Electrochemical Non-Enzymatic Detection of Glucose Based on 3D Electroformed Copper on Ni Foam Nanostructures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Preparation of the 3D Cu Nanoporous Electrodes
2.3. Electrochemical Assessment of 3D Cu Nanoporous Electrodes
3. Results and Discussions
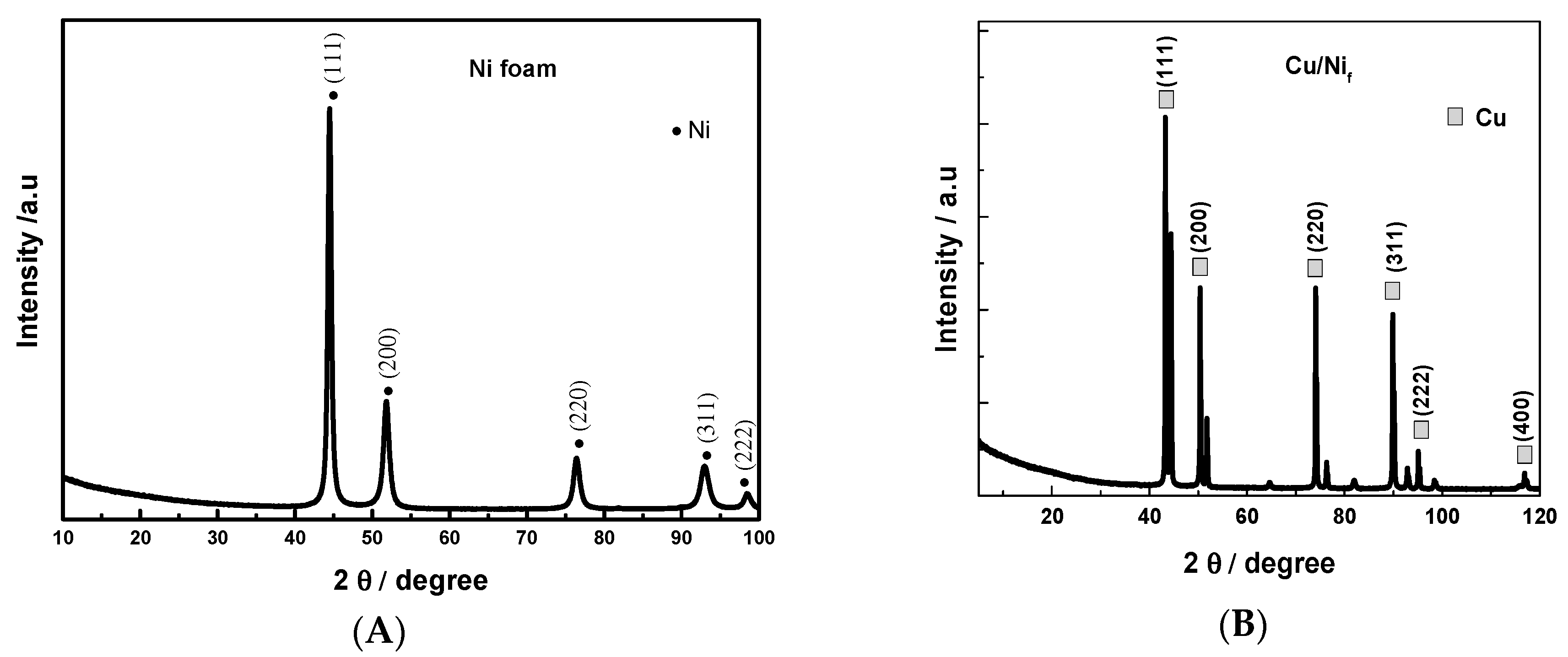
3.1. Preparation and Characterization of 3D Cu Nanoporous Electrodes
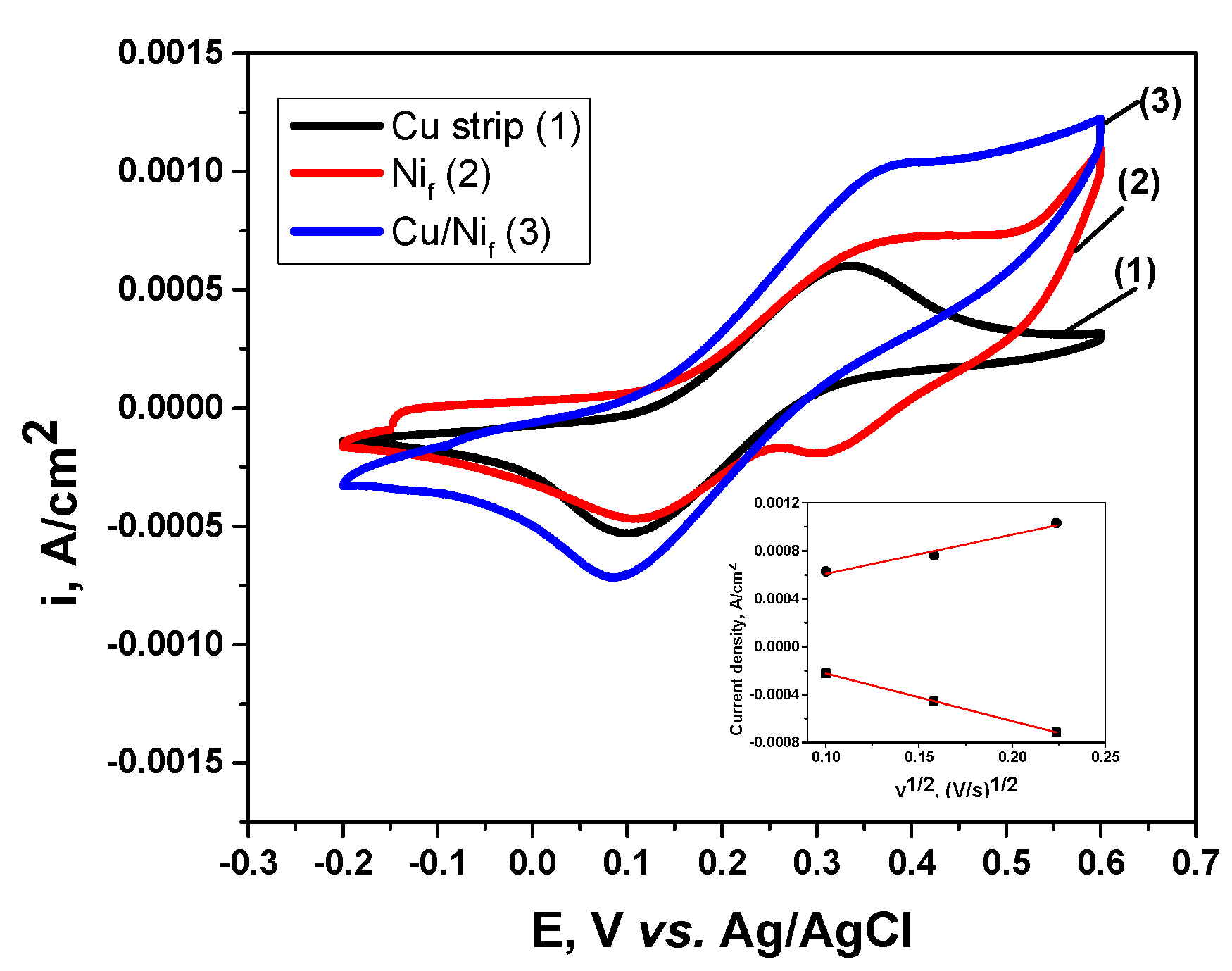
3.2. Electrochemical Characterization of 3D Cu Nanoporous Electrodes
- (i)
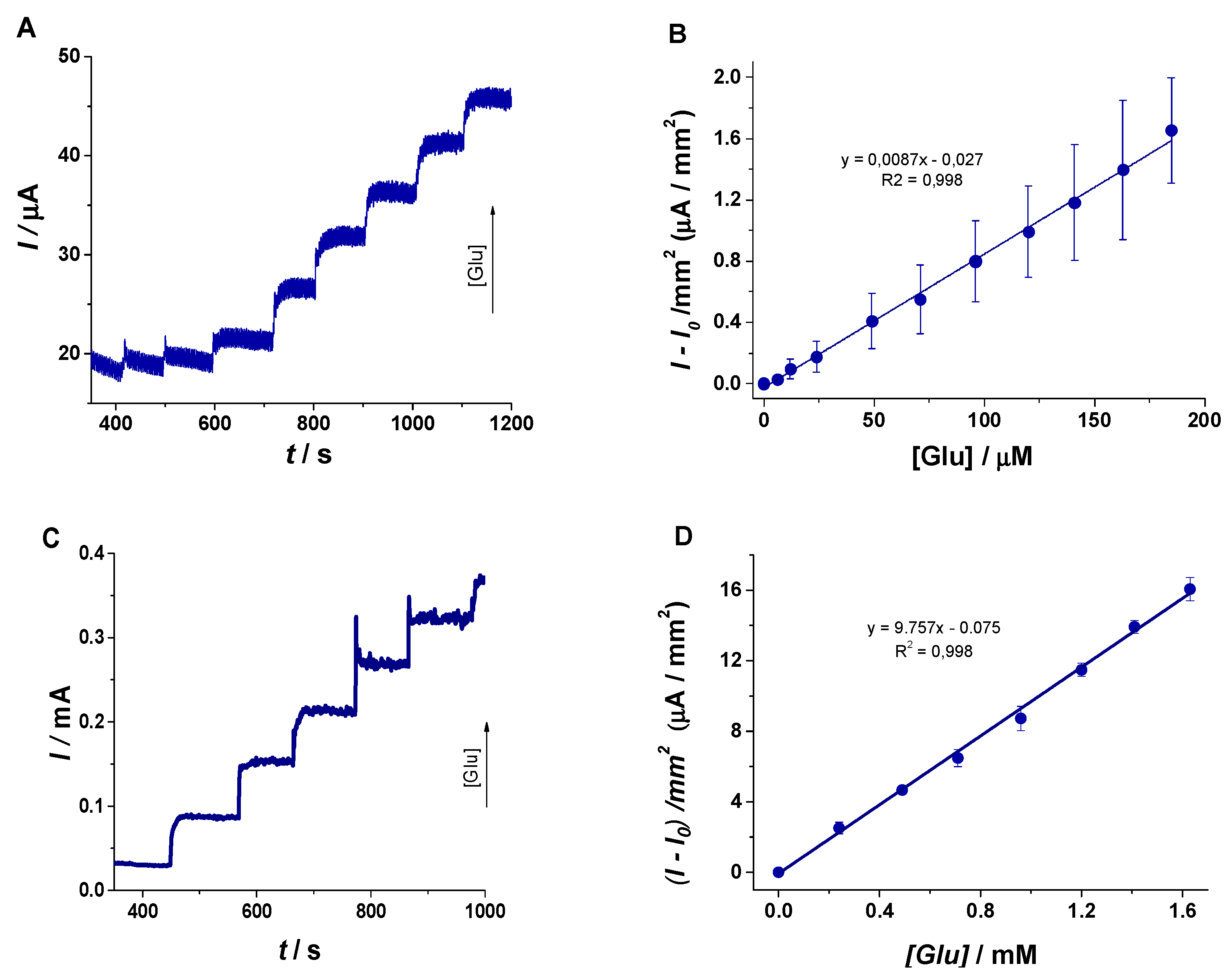
- The first one in the range of 6 – 206 µM defined by the equation y(µA/mm2) = 0.0087 × [Glu(µM)] ‒ 0.027 (R2 = 0.998) has the role to demonstrate the analytical performances of the sensor, with a limit of detection (LOD) calculated as signal/noise = 3 of 2 µM and a limit of quantification (LOQ) of 6 µM.
