Preparation and Characterization of Alumina HDPE Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Surface Morphology Analysis
2.4. Tensile Properties Investigation
2.5. Melt Flow Index Measurement
2.6. Thermal Analyses
2.6.1. DSC
2.6.2. Thermogravimetric analyses (TGAs)
2.7. Chemical Composition Investigation
2.8. Dielectric Properties
3. Results and Discussion
3.1. Morphology Investigation
3.2. Mechanical Properties Analyses
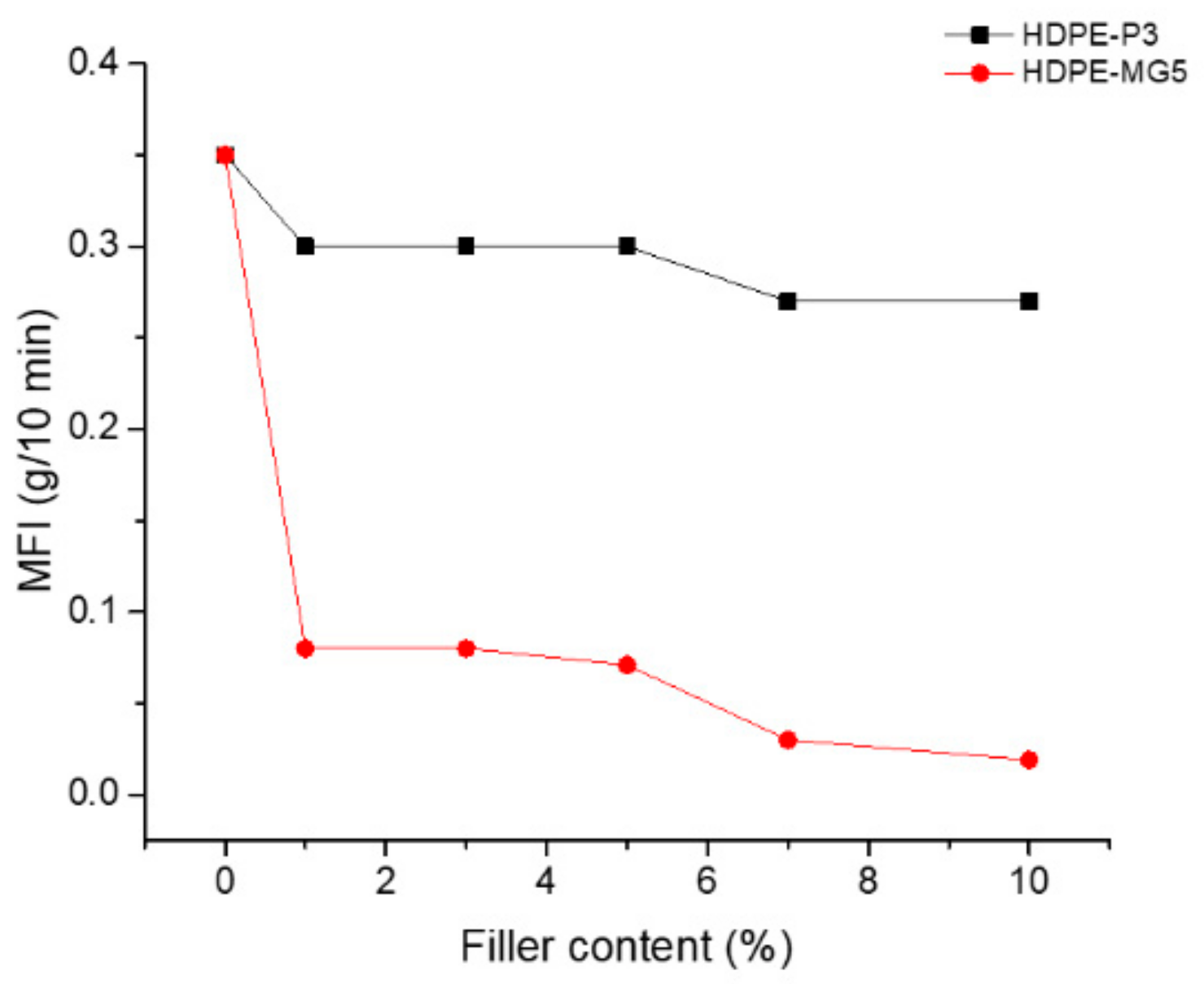
3.3. Melt Flow Index Measurment
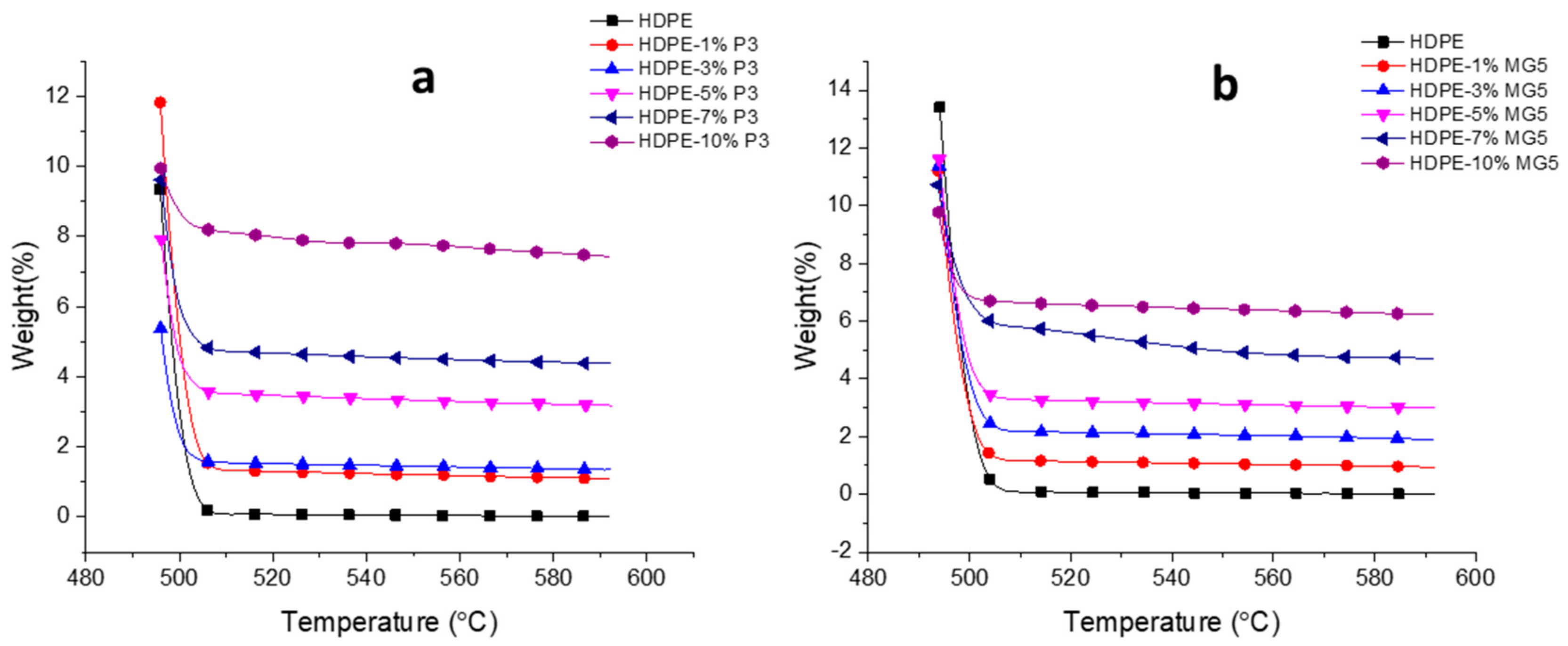
3.4. Thermal Properties Investigation
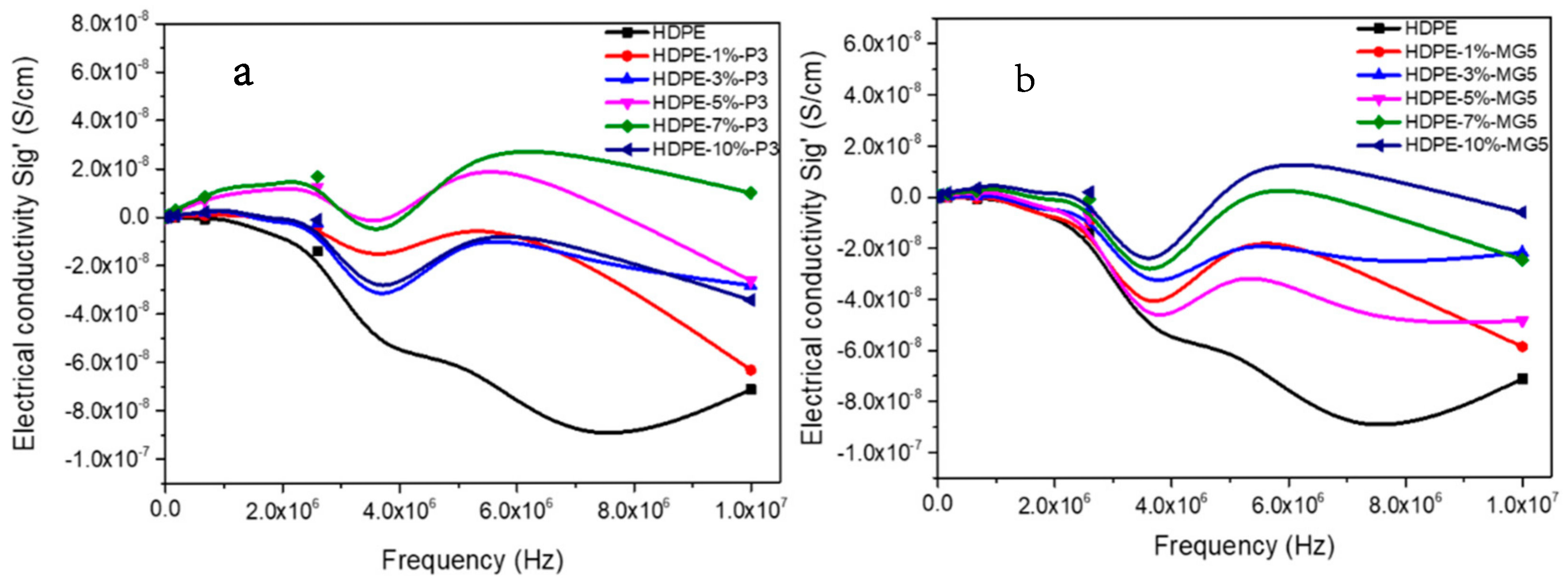
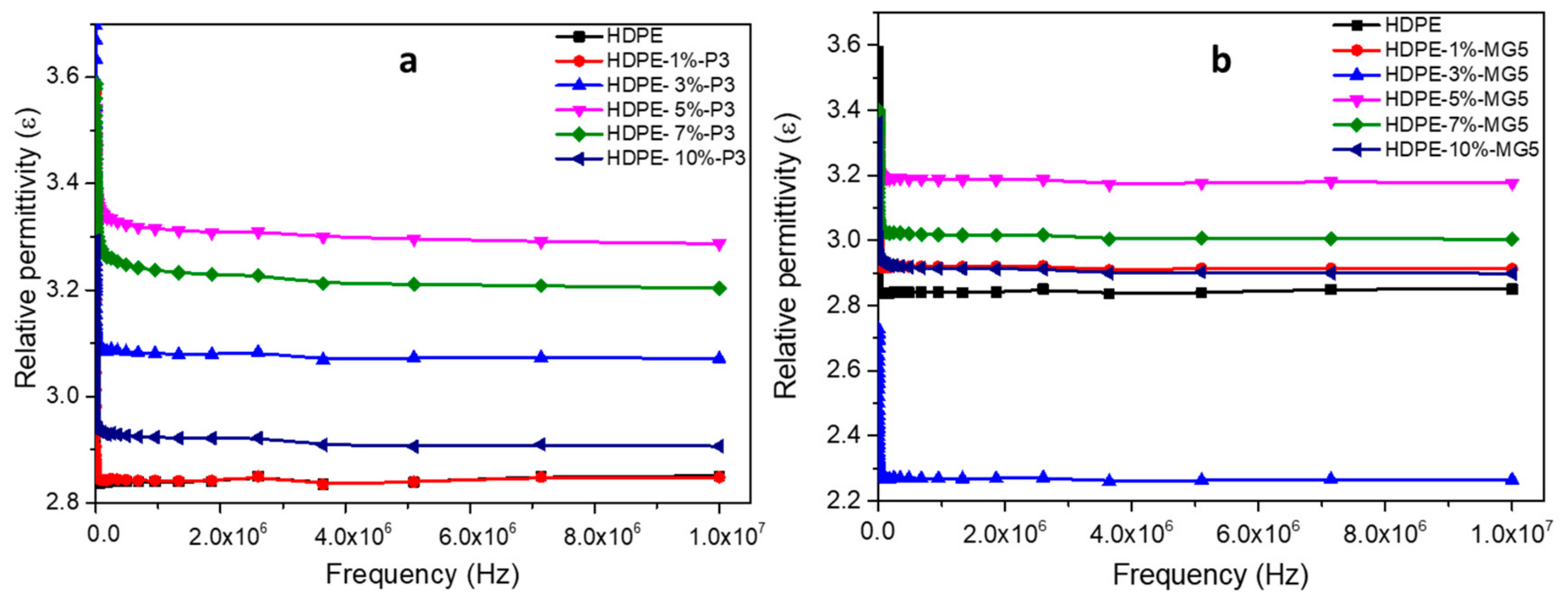
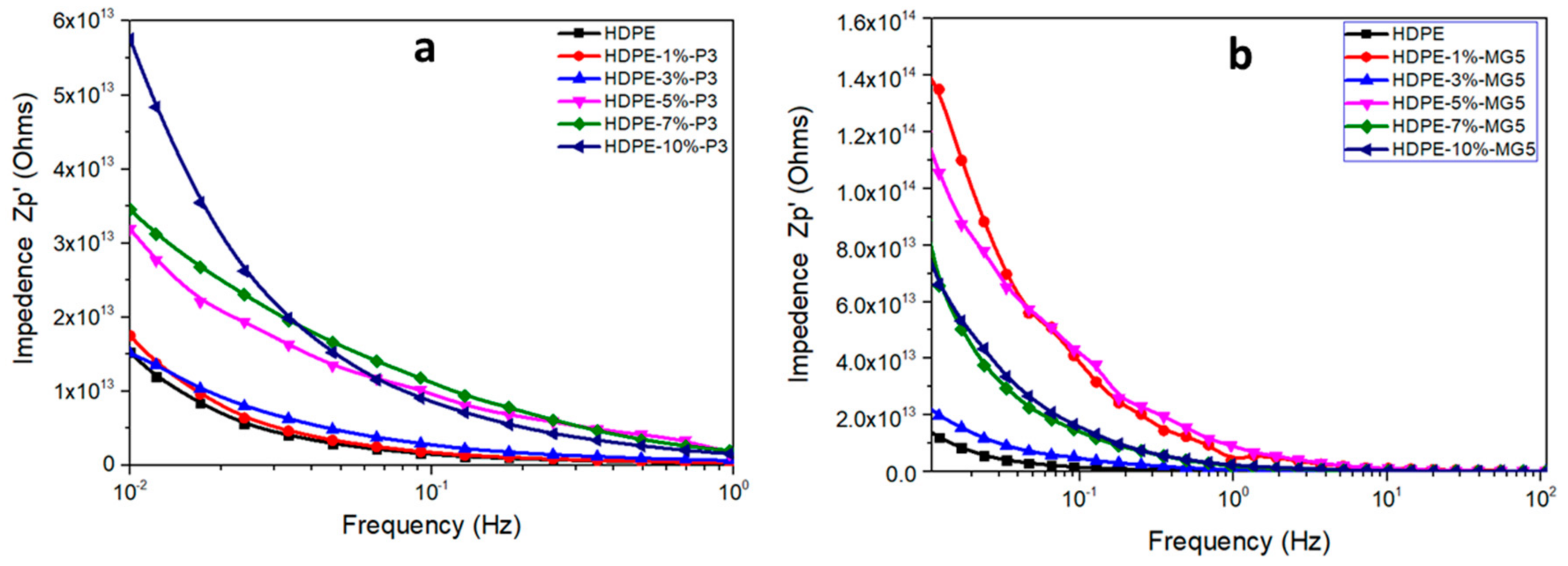
3.5. Dielectric Properties Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tessema, A.; Zhao, D.; Moll, J.; Xu, S.; Yang, R.; Li, C.; Kumar, S.K.; Kidane, A. Effect of filler loading, geometry, dispersion and temperature on thermal conductivity of polymer nanocomposites. Polym. Test. 2017, 57, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Khumalo, V.M.; Karger-Kocsis, J.; Thomann, R. Polyethylene/synthetic boehmite alumina nanocomposites: Structure, mechanical, and perforation impact properties. eXPRESS Polym. Lett. 2010, 4, 264–274. