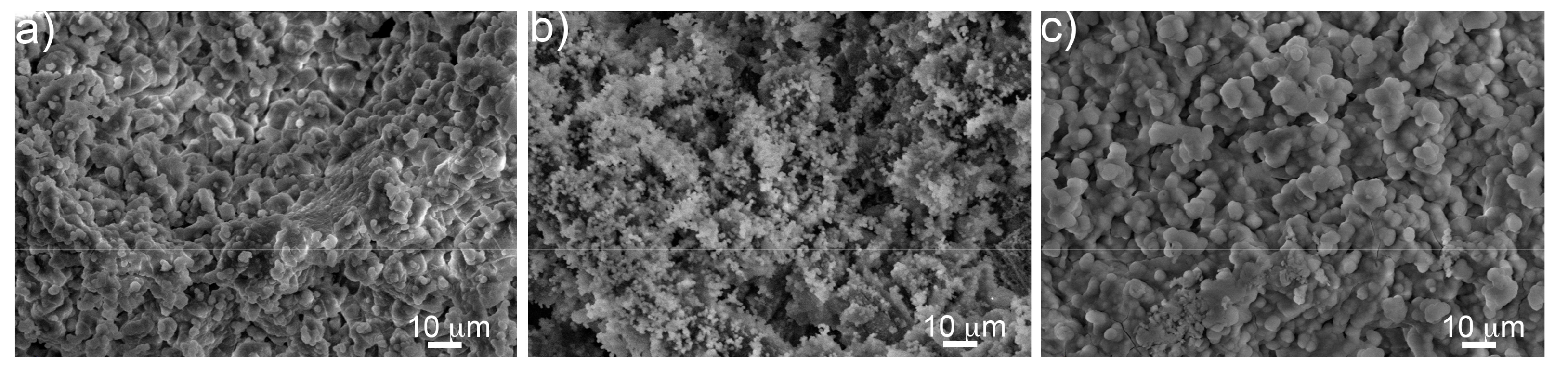
3.2. Morphological Characterization
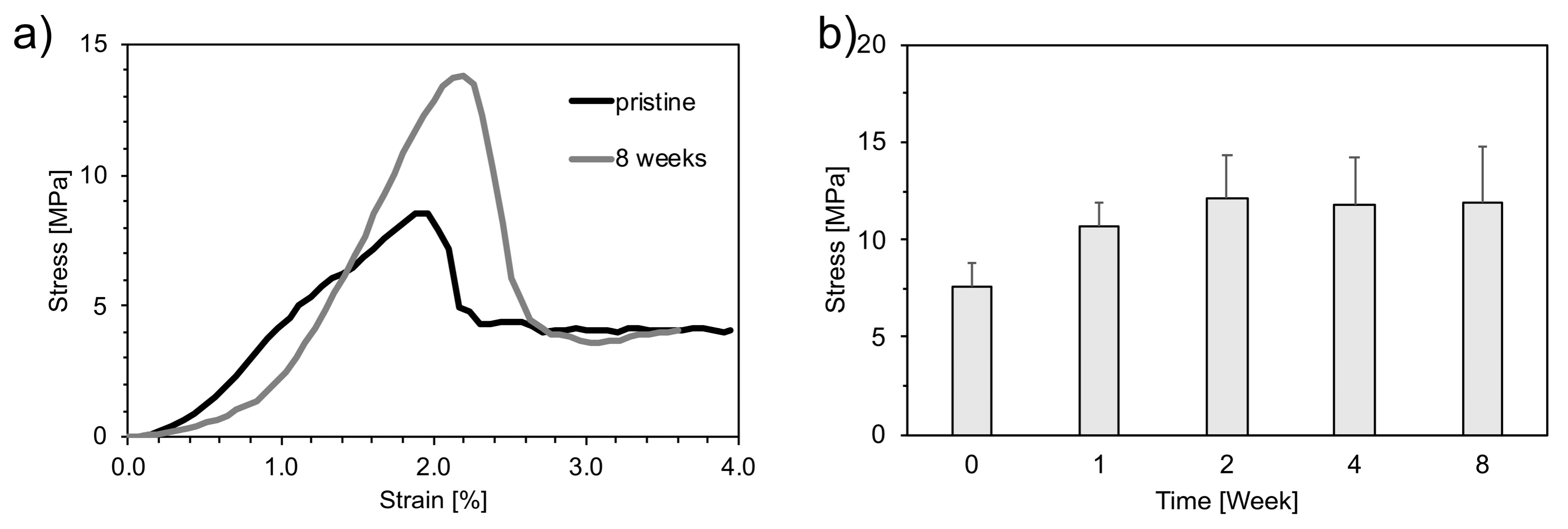
3.3. Mechanical Characterization
3.4. Bioactivity Evaluation
4. Discussion
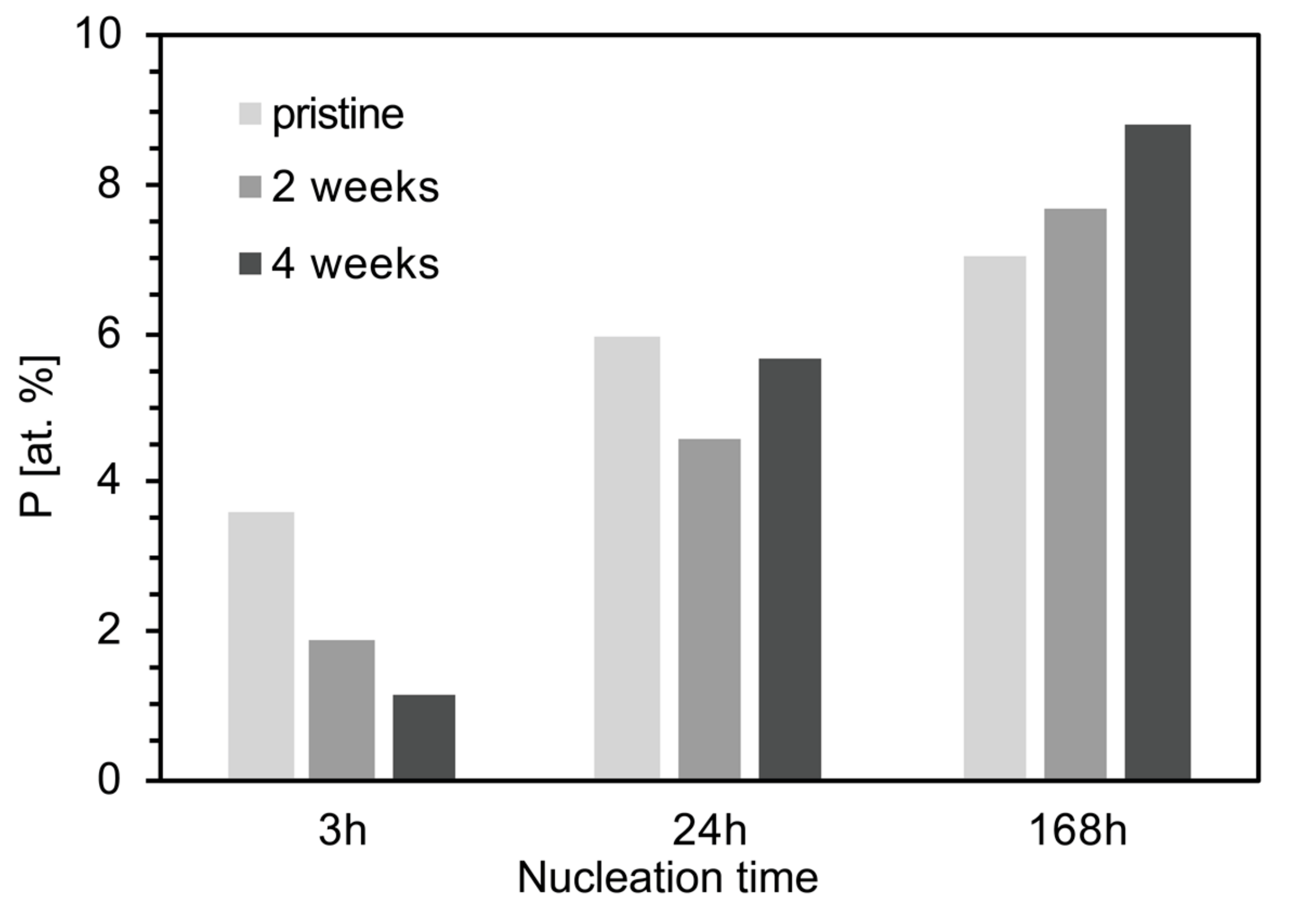
4.1. Nucleation of Carbonates as a Consequence of the Aging Process
4.2. Effects of the Aging Process on Bioactivity
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrington, E.D.; Smith, W.J.; Chambers, H.G.; Bucknell, A.L.; Davino, N.A. Complications of Iliac Crest Bone Graft Harvesting. Clin. Orthop. Relat. Res. 1996, 329, 300–309. [Google Scholar] [CrossRef]
- Cowley, S.P.; Anderson, L.D. Hernias through donor sites for iliac-bone grafts. J. Bone Jt. Surg. 1983, 65, 1023–1025. [Google Scholar] [CrossRef]
- Damien, C.J.; Parsons, J.R. Bone graft and bone graft substitutes: A review of current technology and applications. J. Appl. Biomater. 1991, 2, 187–208. [Google Scholar] [CrossRef] [PubMed]
- Campiglio, C.E.; Marcolin, C.; Draghi, L. Electrospun ECM Macromolecules as Biomimetic Scaffold for Regenerative Medicine: Challenges for Preserving Conformation and Bioactivity. AIMS Mater. Sci. 2017, 4, 638–669. [Google Scholar] [CrossRef]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.M.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef] [PubMed]
- Melli, V.; Lefebvre, L.-P.; Lenci, M.; Mondon, M.; Sao-Joao, S.; Cigada, A.; Delafosse, D.; De Nardo, L. Resorbability of a Bioglass®-based glass-ceramic scaffold produced via a powder metallurgy approach. Ceram. Int. 2017, 43, 8625–8635. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics. J. Am. Ceram. Soc. 2005, 81, 1705–1728. [Google Scholar] [CrossRef]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. N.a. Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef]