- (ii)
- The second one in the range of 0.24–1.63 mM defined by y(µA/mm2) = 9.757 × [Glu(mM)] ‒ 0.075 (R2 = 0.996) underlines the possibility to employ the sensor in real scenarios. The normal level of blood Glu is found to be in the 3–5.5 mM range, and in this case, the sensor could be successfully used to assess even hyperglycemic pathological levels after the dilution of the sample with an electrolyte solution (1:10 or even 1:100). Thus, the calculated concentrations will be found in the linear range and, taking into account the dilutions step, the real value of blood Glu could be determined. Further studies will be performed in order to extend the analytical performances.
3.3. Interferences Studies
3.4. Studies on Real Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization Global Report on Diabetes. Available online: https://www.who.int/diabetes/global-report/en/ (accessed on 4 May 2020).
- Palve, Y.P.; Jha, N. A novel bilayer of copper nanowire and carbon nanotube electrode for highly sensitive enzyme free glucose detection. Mater. Chem. Phys. 2020, 240, 122086. [Google Scholar] [CrossRef]
- Steiner, M.S.; Duerkop, A.; Wolfbeis, O.S. Optical methods for sensing glucose. Chem. Soc. Rev. 2011, 40, 4805–4839. [Google Scholar] [CrossRef] [PubMed]
- Morris, N.A.; Cardosi, M.F.; Birch, B.J.; Turner, A.P.F. An electrochemical capillary fill device for the analysis of glucose incorporating glucose oxidase and ruthenium (III) hexamine as mediator. Electroanalysis 1992, 4, 1–9. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, E.; Yang, X. Capacitive detection of glucose using molecularly imprinted polymers. Biosens. Bioelectron. 2001, 16, 179–185. [Google Scholar] [CrossRef]
- Yoo, E.-H.; Lee, S.-Y. Glucose biosensors: An overview of use in clinical practice. Sensors 2010, 10, 4558–4576. [Google Scholar] [CrossRef] [Green Version]
- Länge, K.; Rapp, B.E.; Rapp, M. Surface acoustic wave biosensors: A review. Anal. Bioanal. Chem. 2008, 391, 1509–1519. [Google Scholar] [CrossRef]
- Miller, P.R.; Skoog, S.A.; Edwards, T.L.; Wheeler, D.R.; Xiao, X.; Brozik, S.M.; Polsky, R.; Narayan, R.J. Hollow Microneedle-based sensor for multiplexed transdermal electrochemical sensing. J. Vis. Exp. 2012, e4067. [Google Scholar] [CrossRef] [Green Version]
- Hwang, D.W.; Lee, S.; Seo, M.; Chung, T.D. Recent advances in electrochemical non-enzymatic glucose sensors—A review. Anal. Chim. Acta 2018, 1033, 1–34. [Google Scholar] [CrossRef]
- Cernat, A.; Le Goff, A.; Holzinger, M.; Sandulescu, R.; Cosnier, S. Micro- to nanostructured poly(pyrrole-nitrilotriacetic acid) films via nanosphere templates: Applications to 3D enzyme attachment by affinity interactions. Anal. Bioanal. Chem. 2014, 406. [Google Scholar] [CrossRef]
- Saei, A.A.; Dolatabadi, J.E.N.; Najafi-Marandi, P.; Abhari, A.; de la Guardia, M. Electrochemical biosensors for glucose based on metal nanoparticles. Trends Anal. Chem. 2013, 42, 216–227. [Google Scholar] [CrossRef]
