Insertion of Iron Decorated Organic–Inorganic Cage-Like Polyhedral Oligomeric Silsesquioxanes between Clay Platelets by Langmuir Schaefer Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Polyhedral Oligomeric Silsesquioxanes (POSS)
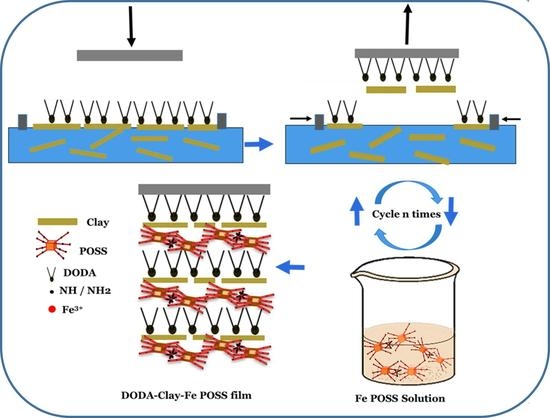
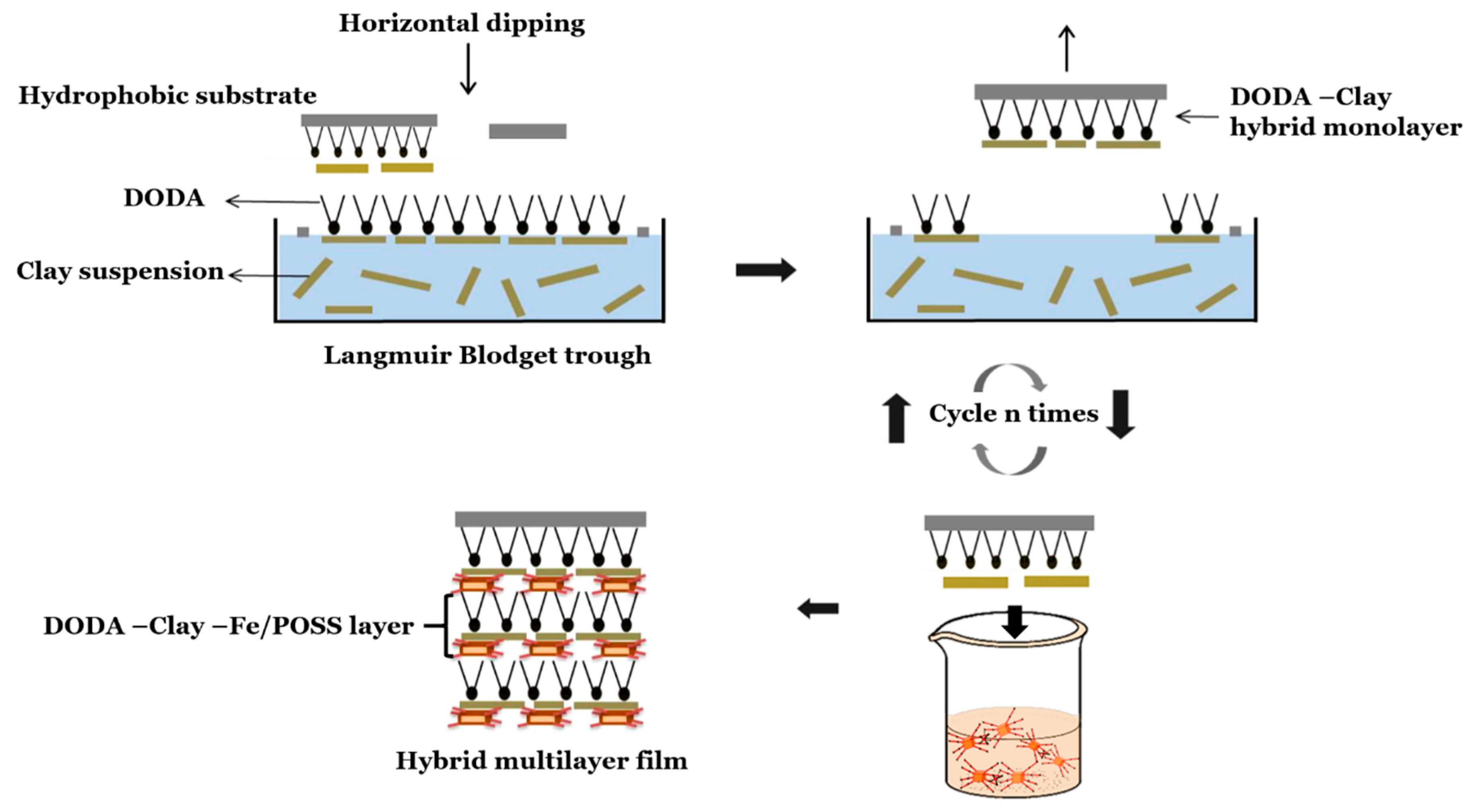
2.3. Preparation of Films by a Modified Langmuir–Schaefer Approach
2.4. Characterization Methods
3. Results and Discussion
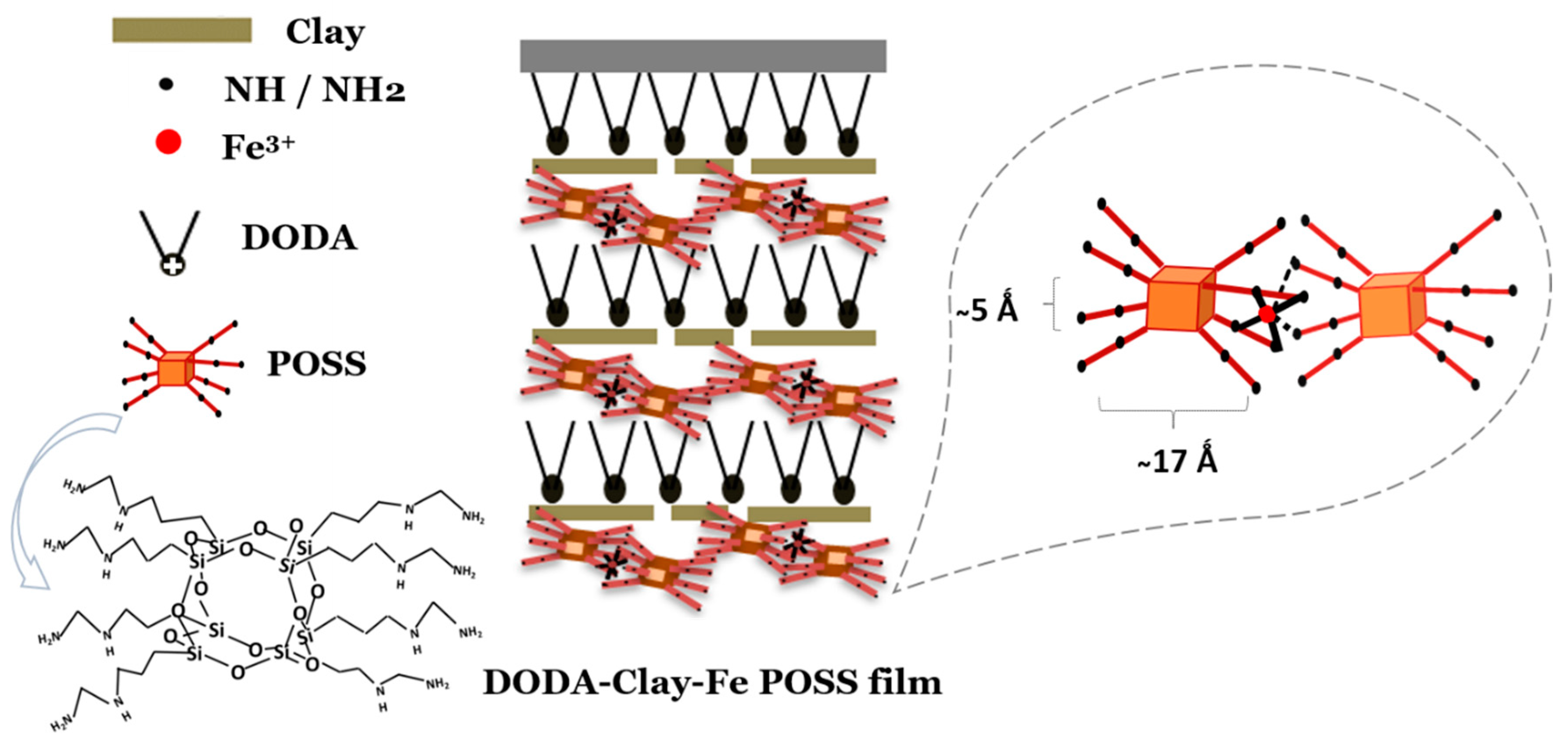
3.1. XRR Pattern of a DODA-Clay-Fe/POSS Hybrid Film
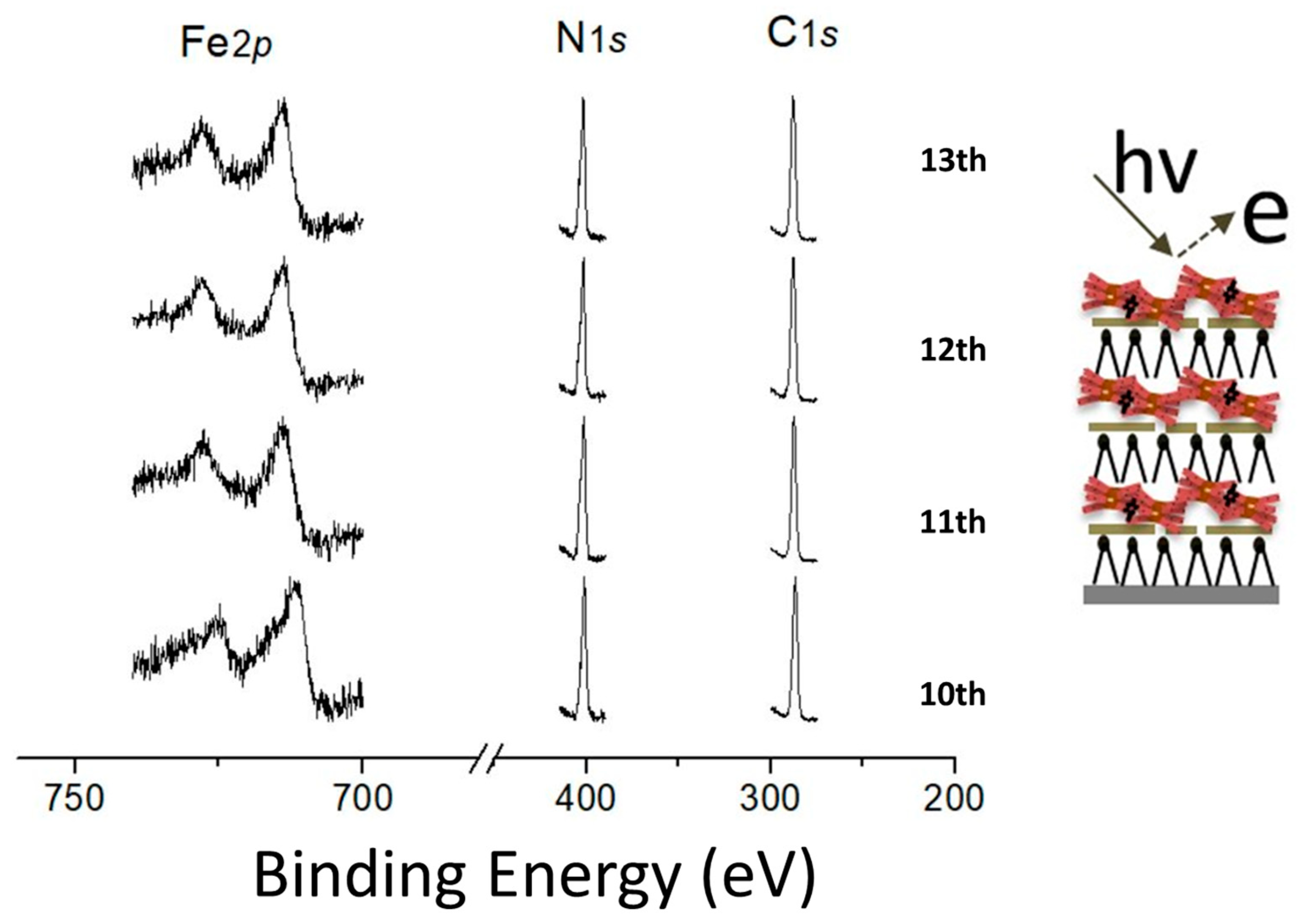
3.2. Probing the Surface of DODA-Clay-Fe/POSS by XPS
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ariga, K.; Hill, J.P.; Lee, M.V.; Vinu, A.; Charvet, R.; Acharya, S. Challenges and breakthroughs in recent research on self-assembly. Sci. Technol. Adv. Mater. 2008, 9, 014109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Qin, R.; Fu, G.; Zheng, N. Surface Coordination Chemistry of Metal Nanomaterials. J. Am. Chem. Soc. 2017, 139, 2122–2131. [Google Scholar] [CrossRef] [PubMed]
- Winfree, E.; Liu, F.; Wenzler, L.A.; Seeman, N.C. Design and self-assembly of two-dimensional DNA crystals. Nature 1998, 394, 539. [Google Scholar] [CrossRef] [PubMed]
- Ercole, F.; Davis, T.P.; Evans, R.A. Photo-responsive systems and biomaterials: Photochromic polymers, light-triggered self-assembly, surface modification, fluorescence modulation and beyond. Polym. Chem. 2010, 1, 37–54. [Google Scholar] [CrossRef]
- Hunt, S.T.; Milina, M.; Alba-Rubio, A.C.; Hendon, C.H.; Dumesic, J.A.; Román-Leshkov, Y. Self-assembly of noble metal monolayers on transition metal carbide nanoparticle catalysts. Science 2016, 352, 974–978. [Google Scholar] [CrossRef] [Green Version]
- Parsonage, E.; Horn, D.J.; Chen, J.J.; Miller, P.J.; Devens, D.A.; Weber, J., Jr. Medical Devices Comprising Nanocomposites. U.S. Patent US10/259,545, 27 September 2002. [Google Scholar]