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Schneider, D.; Fytas, G.; Kumar, S.K.; Zhao, D. Controlling the Thermomechanical Behavior of Nanoparticle/Polymer Films. ACS Nano 2014, 8, 8163–8173. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.C.; Jeong, S.B.; Kim, B.G.; Yoon, P.R. Examination of dispersive properties of alumina treated with silane coupling agents, by using inverse gas chromatography. Powder. Technol. 2009, 191, 117–121. [Google Scholar] [CrossRef]
- Prado, L.A.S.A.; Sriyai, M.; Ghislandi, M.; Barros-Timmons, A.; Schulte, K.A. Surface Modification of Alumina Nanoparticles with Silane Coupling Agents. J. Braz. Chem. Soc. 2010, 21, 2238–2245. [Google Scholar] [CrossRef] [Green Version]
- Amirchakhmaghi, S.; Nia, A.A.; Azizpour, G.; Bamdadi, H. The Effect of surface Treatment of Alumina NanoParticles with a Silane Coupling Agent on the Mechanical Properties of Polymer Nanocomposites. Mech. Compos. Mater. 2015, 51, 347–358. [Google Scholar] [CrossRef]
- Mahmoud, A.K. Production of alumina nanoparticles (Al2O3) using pulsed laser ablation technique in distilled water solution. IJCET 2014, 2, 314–316. [Google Scholar] [CrossRef] [Green Version]
- Piriyawong, V.; Thongpoo, V.; Asanithi, P.; Limsuwan, P. Preparation and Characterization of Alumina Nanoparticles in Deionized Water Using Laser Ablation Technique. J. Nanomater. 2012, 2012, 819403. [Google Scholar] [CrossRef]
- Lukić, I.; Krstić, J.; Jovanović, D.; Skala, D.; Lukić, I. Alumina/silica supported K2CO3 as a catalyst for biodiesel synthesis from sunflower oil. Bioresour. Technol. 2009, 100, 4690–4696. [Google Scholar] [CrossRef]
- Hato, M.J.; Motaung, T.E.; Motloung, S.V.; Koao, L.F. The effect of boehmite alumina nanoparticles on the properties of polylactide/polypropylene blend composites. J. Reinf. Plast. Compos. 2016, 35, 1191–1200. [Google Scholar] [CrossRef]
- Mallakpour, S.; Khadem, E. Recent development in the synthesis of polymer nanocomposites based on nano-alumina. Prog. Polym. Sci. 2015, 51, 74–93. [Google Scholar] [CrossRef]
- Adhikari, R.; Henning, S.; Lebek, W.; Godehardt, R.; Ilisch, S.; Michler, G.H.; Adhikari, R. Structure and Properties of Nanocomposites Based on SBS Block Copolymer and Alumina. Macromol. Symp. 2005, 231, 116–124. [Google Scholar] [CrossRef]