- Xu, G.R.; Ge, C.; Liu, D.; Jin, L.; Li, Y.C.; Zhang, T.H.; Rahman, M.M.; Li, X.B.; Kim, W. In-situ electrochemical deposition of dendritic Cu-Cu2S nanocomposites onto glassy carbon electrode for sensitive and non-enzymatic detection of glucose. J. Electroanal. Chem. 2019, 847, 113177. [Google Scholar] [CrossRef]
- Chakraborty, P.; Dhar, S.; Debnath, K.; Majumder, T.; Mondal, S.P. Non-enzymatic and non-invasive glucose detection using Au nanoparticle decorated CuO nanorods. Sens. Actuators B Chem. 2019, 283, 776–785. [Google Scholar] [CrossRef]
- Ayranci, R.; Demirkan, B.; Sen, B.; Şavk, A.; Ak, M.; Şen, F. Use of the monodisperse Pt/Ni@rGO nanocomposite synthesized by ultrasonic hydroxide assisted reduction method in electrochemical nonenzymatic glucose detection. Mater. Sci. Eng. C 2019, 99, 951–956. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Su, L.; Manuzzi, D.; de los Monteros, H.V.E.; Jia, W.; Huo, D.; Hou, C.; Lei, Y. Ultrasensitive and selective non-enzymatic glucose detection using copper nanowires. Biosens. Bioelectron. 2012, 31, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Mihai, G.; Roşoiu, S.; Costovici, Ş.; Anicăi, L.; Enăchescu, M. Synthesis of copper nanowires using aqueous and ionic liquid electrolytes for electrochemical detection. U.P.B Sci. Bull. Ser. B 2019, 81, 1–16. [Google Scholar]
- Wang, Q.; Subramanian, P.; Li, M.; Yeap, W.S.; Haenen, K.; Coffinier, Y.; Boukherroub, R.; Szunerits, S. Non-enzymatic glucose sensing on long and short diamond nanowire electrodes. Electrochem. Commun. 2013, 34, 286–290. [Google Scholar] [CrossRef]
- Gupta, J.; Arya, S.; Verma, S.; Singh, A.; Sharma, A.; Singh, B.; Prerna; Sharma, R. Performance of template-assisted electrodeposited Copper/Cobalt bilayered nanowires as an efficient glucose and Uric acid senor. Mater. Chem. Phys. 2019, 238, 121969. [Google Scholar] [CrossRef]
- Çiftçi, H.; Tamer, U. Functional gold nanorod particles on conducting polymer poly(3-octylthiophene) as non-enzymatic glucose sensor. React. Funct. Polym. 2012, 72, 127–132. [Google Scholar] [CrossRef]
- Kim, K.; Kim, S.; Lee, H.N.; Park, Y.M.; Bae, Y.S.; Kim, H.J. Electrochemically derived CuO nanorod from copper-based metal-organic framework for non-enzymatic detection of glucose. Appl. Surf. Sci. 2019, 479, 720–726. [Google Scholar] [CrossRef]
- Weremfo, A.; Fong, S.T.C.; Khan, A.; Hibbert, D.B.; Zhao, C. Electrochemically roughened nanoporous platinum electrodes for non-enzymatic glucose sensors. Electrochim. Acta 2017, 231, 20–26. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Park, S.; Boo, H.; Kim, H.C.; Chung, T.D. Disposable non-enzymatic blood glucose sensing strip based on nanoporous platinum particles. Appl. Mater. Today 2018, 10, 24–29. [Google Scholar] [CrossRef]
- Guo, M.Q.; Hong, H.S.; Tang, X.N.; Fang, H.D.; Xu, X.H. Ultrasonic electrodeposition of platinum nanoflowers and their application in nonenzymatic glucose sensors. Electrochim. Acta 2012, 63, 1–8. [Google Scholar] [CrossRef]