- Wan, C.; Zhao, F.; Bao, X.; Kandasubramanian, B.; Duggan, M. Surface Characteristics of Polyhedral Oligomeric Silsesquioxane Modified Clay and Its Application in Polymerization of Macrocyclic Polyester Oligomers. J. Phys. Chem. B 2008, 112, 11915–11922. [Google Scholar] [CrossRef]
- He, F.-A.; Zhang, L.-M. Using inorganic POSS-modified laponite clay to support a nickel α-diimine catalyst forin situformation of high performance polyethylene nanocomposites. Nanotechnology 2006, 17, 5941–5946. [Google Scholar] [CrossRef]
- Petruska, M.A.; Watson, B.C.; Meisel, M.W.; Talham, D.R. A magnetic manganese phosphonate Langmuir-blodgett film containing a tetrathiafulvalene amphiphile. Mol. Cryst. Liq. Cryst. 2002, 376, 121–126. [Google Scholar] [CrossRef]
- Clemente-leo, M.; Coronado, E.; Soriano-portillo, A.; Colacio, E.; Domı, M.; Ga, N.; Maduen, R. Magnetic Langmuir—Blodgett Films of Ferritin with Different Iron Contents. Langmuir 2006, 22, 6993–7000. [Google Scholar] [CrossRef]
- Kouloumpis, A.; Vourdas, N.; Zygouri, P.; Chalmpes, N.; Potsi, G.; Kostas, V.; Spyrou, K.; Stathopoulos, V.N.; Gournis, D.; Rudolf, P. Controlled deposition of fullerene derivatives within a graphene template by means of a modified Langmuir-Schaefer method. J. Colloid Interface Sci. 2018, 524, 388–398. [Google Scholar] [CrossRef] [Green Version]
- Kouloumpis, A.; Spyrou, K.; Dimos, K.; Georgakilas, V.; Rudolf, P.; Gournis, D. A Bottom-Up Approach for the Synthesis of Highly Ordered Fullerene-Intercalated Graphene Hybrids. Front. Mater. 2015, 2, 10. [Google Scholar]
- Kouloumpis, A.; Thomou, E.; Chalmpes, N.; Dimos, K.; Spyrou, K.; Bourlinos, A.B.; Koutselas, I.; Gournis, D.; Rudolf, P. Graphene/Carbon Dot Hybrid Thin Films Prepared by a Modified Langmuir–Schaefer Method. ACS Omega 2017, 2, 2090–2099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, S.W.; Chang, F.C. POSS related polymer nanocomposites. Prog. Polym. Sci. 2011, 36, 1649–1696. [Google Scholar] [CrossRef]