- Wang, S.J.; Zha, J.W.; Wu, Y.H.; Ren, L.; Dang, Z.M.; Wu, J. Preparation, microstructure and properties of polyethylene/alumina nanocomposites for HVDC insulation. IEEE. Trans. Dielectr. Electr. Insul. 2015, 22, 3350–3356. [Google Scholar] [CrossRef]
- Li, B.; Li, R. Preparation and property of ultrahigh molecular weight polyethylene/halloysite nanotube fiber. Fibers Polym. 2016, 17, 1043–1047. [Google Scholar] [CrossRef]
- Szpilska, K.; Kudla, S.; Czaja, K. Polyolefin-matrix composites with modified halloysite nanotubes. Przem. Chem. 2015, 94, 2130–2132. [Google Scholar]
- Qiao, X.; Na, M.; Gao, P.; Sun, K. Halloysite nanotubes reinforced ultrahigh molecular weight polyethylene nanocomposite films with different filler concentration and modification. Polym. Test. 2017, 57, 133–140. [Google Scholar] [CrossRef]
- Sikora, J.W.; Gajdoš, I.; Puszka, A. Polyethylene-Matrix Composites with Halloysite Nanotubes with Enhanced Physical/Thermal Properties. Polymers 2019, 11, 787. [Google Scholar] [CrossRef] [Green Version]
