New Proposal for Size and Size-Distribution Evaluation of Nanoparticles Synthesized via Ultrasonic Spray Pyrolysis Using Search Algorithm Based on Image-Processing Technique
Abstract
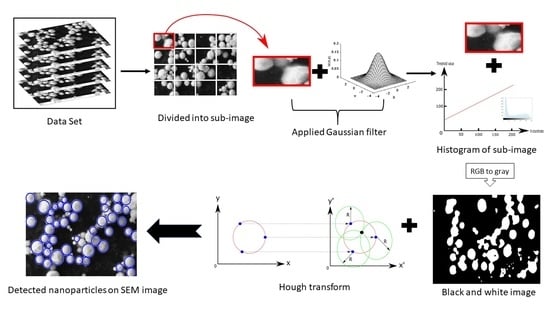
:1. Introduction
2. Materials and Methods
| Algorithm 1 Searching particle Algorithm 1. | |
| 1: | Definition of min and max value for subimages |
| 2: | Image reading |
| 3: | for each defined value in size of image do |
| 4: | Obtain grey code of subimage |
| 5: | Compute histogram of subimage |
| 6: | Define threshold value |
| 7: | Obtain black and white of subimage |
| 8: | Search the particle |
| 9: | while (detecting particle = 1) do |
| 10: | Measure particle size |
| 11: | Find location |
| 12: | Label particle |
| 13: | end while |
| 14: | end for |
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kruis, F.E.; Fissan, H.; Peled, A. Synthesis of nanoparticles in the gas phase for electronic, optical and magnetic application. J. Aerosol Sci. 1998, 29, 511–535. [Google Scholar] [CrossRef]
- Mohammed, L.; Gomaa, H.G.; Ragab, D.; Zhu, J. Magnetic nanoparticles for environmental and biomedical applications: A review. Particuology 2017, 30, 1–14. [Google Scholar] [CrossRef]
- De Crozals, G.; Bonnet, R.; Farre, C.; Chaix, C. Nanoparticles with multiple properties for biomedical applications: A strategic guide. Nano Today 2016, 11, 435–463. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2017, 12, 908–931. [Google Scholar] [CrossRef]
- Campelo, J.M.; Luna, D.; Luque, R.; Marinas, J.M.; Romero, A.A. Sustainable preparation of supported metal nanoparticles and their applications in catalysis. ChemSusChem 2009, 2, 18–45. [Google Scholar] [CrossRef]
- Ma, P.C.; Tang, B.Z.; Kim, J.K. Effect of CNT decoration with silver nanoparticles on electrical conductivity of CNT-polymer composites. Carbon 2008, 46, 1497–1505. [Google Scholar] [CrossRef]
- Sharma, P.; Baek, I.H.; Cho, T.; Park, S.; Lee, K.B. Enhancement of thermal conductivity of ethylene glycol based silver nanofluids. Powder Technol. 2011, 208, 7–19. [Google Scholar] [CrossRef]
- Deshmukh, S.P.; Patil, S.M.; Mullani, S.B.; Delekar, S.D. Silver nanoparticles as an effective disinfectant: A review. Mater. Sci. Eng. C 2019, 97, 954–965. [Google Scholar] [CrossRef]
- Beyene, H.D.; Werkneh, A.A.; Bezabh, H.K.; Ambaye, T.G. Synthesis paradigm and applications of silver nanoparticles (AgNPs), a review. Sustain. Mater. Technol. 2017, 13, 18–23. [Google Scholar] [CrossRef]
- Simakin, A.V.; Voronov, V.V.; Shafeev, G.A.; Brayner, R.; Bozon-Verduraz, F. Nanodisks of Au and Ag produced by laser ablation in liquid environment. Chem. Phys. Lett. 2001, 348, 182–186. [Google Scholar] [CrossRef]
- Chen, Y.H.; Yeh, C.S. Laser ablation method: Use of surfactants to form the dispersed Ag nanoparticles. Colloids Surf. A 2002, 197, 133–139. [Google Scholar] [CrossRef]
- Sharma, P.; Lotey, G.S.; Singh, S. Solution-combustion: The versatile route to synthesize silver nanoparticles. J. Nanoparticle Res. 2011, 13, 2553–2561. [Google Scholar] [CrossRef]