- Su, C.; Zhang, C.; Lu, G.; Ma, C. Nonenzymatic electrochemical glucose sensor based on pt nanoparticles/mesoporous carbon matrix. Electroanalysis 2010, 22, 1901–1905. [Google Scholar] [CrossRef]
- Zhou, Y.G.; Yang, S.; Qian, Q.Y.; Xia, X.H. Gold nanoparticles integrated in a nanotube array for electrochemical detection of glucose. Electrochem. Commun. 2009, 11, 216–219. [Google Scholar] [CrossRef]
- Cherevko, S.; Chung, C.H. Gold nanowire array electrode for non-enzymatic voltammetric and amperometric glucose detection. Sens. Actuators B Chem. 2009, 142, 216–223. [Google Scholar] [CrossRef]
- Chang, G.; Shu, H.; Ji, K.; Oyama, M.; Liu, X.; He, Y. Gold nanoparticles directly modified glassy carbon electrode for non-enzymatic detection of glucose. Appl. Surf. Sci. 2014, 288, 524–529. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, Y.; Zhang, Y.; Lei, Y. Pt-Au nanocorals, Pt nanofibers and Au microparticles prepared by electrospinning and calcination for nonenzymatic glucose sensing in neutral and alkaline environment. Sens. Actuators B Chem. 2012, 171, 954–961. [Google Scholar] [CrossRef]
- Niu, X.; Lan, M.; Chen, C.; Zhao, H. Nonenzymatic electrochemical glucose sensor based on novel Pt-Pd nanoflakes. Talanta 2012, 99, 1062–1067. [Google Scholar] [CrossRef]
- Xu, C.; Liu, Y.; Su, F.; Liu, A.; Qiu, H. Nanoporous PtAg and PtCu alloys with hollow ligaments for enhanced electrocatalysis and glucose biosensing. Biosens. Bioelectron. 2011, 27, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Li, X.; Pan, J.; He, Y.; Qiu, F.; Yan, Y. Recent advances in non-enzymatic electrochemical glucose sensors based on non-precious transition metal materials: Opportunities and challenges. RSC Adv. 2016, 6, 84893–84905. [Google Scholar] [CrossRef]
- Kurt Urhan, B.; Demir, Ü.; Öznülüer Özer, T.; Öztürk Doğan, H. Electrochemical fabrication of Ni nanoparticles-decorated electrochemically reduced graphene oxide composite electrode for non-enzymatic glucose detection. Thin Solid Films 2020, 693. [Google Scholar] [CrossRef]
- Sim, H.; Kim, J.H.; Lee, S.K.; Song, M.J.; Yoon, D.H.; Lim, D.S.; Hong, S.I. High-sensitivity non-enzymatic glucose biosensor based on Cu(OH) 2 nanoflower electrode covered with boron-doped nanocrystalline diamond layer. Thin Solid Films 2012, 520, 7219–7223. [Google Scholar] [CrossRef]
- Zhou, S.; Feng, X.; Shi, H.; Chen, J.; Zhang, F.; Song, W. Direct growth of vertically aligned arrays of Cu(OH)2 nanotubes for the electrochemical sensing of glucose. Sens. Actuators B Chem. 2013, 177, 445–452. [Google Scholar] [CrossRef]
- Zhou, X.; Nie, H.; Yao, Z.; Dong, Y.; Yang, Z.; Huang, S. Facile synthesis of nanospindle-like Cu 2O/straight multi-walled carbon nanotube hybrid nanostructures and their application in enzyme-free glucose sensing. Sens. Actuators B Chem. 2012, 168, 1–7. [Google Scholar] [CrossRef]
- Cherevko, S.; Chung, C.H. The porous CuO electrode fabricated by hydrogen bubble evolution and its application to highly sensitive non-enzymatic glucose detection. Talanta 2010, 80, 1371–1377. [Google Scholar] [CrossRef]