- ASTM International. D638-02 Standard Test Method for Tensile Properties of Plastics; ASTM International: West Conshohocken, PA, USA, 2002. [Google Scholar]
- ASTM International. D1238-13 Standard Test Method for Melt Flow Rates of Thermoplastics by Extrusion Plastometer; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Reiter, G.; Strobl, G.R. Progress in Understanding of Polymer Crystallization, 2007 ed.; Springer: New York, NY, USA, 2007. [Google Scholar]
- Pedrazzoli, D.; Khumalo, V.M.; Karger-Kocsis, J.; Pegoretti, A. Thermal, viscoelastic and mechanical behavior of polypropylene with synthetic boehmite alumina nanoparticles. Polym. Test. 2014, 35, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Farzad, R.H.; Hassan, A.; Jawaid, M.; Piah, M.A.M. Mechanical properties of alumina trihydrate filled polypropylene/ethylene propylene diene monomer composites for cable applications. Sains Malays. 2013, 42, 801–810. [Google Scholar]
- Hamzah, M.S.; Mariatti, M.; Kamarol, M. Tensile properties and melt flow index of polypropylene/ethylene propylene diene monomer nanocomposites. Adv. Mater. Res. 2015, 1107, 125–130. [Google Scholar] [CrossRef]
- Wypych, G. Handbook of Fillers, 4th ed.; ChemTec Publishing: Toronto, ON, Canada, 2000. [Google Scholar]