- Yin, B.; Ma, H.; Wang, S.; Chen, S. Electrochemical synthesis of silver nanoparticles under protection of poly(N-vinylpyrrolidone). J. Phys. Chem. B 2003, 107, 8898–8904. [Google Scholar] [CrossRef]
- Ebin, B.; Yazici, E.; Gürmen, S. Production of nanocrystalline silver particles by hydrogen reduction of silver nitrate aerosol droplets. Transact. Nonferrous Met. Soc. China 2013, 23, 841–848. [Google Scholar] [CrossRef]
- Alkan, G.; Mancic, L.; Tamura, S.; Tomita, K.; Tan, Z.; Sun, F.; Rudolf, R.; Ohara, S.; Friedrich, B.; Milosevic, O. Plasmon enhanced luminescence in hierarchically structured Ag@ (Y0.95Eu0.05)2O3 nanocomposites synthesized by ultrasonic spray pyrolysis. Adv. Powder Technol. 2019, 30, 1409–1418. [Google Scholar] [CrossRef]
- Emil, E.; Gürmen, S. Estimation of yttrium oxide microstructural parameters using the Williamson–Hall analysis. Mater. Sci. Technol. 2018, 34, 1549–1557. [Google Scholar] [CrossRef]
- Alkan, G.; Rudolf, R.; Bogovic, J.; Jenko, D.; Friedrich, B. Structure and formation model of Ag/TiO2 and Au/TiO2 nanoparticles synthesized through ultrasonic spray pyrolysis. Metals 2017, 7, 389. [Google Scholar] [CrossRef] [Green Version]
- Emil, E.; Alkan, G.; Gurmen, S.; Rudolf, R.; Jenko, D.; Friedrich, B. Tuning the morphology of ZnO nanostructures with the ultrasonic spray pyrolysis process. Metals 2018, 8, 569. [Google Scholar] [CrossRef] [Green Version]
- Ebin, B.; Lindbergh, G.; Gürmen, S. Preparation and electrochemical properties of nanocrystalline LiBxMn2-xO4 cathode particles for Li-ion batteries by ultrasonic spray pyrolysis method. J. Alloy. Compd. 2015, 620, 399–406. [Google Scholar] [CrossRef]
- Toparli, C.; Ebin, B.; Gürmen, S. Synthesis, structural and magnetic characterization of soft magnetic nanocrystalline ternary FeNiCo particles. J. Magn. Magn. Mater. 2017, 423, 133–139. [Google Scholar] [CrossRef]
- Nedeljković, J.M.; Šaponjić, Z.V.; Rakočević, Z.; Jokanović, V.; Uskoković, D.P. Ultrasonic spray pyrolysis of TiO2 nanoparticles. Nanostructured Mater. 1997, 9, 125–128. [Google Scholar] [CrossRef]
- Gürmen, S.; Stopić, S.; Friedrich, B. Synthesis of nanosized spherical cobalt powder by ultrasonic spray pyrolysis. Mater. Res. Bull. 2006, 41, 1882–1890. [Google Scholar] [CrossRef]
- Fisker, R.; Carstensen, J.M.; Hansen, M.F.; Bødker, F.; Mørup, S. Estimation of nanoparticle size distributions by image analysis. J. Nanoparticle Res. 2000, 2, 267–277. [Google Scholar] [CrossRef]
- Woehrle, G.H.; Hutchison, J.E.; Özkar, S.; Finke, R.G. Analysis of nanoparticle transmission electron microscopy data using a public- domain image-processing program, Image. Turk. J. Chem. 2006, 30, 1–13. [Google Scholar]
- Ziel, R.; Haus, A.; Tulke, A. Quantification of the pore size distribution (porosity profiles) in microfiltration membranes by SEM, TEM and computer image analysis. J. Membr. Sci. 2008, 323, 241–246. [Google Scholar] [CrossRef]
- Rajeshwari, A.; Prathna, T.C.; Balajee, J.; Chandrasekaran, N.; Mandal, A.B.; Mukherjee, A. Computational approach for particle size measurement of silver nanoparticle from electron microscopic image. Int. J. Pharm. Pharm. Sci. 2013, 5, 619–623. [Google Scholar]