- Chen, Q.; Mohn, D.; Stark, W.J. Optimization of Bioglass® Scaffold Fabrication Process. J. Am. Ceram. Soc. 2011, 94, 4184–4190. [Google Scholar] [CrossRef]
- Bellucci, D.; Cannillo, V.; Sola, A.; Chiellini, F.; Gazzarri, M.; Migone, C. Macroporous Bioglass®-derived scaffolds for bone tissue regeneration. Ceram. Int. 2011, 37, 1575–1585. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Efthymiou, A.; Salih, V.; Boccaccini, A.R. Bioglass®-derived glass–ceramic scaffolds: Study of cell proliferation and scaffold degradationin vitro. J. Biomed. Mater. Res. A 2008, 84, 1049–1060. [Google Scholar] [CrossRef]
- Baino, F.; Fiume, E.; Barberi, J.; Kargozar, S.; Marchi, J.; Massera, J.; Verné, E. Processing Methods for Making Porous Bioactive Glass-Based Scaffolds-A State-of-the-Art Review. Int. J. Appl. Ceram. Technol. 2019. [Google Scholar] [CrossRef]
- El-Rashidy, A.A.; Roether, J.A.; Harhaus, L.; Kneser, U.; Boccaccini, A.R. Regenerating bone with bioactive glass scaffolds: A review of in vivo studies in bone defect models. Acta Biomater. 2017, 62, 1–28. [Google Scholar] [CrossRef]
- Esfahani Gahalayani, A.; Soleimanzade, M.; Campiglio, C.E.; Federici, A.; Altomare, L.; Draghi, L.; Boccaccini, A.R.; De Nardo, L. Hierarchical microchannel architecture in chitosan/bioactive glass scaffolds via electrophoretic deposition positive-replica. J. Biomed. Mater. Res. A 2019. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Reyes, E.A.; Leon-Patiño, C.A.; Jacinto-Diaz, B.; Lefebvre, L.-P.; Aguilar-Reyes, E.A.; León-Patiño, C.A.; Jacinto-Diaz, B. Structural Characterization and Mechanical Evaluation of Bioactive Glass 45S5 Foams Obtained by a Powder Technology Approach. J. Am. Ceram. Soc. 2012, 95, 3776–3780. [Google Scholar] [CrossRef]
- Tognonvi, M.T.; Zidol, A.; Aïtcin, P.-C.; Tagnit-Hamou, A. Aging of glass powder surface. J. Non-Crystalline Solids 2015, 427, 175–183. [Google Scholar] [CrossRef]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Boccardi, E.; Melli, V.; Catignoli, G.; Altomare, L.; Jahromi, M.T.; Cerruti, M.; Lefebvre, L.-P.; De Nardo, L. Study of the mechanical stability and bioactivity of Bioglass® based glass-ceramic scaffolds produced via powder metallurgy-inspired technology. Biomed. Mater. 2016, 11, 15005. [Google Scholar] [CrossRef]
- Hench, L.L.; Wilson, J. An Introduction to Bioceramics; World Scientific: Singapore, 1993; pp. 41–75. [Google Scholar]
- ISO. Implants for surgery—In vivo evaluation for apatite-forming ability of implant; ISO/FDIS 23317:2014; The British Standards Institution; BSI Standards Limited: London, UK, 2014; ISBN 978 0 580 84724 0. [Google Scholar]
- Lefebvre, L.-P.; Vanier, F.; Charbonneau, C. Stability of Bioactive Bone Graft Substitutes Exposed to Different Aging and Sterilization Conditions. J. Biomed. Mater. Res. B. 2019. Submitted. [Google Scholar]