- Mohammadnezhad, G.; Dinari, M.; Soltani, R. The preparation of modified boehmite/PMMA nanocomposites by in situ polymerization and the assessment of their capability for Cu2+ ion removal. New J. Chem. 2016, 40, 3612–3621. [Google Scholar] [CrossRef]
- Streller, R.C.; Thomann, R.; Mülhaupt, T.R. Isotactic poly(propylene) nanocomposites based upon boehmite nanofillers. Macromol. Mater. Eng. 2008, 293, 218–227. [Google Scholar] [CrossRef]
- Vuorinen, E.; Nhlapo, N.; Mafa, T.; Karger-Kocsis, J. Thermooxidative degradation of LDPE nanocomposites: Effect of surface treatments of fumed silica and boehmite alumina. Polym. Degrad. Stab. 2013, 98, 2297–2305. [Google Scholar] [CrossRef] [Green Version]
- Gulmine, J.V.; Janissek, P.R.; Heise, H.M.; Akcelrud, L. Polyethylene characterization by FTIR. Polym. Test. 2002, 21, 557–563. [Google Scholar] [CrossRef]
- Sengwa, R.J.; Choudhary, S. Dielectric and electrical properties of PEO—Al2O3 nanocomposites. J. Alloys. Compd. 2017, 701, 652–659. [Google Scholar] [CrossRef]
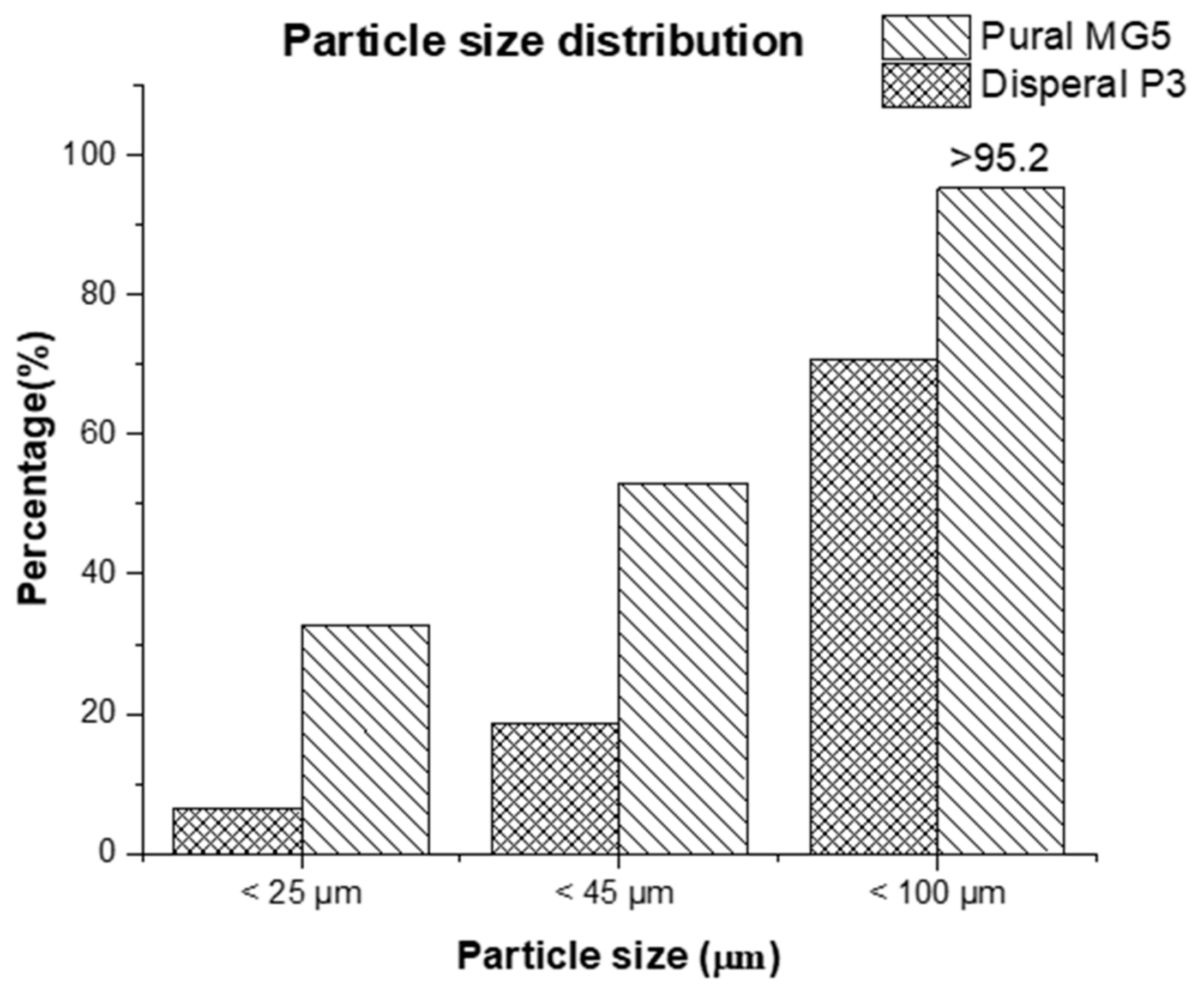
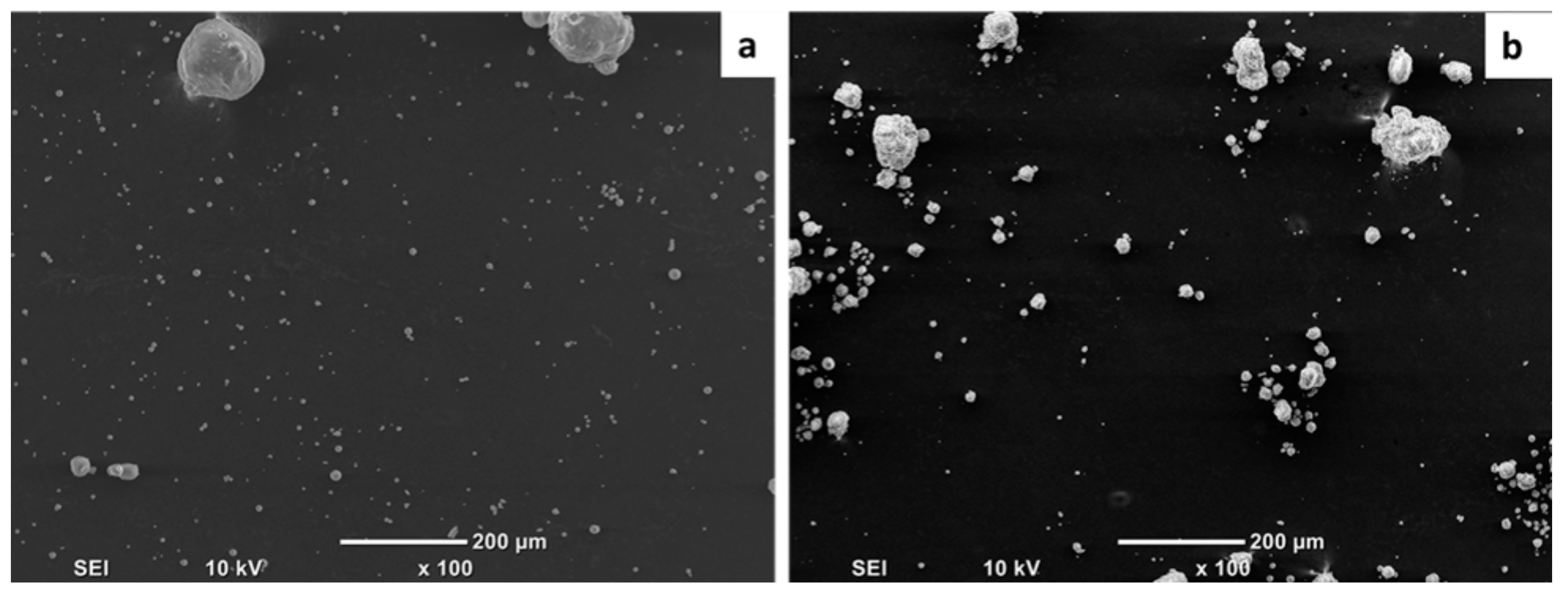