- Benitez, D.S.; Debut, A.; Cumbal, L. A texture based image processing algorithm for nanoparticles analysis. In Proceedings of the 2015 IEEE International Autumn Meeting on Power, Electronics and Computing, Ixtapa, Mexico, 4–6 November 2015. [Google Scholar]
- Stopic, S. Synthesis of Metallic Nanosized Particles by Ultrasonic Spray Pyrolysis; Shaker Verlag: Herzogenrath, Germany, 2015; p. 120. [Google Scholar]
- Stopic, S.; Friedrich, B.; Dvorak, P. Synthesis of nanosized spherical silver powder by ultrasonic spray pyrolysis. Metall 2006, 60, 377–382. [Google Scholar]
- Smith, S. Digital Signal Processing: A Practical Guide for Engineers and Scientists; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Gonzalez, R.C.; Richard, E. Woods. In Digital Image Processing; Addison-Wesley Longman Publishing Co., Inc.: Boston, MA, USA, 2018. [Google Scholar]
- Gonzalez, R.C.; Richard, E. Woods. In Digital Image Processing, 2nd ed.; Addison-Wesley Longman Publishing Co., Inc.: Boston, MA, USA, 2001. [Google Scholar]
- Parsania, P.S.; Virpari, P.V. A review: Image interpolation techniques for image scaling. Int. J. Innov. Res. Comput. Commun. Eng. 2014, 2, 7409–7413. [Google Scholar] [CrossRef]
- Boyat, A.K.; Joshi, B.K. A review paper: Noise models in digital image processing. arXiv 2015, arXiv:1505.03489. [Google Scholar] [CrossRef]
- Takeda, H.; Farsiu, S.; Milanfar, P. Kernel Regression for Image Processing and Reconstruction. IEEE Trans. Image Process. 2007, 16, 349–366. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Oja, E.; Kultanen, P. A new curve detection method: Randomized Hough transform (RHT). Pattern Recognit. Lett. 1990, 11, 331–338. [Google Scholar] [CrossRef]






| Sample Codes | Theoretical Mean Particle Diameter | DLS Analysis | Mean Particle Diameter Calculated by Proposed Method |
|---|---|---|---|
| S1 | 410 nm | 258 nm | 392 nm |
| S2 | 520 nm | 526 nm | 503 nm |
| S3 | 650 nm | 653 nm | 622 nm |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emil Kaya, E.; Kaya, O.; Alkan, G.; Gürmen, S.; Stopic, S.; Friedrich, B. New Proposal for Size and Size-Distribution Evaluation of Nanoparticles Synthesized via Ultrasonic Spray Pyrolysis Using Search Algorithm Based on Image-Processing Technique. Materials 2020, 13, 38. https://doi.org/10.3390/ma13010038
Emil Kaya E, Kaya O, Alkan G, Gürmen S, Stopic S, Friedrich B. New Proposal for Size and Size-Distribution Evaluation of Nanoparticles Synthesized via Ultrasonic Spray Pyrolysis Using Search Algorithm Based on Image-Processing Technique. Materials. 2020; 13(1):38. https://doi.org/10.3390/ma13010038
Chicago/Turabian StyleEmil Kaya, Elif, Ozan Kaya, Gözde Alkan, Sebahattin Gürmen, Srecko Stopic, and Bernd Friedrich. 2020. "New Proposal for Size and Size-Distribution Evaluation of Nanoparticles Synthesized via Ultrasonic Spray Pyrolysis Using Search Algorithm Based on Image-Processing Technique" Materials 13, no. 1: 38. https://doi.org/10.3390/ma13010038