- Mengel, C.; Meyer, W.H.; Wegner, G. Photocrosslinkable star polymers: Precursors for model polyelectrolyte networks. Macromol. Chem. Phys. 2001, 202, 1138–1149. [Google Scholar] [CrossRef]
- Blanco, I. The Rediscovery of POSS: A Molecule Rather than a Filler. Polym. 2018, 10, 904. [Google Scholar] [CrossRef] [Green Version]
- Feher, F.J.; Newman, D.A.; Walzer, J.F. Silsesquioxanes as models for silica surfaces. J. Am. Chem. Soc. 1989, 111, 1741–1748. [Google Scholar] [CrossRef]
- Duchateau, R.; Abbenhuis, H.C.L.; van Santen, R.A.; Meetsma, A.; Thiele, S.K.-H.; van Tol, M.F.H. Ethylene Polymerization with Dimeric Zirconium and Hafnium Silsesquioxane Complexes. Organometallics 1998, 17, 5663–5673. [Google Scholar] [CrossRef]
- Ropartz, L.; Foster, D.F.; Morris, R.E.; Slawin, A.M.Z.; Cole-Hamilton, D.J. Hydrocarbonylation reactions using alkylphosphine-containing dendrimers based on a polyhedral oligosilsesquioxane core. J. Chem. Soc. Dalt. Trans. 2002, 1997–2008. [Google Scholar] [CrossRef]
- Gonzalez, R.I.; Phillips, S.H.; Hoflund, G.B. In Situ Oxygen-Atom Erosion Study of Polyhedral Oligomeric Silsesquioxane-Siloxane Copolymer. J. Spacecr. Rocket. 2000, 37, 463–467. [Google Scholar] [CrossRef]
- Hoflund, G.B.; Gonzalez, R.I.; Phillips, S.H. In situ oxygen atom erosion study of a polyhedral oligomeric silsesquioxane-polyurethane copolymer. J. Adhes. Sci. Technol. 2001, 15, 1199–1211. [Google Scholar] [CrossRef] [Green Version]
- Paquet, O.; Brochier Salon, M.-C.; Zeno, E.; Belgacem, M.N. Hydrolysis-condensation kinetics of 3-(2-amino-ethylamino)propyl-trimethoxysilane. Mater. Sci. Eng. C 2012, 32, 487–493. [Google Scholar] [CrossRef]