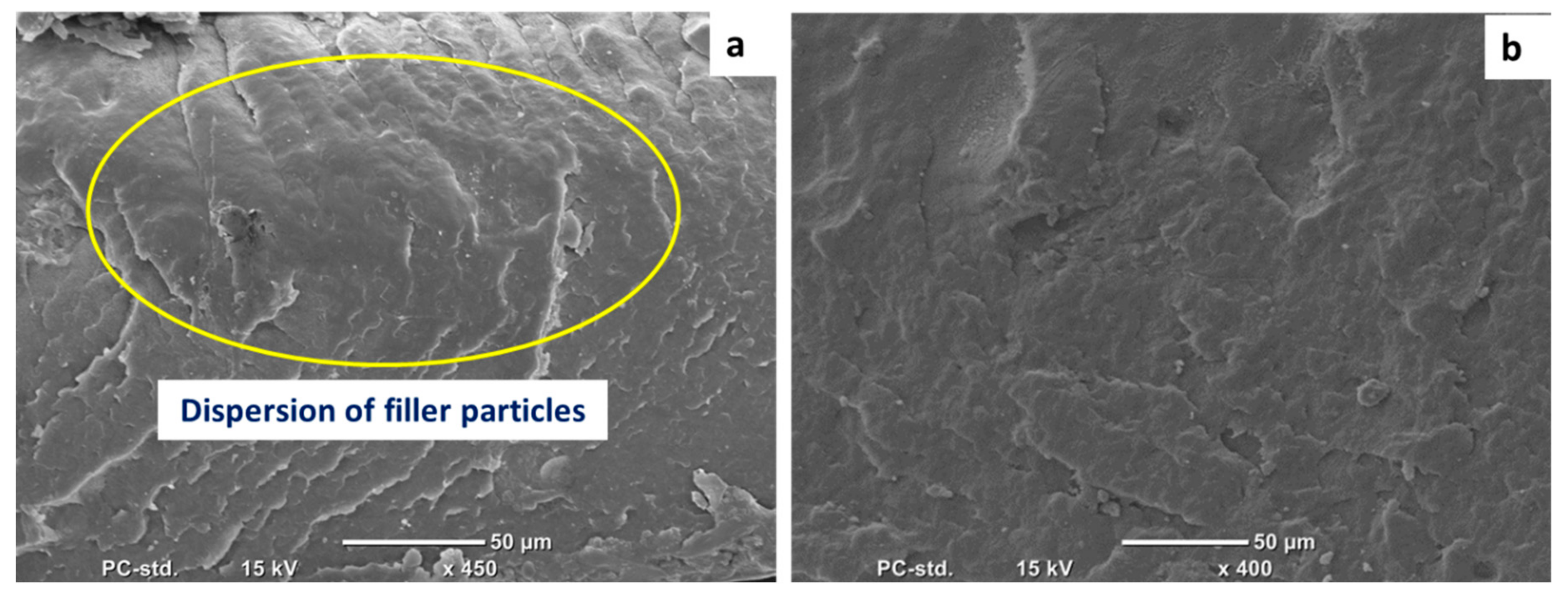
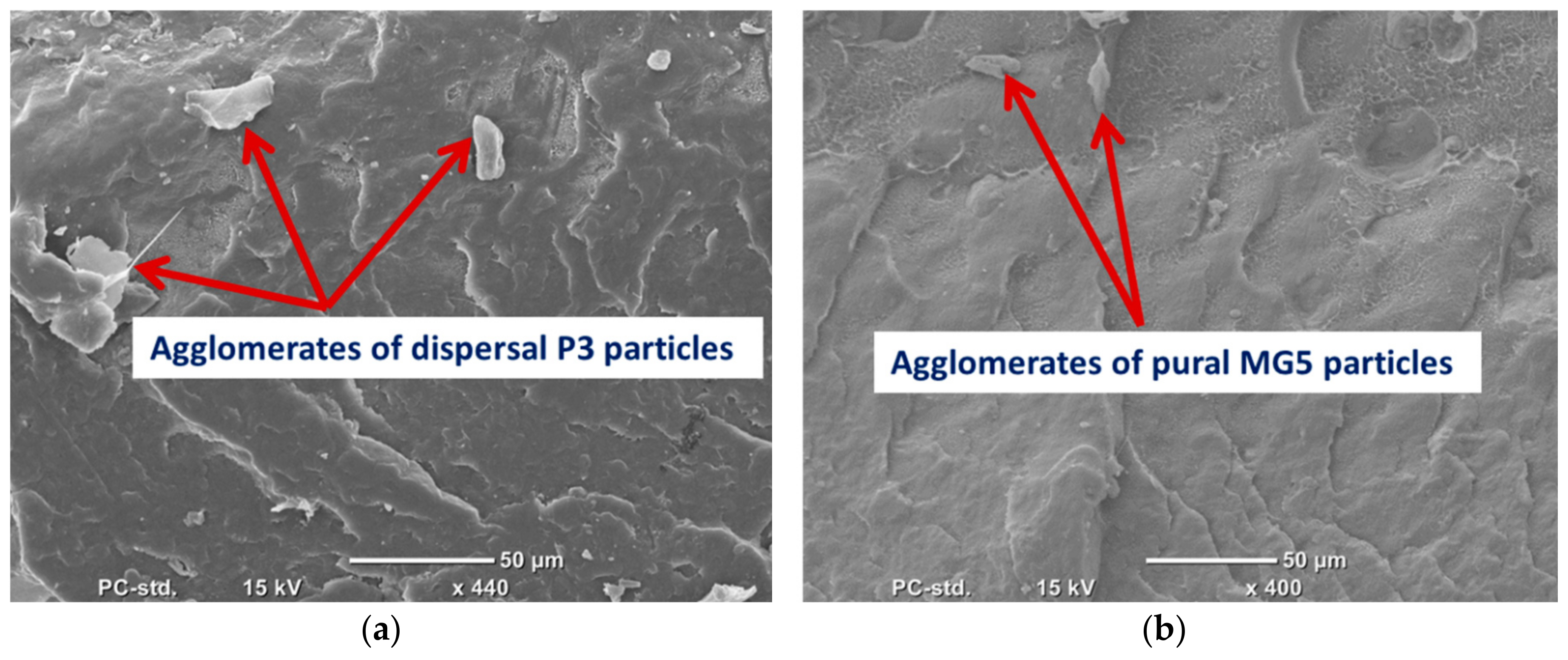




| Physical Properties | Standard | Value |
|---|---|---|
| Density (g/cm3) | ASTM D1505 | 0.951 |
| Melt Index [190 °C; 2.16 kg] (g/10 min) | ASTM D1238 | 0.35 |
| Tensile yield strength [50 mm/min] (MPa) | ASTM D638 | 26 |
| Elongation at break [50 mm/min] (%) | ASTM D638 | >600 |
| Brittleness temperature (°C) | ASTM D746 | ≤75 |
| Analysis | Disperal P3 | Pural MG5 |
|---|---|---|
| Surface area [550 °C/3 h] (m2/g) | 317 | 355 |
| Loose bulk density (g/mL) | 0.92 | 0.52 |
| Sample | Crystallization Cycle | 2nd Heating Cycle | ||||
|---|---|---|---|---|---|---|
| TC, max (°C) | TC, initial (°C) | Tm, max (°C) | Tm, final (°C) | |||
| Pure HDPE | 116.3 | 119.6 | 62.8 | 130.8 | 133.7 | 61.2 |
| HDPE-1%P3 | 117 | 120.3 | 57 | 132 | 135.5 | 58.3 |
| HDPE-3%P3 | 116.2 | 119.5 | 61.8 | 130.5 | 134.7 | 61.2 |
| HDPE-5%P3 | 116.3 | 119.5 | 60.2 | 131 | 135 | 59 |
| HDPE-7%P3 | 116.3 | 119.5 | 58.3 | 131.2 | 134.6 | 57.9 |
| HDPE-10%P3 | 116.5 | 119.4 | 57.9 | 130.7 | 133.3 | 57 |
| HDPE-1%MG5 | 116.1 | 119.1 | 63.1 | 130.7 | 135.2 | 61.5 |
| HDPE-3%MG5 | 116.3 | 119.2 | 64.4 | 130 | 133.3 | 59.1 |