- Lin, K.C.; Lin, Y.C.; Chen, S.M. A highly sensitive nonenzymatic glucose sensor based on multi-walled carbon nanotubes decorated with nickel and copper nanoparticles. Electrochim. Acta 2013, 96, 164–172. [Google Scholar] [CrossRef]
- Yi, W.; Liu, J.; Chen, H.; Gao, Y.; Li, H. Copper/nickel nanoparticle decorated carbon nanotubes for nonenzymatic glucose biosensor. J. Solid State Electrochem. 2015, 19, 1511–1521. [Google Scholar] [CrossRef]
- Guo, M.M.; Wang, P.S.; Zhou, C.H.; Xia, Y.; Huang, W.; Li, Z. An ultrasensitive non-enzymatic amperometric glucose sensor based on a Cu-coated nanoporous gold film involving co-mediating. Sens. Actuators B Chem. 2014, 203, 388–395. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.C.; Chung, T.D. Electrochemical analysis based on nanoporous structures. Analyst 2012, 137, 3891–3903. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.F. Facile preparation of an ultrathin nickel film coated nanoporous gold electrode with the unique catalytic activity to oxidation of glucose. Chem. Commun. 2009, 1270–1272. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wu, X.; Li, X. Gold nanorods on three-dimensional nickel foam: A non-enzymatic glucose sensor with enhanced electro-catalytic performance. RSC Adv. 2017, 7, 36744–36749. [Google Scholar] [CrossRef] [Green Version]
- Eugénio, S.; Silva, T.M.; Carmezim, M.J.; Duarte, R.G.; Montemor, M.F. Electrodeposition and characterization of nickel-copper metallic foams for application as electrodes for supercapacitors. J. Appl. Electrochem. 2013, 44, 455–465. [Google Scholar] [CrossRef]
- Golgovici, F.; Pumnea, A.; Petica, A.; Manea, A.C.; Brincoveanu, O.; Enachescu, M.; Anicai, L. Ni–Mo alloy nanostructures as cathodic materials for hydrogen evolution reaction during seawater electrolysis. Chem. Pap. 2018, 72, 1889–1903. [Google Scholar] [CrossRef]
- Marozzi, C.A.; Chialvo, A.C. Development of electrode morphologies of interest in electrocatalysis. Part 1: Electrodeposited porous nickel electrodes. Electrochim. Acta 2000, 45, 2111–2120. [Google Scholar] [CrossRef]
- Saitou, M. Characterization of electrodeposited Ni and Ni-Mo thin films by X-ray diffraction. Int. J. Electrochem. Sci. 2014, 9, 6033–6042. [Google Scholar]
- Gao, Z.; Chen, L.; Chang, J.; Wang, Z.; Wu, D.; Xu, F.; Jiang, K. Bare Ni foam electrode-ferricyanides redox electrolyte system with high capacitive performance. Int. J. Hydrogen Energy 2019, 44, 10554–10560. [Google Scholar] [CrossRef]
- González-Buch, C.; Herraiz-Cardona, I.; Ortega, E.; García-Antón, J.; Pérez-Herranz, V. Study of the catalytic activity of 3D macroporous Ni and NiMo cathodes for hydrogen production by alkaline water electrolysis. J. Appl. Electrochem. 2016, 46, 791–803. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Wang, Z.; Yu, X.; Guo, Z. Facile one-step electrodeposition preparation of porous NiMo film as electrocatalyst for hydrogen evolution reaction. Int. J. Hydrogen Energy 2015, 40, 2173–2181. [Google Scholar] [CrossRef]