- Potsi, G.; Rossos, A.; Kouloumpis, A.; Antoniou, M.K.; Spyrou, K.; Karakassides, M.A.; Gournis, D.; Rudolf, P. Carbon Nanostructures Containing Polyhedral Oligomeric Silsesquioxanes (POSS). Curr. Org. Chem. 2016, 20, 662–673. [Google Scholar] [CrossRef] [Green Version]
- Potsi, G.; Ladavos, A.K.; Petrakis, D.; Douvalis, A.P.; Sanakis, Y.; Katsiotis, M.S.; Papavassiliou, G.; Alhassan, S.; Gournis, D.; Rudolf, P. Iron-substituted cubic silsesquioxane pillared clays: Synthesis, characterization and acid catalytic activity. J. Colloid Interface Sci. 2018, 510, 395–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rethinasabapathy, M.; Kang, S.-M.; Lee, I.; Lee, G.-W.; Hwang, S.K.; Roh, C.; Huh, Y.S. Layer-Structured POSS-Modified Fe-Aminoclay/Carboxymethyl Cellulose Composite as a Superior Adsorbent for the Removal of Radioactive Cesium and Cationic Dyes. Ind. Eng. Chem. Res. 2018, 57, 13731–13741. [Google Scholar] [CrossRef]
- Alexander, R.; Kagi, R.I.; Larcher, A.V. Clay catalysis of alkyl hydrogen exchange reactions-reaction mechanisms. Org. Geochem. 1984, 6, 755–760. [Google Scholar] [CrossRef]
- Alexander, R.; Kagi, R.I.; Larcher, A.V. Clay catalysis of aromatic hydrogen-exchange reactions. Geochim. Cosmochim. Acta 1982, 46, 219–222. [Google Scholar] [CrossRef]
- Georgakilas, V.; Gournis, D.; Bourlinos, A.B.; Karakassides, M.A.; Petridis, D. Clays as a host matrix in the synthesis of organic macrocycles. Chem. A Eur. J. 2003, 9, 3904–3908. [Google Scholar] [CrossRef]
- Georgakilas, V.; Gournis, D.; Petridis, D. Organoclay derivatives in the synthesis of macrocycles. Angew. Chem. Int. Ed. 2001, 40, 4286–4288. [Google Scholar] [CrossRef]
- Gopakumar, T.G.; Lee, J.A.; Kontopoulou, M.; Parent, J.S. Influence of clay exfoliation on the physical properties of montmorillonite/polyethylene composites. Polymer 2002, 43, 5483–5491. [Google Scholar] [CrossRef]