- Rumble, J.R. Handbook of Chemistry and Physics, 99th ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Fu, Q.; Saiz, E.; Rahaman, M.N.; Tomsia, A.P. Bioactive glass scaffolds for bone tissue engineering: State of the art and future perspectives. Mater. Sci. Eng. C 2011, 31, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Cerruti, M.; Greenspan, D.; Powers, K. Effect of pH and ionic strength on the reactivity of Bioglass® 45S5. Biomaterials 2005, 26, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Boccaccini, A.R.; Chen, Q.; Lefebvre, L.; Gremillard, L.; Chevalier, J. Sintering, crystallisation and biodegradation behaviour of Bioglass®-derived glass-ceramics. Faraday Discuss. 2007, 136, 27–44. [Google Scholar] [CrossRef] [PubMed]


| Carbonate | pH (50 g L−1 at 25 °C) | Solubility in Water (g L−1) |
|---|---|---|
| Na2CO3 | 11.5 | 220 |
| CaCO3 | 9.5 | 0.013 |
| NaHCO3 | 8.6 | 96 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menci, P.F.; Mari, A.; Charbonneau, C.; Lefebvre, L.-P.; De Nardo, L. Aging of Bioactive Glass-Based Foams: Effects on Structure, Properties, and Bioactivity. Materials 2019, 12, 1485. https://doi.org/10.3390/ma12091485
Menci PF, Mari A, Charbonneau C, Lefebvre L-P, De Nardo L. Aging of Bioactive Glass-Based Foams: Effects on Structure, Properties, and Bioactivity. Materials. 2019; 12(9):1485. https://doi.org/10.3390/ma12091485
Chicago/Turabian StyleMenci, Pier Francesco, Andrea Mari, Cindy Charbonneau, Louis-Philippe Lefebvre, and Luigi De Nardo. 2019. "Aging of Bioactive Glass-Based Foams: Effects on Structure, Properties, and Bioactivity" Materials 12, no. 9: 1485. https://doi.org/10.3390/ma12091485