- Marro, J.B.; Okoro, C.A.; Obeng, Y.S.; Richardson, K.C. The impact of organic additives on copper trench microstructure. J. Electrochem. Soc. 2017, 164, D543–D550. [Google Scholar] [CrossRef]
- Scialdone, O.; Guarisco, C.; Grispo, S.; Angelo, A.D.; Galia, A. Investigation of electrode material-Redox couple systems for reverse electrodialysis processes. Part I: Iron redox couples. J. Electroanal. Chem. 2012, 681, 66–75. [Google Scholar] [CrossRef]
- Yang, F.; Cheng, K.; Xue, X.; Yin, J.; Wang, G.; Cao, D. Three-dimensional porous Ni film electrodeposited on Ni foam: High performance and low-cost catalytic electrode for H2O2 electrooxidation in KOH solution. Electrochim. Acta 2013, 107, 194–199. [Google Scholar] [CrossRef]








| Coating System | Electrolyte Composition | Operating Conditions |
|---|---|---|
| Cu/Ni foam (denoted Cu/Nif) | (i) 0.1 M NiCl2∙6H2O + 2M NH4Cl | pH = 4–4.5 T = 25 °C i = 1 A/cm2 time = 3 min. |
| (ii) 0.8 M CuSO4∙5H2O + 0.6 M H2SO4 | T = 25–30 °C i = 28–35 mA/cm2 time = 15–20 min. |
| Working Electrode Type | Rs / ohm cm2 | Rct / ohm cm2 | Cdl /µF | RW / ohm cm2 | The Used Model |
|---|---|---|---|---|---|
| Cu strip | 80.6 | 20 | 21.5 | 521 | (A) |
| Nif | 111.2 | 4473 | 210.1 | - | (B) |
| Cu/Nif | 185.4 | 10 | 291.5 | 348 | (A) |
| Working Electrode Type | CPEdl/µF | Areal/cm2 | σ |
|---|---|---|---|
| Cu strip | 21.5 | 1.07 | 1.69 |
| Nif | 210.1 | 10.5 | 16.67 |
| Cu/Nif | 291.5 | 14.5 | 23.1 |
| No. | Platform | Detection Method | LOD µM | Linearity µM | Interfering Species | Ref. |
|---|---|---|---|---|---|---|
| 1. | NiNPs/ERGO | CA | 0.04 | 0.5–244 | Dopamine, UA, AA | [33] |
| 2. | Cu-NW-CNT-BL/GCE | EIS, CV, CA | 0.33 × 10‒3 | 10–2000 | Sucrose, Fructose, Maltose, AA, Dopamine | [2] |
| 3. | CuO nanorod | CA | 1 | 1–1250 | UA, AA Dopamine | [21] |
| 4. | Cu-Cu2S nanocomposite/GCE | CV, CA | 0.33 | 0.5–500 | Fructose, Sucrose, AA, UA, Dopamine | [12] |
| 5 | CuO nanorods - AuNP | LSV | 0.17 | 5–1325 | AA, UA, Dopamine, Ureea, Sucrose | [13] |
| 6. | Pt/Ni@rGO | CA | 6.3 | 20–5000 | AA, Phenol, Ethanol | [14] |
| 7. | Cu/Ni Foam | CA | 2 | 6–206 240–1630 | UA, AA, R, P | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melinte, G.; Cernat, A.; Petica, A.; Lazar, O.; Enachescu, M.; Anicai, L.; Cristea, C. Electrochemical Non-Enzymatic Detection of Glucose Based on 3D Electroformed Copper on Ni Foam Nanostructures. Materials 2020, 13, 2752. https://doi.org/10.3390/ma13122752
Melinte G, Cernat A, Petica A, Lazar O, Enachescu M, Anicai L, Cristea C. Electrochemical Non-Enzymatic Detection of Glucose Based on 3D Electroformed Copper on Ni Foam Nanostructures. Materials. 2020; 13(12):2752. https://doi.org/10.3390/ma13122752
Chicago/Turabian StyleMelinte, Gheorghe, Andreea Cernat, Aurora Petica, Oana Lazar, Marius Enachescu, Liana Anicai, and Cecilia Cristea. 2020. "Electrochemical Non-Enzymatic Detection of Glucose Based on 3D Electroformed Copper on Ni Foam Nanostructures" Materials 13, no. 12: 2752. https://doi.org/10.3390/ma13122752