- Zhao, C.; Qin, H.; Gong, F.; Feng, M.; Zhang, S.; Yang, M. Mechanical, thermal and flammability properties of polyethylene/clay nanocomposites. Polym. Degrad. Stab. 2005, 87, 183–189. [Google Scholar] [CrossRef]
- Fu, X.; Qutubuddin, S. Polymer–clay nanocomposites: Exfoliation of organophilic montmorillonite nanolayers in polystyrene. Polymer 2001, 42, 807–813. [Google Scholar] [CrossRef]
- Wang, K.H.; Choi, M.H.; Koo, C.M.; Choi, Y.S.; Chung, I.J. Synthesis and characterization of maleated polyethylene/clay nanocomposites. Polymer 2001, 42, 9819–9826. [Google Scholar] [CrossRef]
- Toma, L.M.; Gengler, R.Y.N.; Prinsen, E.B.; Gournis, D.; Rudolf, P. A Langmuir-Schaefer approach for the synthesis of highly ordered organoclay thin films. Phys. Chem. Chem. Phys. 2010, 12, 12188–12197. [Google Scholar] [CrossRef] [PubMed]
- Balomenou, G.; Stathi, P.; Enotiadis, A.; Gournis, D.; Deligiannakis, Y. Physicochemical study of amino-functionalized organosilicon cubes intercalated in montmorillonite clay: H-binding and metal uptake. J. Colloid Interface Sci. 2008, 325, 74–83. [Google Scholar] [CrossRef]
- Yei, D.R.; Kuo, S.W.; Su, Y.C.; Chang, F.C. Enhanced thermal properties of PS nanocomposites formed from inorganic POSS-treated montmorillonite. Polymer 2004, 45, 2633–2640. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, W.; Zheng, S. Montmorillonite intercalated by ammonium of octaaminopropyl polyhedral oligomeric silsesquioxane and its nanocomposites with epoxy resin. Polymer 2005, 46, 157–165. [Google Scholar] [CrossRef]
- Toma, L.M.; Gengler, R.Y.N.; Cangussu, D.; Pardo, E.; Lloret, F.; Rudolf, P. New magnetic thin film hybrid materials built by the incorporation of octanickel(II)-oxamato clusters between clay mineral platelets. J. Phys. Chem. Lett. 2011, 2, 2004–2008. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, N.; Blake, G.R.; Felici, R.; Amenitsch, H.; Palstra, T.T.M.; Rudolf, P. Design of molecule-based magnetic conductors. Nano Res. 2014, 7, 1832–1842. [Google Scholar] [CrossRef]