| HDPE-5%MG5 | 116.2 | 119.6 | 60.7 | 131.3 | 134.7 | 57.7 |
| HDPE-7%MG5 | 116.4 | 119.8 | 60.3 | 131.7 | 136 | 56.1 |
| HDPE-10%MG5 | 116.2 | 119.6 | 58.5 | 131.5 | 134.6 | 55.5 |
| Material | Temperature at Which—95 wt. % Loss (°C) | Residue at 592 °C (%) |
|---|---|---|
| HDPE | 498 | 0.01 |
| HDPE-1%P3 | 500 | 1.10 |
| HDPE-3%P3 | 497 | 1.35 |
| HDPE-5%P3 | 499 | 3.18 |
| HDPE-7%P3 | 504 | 4.38 |
| HDPE-10%P3 | ˃592 | 7.44 |
| HDPE-1%MG5 | 498 | 0.95 |
| HDPE-3%MG5 | 499 | 1.90 |
| HDPE-5%MG5 | 499 | 2.99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleh, M.; Al-Hajri, Z.; Popelka, A.; Javaid Zaidi, S. Preparation and Characterization of Alumina HDPE Composites. Materials 2020, 13, 250. https://doi.org/10.3390/ma13010250
Saleh M, Al-Hajri Z, Popelka A, Javaid Zaidi S. Preparation and Characterization of Alumina HDPE Composites. Materials. 2020; 13(1):250. https://doi.org/10.3390/ma13010250
Chicago/Turabian StyleSaleh, Mohamed, Zainab Al-Hajri, Anton Popelka, and Syed Javaid Zaidi. 2020. "Preparation and Characterization of Alumina HDPE Composites" Materials 13, no. 1: 250. https://doi.org/10.3390/ma13010250