- Akhtar, N.; Polyakov, A.O.; Aqeel, A.; Gordiichuk, P.; Blake, G.R.; Baas, J.; Amenitsch, H.; Herrmann, A.; Rudolf, P.; Palstra, T.T.M. Self-assembly of ferromagnetic organic-inorganic perovskite-like films. Small 2014, 10, 4912–4919. [Google Scholar] [CrossRef]
- Kim, J.-H.; Fujita, S.; Shiratori, S. Design of a thin film for optical applications, consisting of high and low refractive index multilayers, fabricated by a layer-by-layer self-assembly method. Colloids Surfaces A Physicochem. Eng. Asp. 2006, 284–285, 290–294. [Google Scholar] [CrossRef]
- Yamamoto, T.; Umemura, Y.; Sato, O.; Einaga, Y. Photoswitchable Magnetic Films: Prussian Blue Intercalated in Langmuir−Blodgett Films Consisting of an Amphiphilic Azobenzene and a Clay Mineral. Chem. Mater. 2004, 16, 1195–1201. [Google Scholar] [CrossRef]
- Andreatta, G.; Jian Wang, Y.; Kay Lee, F.; Polidori, A.; Tong, P.; Pucci, B.; Benattar, J.-J. Molecular Transfer of Surfactant Bilayers: Widening the Range of Substrates. Langmuir 2008, 24, 6072–6078. [Google Scholar] [CrossRef] [PubMed]
- Roziere, J.; Jones, D.J.; Cassagneau, T. Crosslinked layered materials formed by intercalation of octameric siloxanes in metal(IV) hydrogen phosphates. J. Mater. Chem. 1991, 1, 1081–1082. [Google Scholar] [CrossRef]
- Cassagneau, T.; Jones, D.J.; Roziere, J. Novel inorganic oxide pillared.gamma.-zirconium phosphate formed by intercalation of octameric siloxanes. J. Phys. Chem. 1993, 97, 8678–8680. [Google Scholar] [CrossRef]
- Umemura, Y.; Yamagishi, A.; Schoonheydt, R.; Persoons, A.; De Schryver, F. Formation of hybrid monolayers of alkylammonium cations and a clay mineral at an air-water interface: Clay as an inorganic stabilizer for water-soluble amphiphiles. Thin Solid Films 2001, 388, 5–8. [Google Scholar] [CrossRef]
- Ras, R.H.A.; Nemeth, J.; Johnston, C.T.; DiMasi, E.; Dekany, I.; Schoonheydt, R.A. Hybrid Langmuir?Blodgett monolayers containing clay minerals: Effect of clay concentration and surface charge density on the film formation. Phys. Chem. Chem. Phys. 2004, 6, 4174. [Google Scholar] [CrossRef]
- Umemura, Y.; Yamagishi, A.; Schoonheydt, R.; Persoons, A.; De Schryver, F. Fabrication of hybrid films of alkylammonium cations (CnH2n+1NH3+; n = 4-18) and a smectite clay by the Langmuir-Blodgett method. Langmuir 2001, 17, 449–455. [Google Scholar] [CrossRef]
- Umemura, Y.; Onodera, Y.; Yamagishi, A. Layered structure of hybrid films of an alkylammonium cation and a clay mineral as prepared by the Langmuir–Blodgett method. Thin Solid Films 2003, 426, 216–220. [Google Scholar] [CrossRef]
- Moulder, J.F.; Chastain, J. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Physical Electronics: Eden Prairie, MN, USA, 1995; ISBN 096481241X 9780964812413. [Google Scholar]
- Johnston, C.T.; Khan, B.; Barth, E.F.; Chattopadhyay, S.; Boyd, S.A. Nature of the interlayer environment in an organoclay optimized for the sequestration of dibenzo-p-dioxin. Environ. Sci. Technol. 2012, 46, 9584–9591. [Google Scholar] [CrossRef] [Green Version]
- Chalmpes, N.; Kouloumpis, A.; Zygouri, P.; Karouta, N.; Spyrou, K.; Stathi, P.; Tsoufis, T.; Georgakilas, V.; Gournis, D.; Rudolf, P. Layer-by-Layer Assembly of Clay–Carbon Nanotube Hybrid Superstructures. ACS Omega 2019, 4, 18100–18107. [Google Scholar] [CrossRef]
- Spyrou, K.; Potsi, G.; Diamanti, E.K.; Ke, X.; Serestatidou, E.; Verginadis, I.I.; Velalopoulou, A.P.; Evangelou, A.M.; Deligiannakis, Y.; Van Tendeloo, G.; et al. Towards Novel Multifunctional Pillared Nanostructures: Effective Intercalation of Adamantylamine in Graphene Oxide and Smectite Clays. Adv. Funct. Mater. 2014, 24, 5841–5850. [Google Scholar] [CrossRef]
- Kataoka, S.; Banerjee, S.; Kawai, A.; Kamimura, Y.; Choi, J.-C.; Kodaira, T.; Sato, K.; Endo, A. Layered Hybrid Perovskites with Micropores Created by Alkylammonium Functional Silsesquioxane Interlayers. J. Am. Chem. Soc. 2015, 137, 4158–4163. [Google Scholar] [CrossRef] [PubMed]
- Dimos, K.; Panagiotopoulos, I.; Tsoufis, T.; Gengler, R.Y.N.; Moukarika, A.; Rudolf, P.; Karakassides, M.A.; Bakas, T.; Gournis, D. Effect of [Fe(CN)6]4– Substitutions on the Spin-Flop Transition of a Layered Nickel Phyllosilicate. Langmuir 2012, 28, 10289–10295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashid, S.; Shen, C.; Chen, X.; Li, S.; Chen, Y.; Wen, Y.; Liu, J. Enhanced catalytic ability of chitosan–Cu–Fe bimetal complex for the removal of dyes in aqueous solution. RSC Adv. 2015, 5, 90731–90741. [Google Scholar] [CrossRef]
- Noriega, R.; Rivnay, J.; Vandewal, K.; Koch, F.P.V.; Stingelin, N.; Smith, P.; Toney, M.F.; Salleo, A. A general relationship between disorder, aggregation and charge transport in conjugated polymers. Nat. Mater. 2013, 12, 1038–1044. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Potsi, G.; Gengler, R.Y.N.; Gournis, D.; Rudolf, P. Insertion of Iron Decorated Organic–Inorganic Cage-Like Polyhedral Oligomeric Silsesquioxanes between Clay Platelets by Langmuir Schaefer Deposition. Materials 2020, 13, 216. https://doi.org/10.3390/ma13010216
Wu J, Potsi G, Gengler RYN, Gournis D, Rudolf P. Insertion of Iron Decorated Organic–Inorganic Cage-Like Polyhedral Oligomeric Silsesquioxanes between Clay Platelets by Langmuir Schaefer Deposition. Materials. 2020; 13(1):216. https://doi.org/10.3390/ma13010216
Chicago/Turabian StyleWu, Jiquan, Georgia Potsi, Regis Y. N. Gengler, Dimitrios Gournis, and Petra Rudolf. 2020. "Insertion of Iron Decorated Organic–Inorganic Cage-Like Polyhedral Oligomeric Silsesquioxanes between Clay Platelets by Langmuir Schaefer Deposition" Materials 13, no. 1: 216. https://doi.org/10.3390/ma13010216