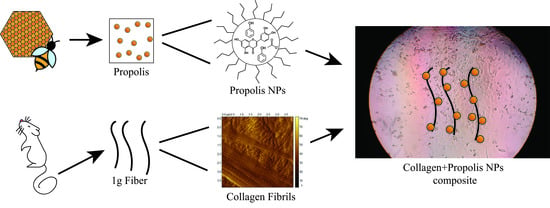
Increased Fibroblast Metabolic Activity of Collagen Scaffolds via the Addition of Propolis Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Type I Collagen Extraction
2.2. Propolis Extraction Process and NP Formation
2.3. Preparation of Collagen Scaffolds Impregnated with Propolis NPs
2.4. Total Polyphenol Content
2.5. Chemical and Physical Characterization of Collagen-Propolis NP Scaffolds
2.6. Cellular Response to Propolis-Enriched Collagen Scaffolds: Cell Viability and Proliferation
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reyes, F. Apósito Para Cicatrización de Heridas Comprometidas. España Patent 076336, 22 May 2014. [Google Scholar]
- Naik, G.; Priyadarsini, K.; Satav, J.; Banavalikar, M.; Sohoni, D.; Biyani, M.; Mohan, H. Comparative antioxidant activity of individual herbal. Phytochemistry 2003, 63, 97–104. [Google Scholar] [CrossRef]
- Rodeiro, I.; Donato, M.T.; Jimenez, N.; Garrido, G.; Molina-Torres, J.; Menendez, R.; Castell, J.V.; Gómez-Lechón, M.J. Inhibition of Human P450 Enzymes by Natural. Phitotherapy Res. 2009, 23, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, S.; Capasso, F. Propolis, an old remedy used in modern medicine. Fitoterapia 2002, 73, S1–S6. [Google Scholar] [CrossRef]
- Kuropatnicki, A.K.; Szliszka, E.; Krol, W. Historical Aspects of Propolis Research in Modern Times. Evid. Based Complement. Altern. Med. 2013, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.C.; Rodrigues, S.; Feás, X.; Estevinho, L. Antimicrobial activity, phenolic profile and role in the inflammation of propolis. Food Chem. Toxicol. 2012, 50, 1790–1795. [Google Scholar] [CrossRef]
- Sulaiman, G.M.; al Sammarrae, K.W.; Ad’hiah, A.H.; Zucchetti, M.; Frapolli, R.; Bello, E.; Erba, E.; D’Incalci, M.; Bagnati, R. Chemical characterization of Iraqi propolis samples and assessing their antioxidant potentials. Food Chem. Toxicol. 2011, 49, 2415–2421. [Google Scholar] [CrossRef]
- Sodagar, A.; Akhavan, A.; Arab, S.; Bahador, A.; Pourhajibagher, M.; Soudi, A. Evaluation of the Effect of Propolis Nanoparticles on Antimicrobial Properties and Shear Bond Strength of Orthodontic Composite Bonded to Bovine Enamel. Front. Dent. 2019, 2, 96–104. [Google Scholar] [CrossRef]
- Mocanu, A.; Isopencu, G.; Busuioc, C.; Popa, O.M.; Socaciu-Siebert, L. Bacterial cellulose films with ZnO nanoparticles and propolis extracts: Synergistic antimicrobial effect. Sci. Rep. 2019, 9, 17687. [Google Scholar] [CrossRef]
- Machado, G.T.P.; Veleirinho, M.B.; Mazzarino, L.; Filho, L.C.P.M.; Cerri, M.M.R.L.A.; Kuhnen, S. Development of propolis nanoparticles for the treatment. Can. J. Anim. Sci. 2019, 99, 713–723. [Google Scholar]
- Kazemi, F.; Divsalar, A.; Saboury, A.A.; Seyedarabi, A. Propolis nanoparticles prevent structural changes in human hemoglobin. Colloids Surf. B Biointerfaces 2019, 177, 188–195. [Google Scholar] [CrossRef]
- Dota, K.F.D.; Consolaro, M.E.L.; Svidzinski, T.I.E.; Bruschi, M.L. Antifungal Activity of Brazilian Propolis Microparticles Against. Evid. -Based Complement. Altern. Med. 2011, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Athikomkulchai, S.; Awale, S.; Ruangrungsi, N.; Ruchirawat, S.; Kadota, S. Chemical constituents of Thai propolis. Fitoterapia 2013, 88, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Olczyk, P.; Wisowski, G.; Komosinska-Vassev, K.; Stojko, J.; Klimek, K.; Olczyk, M.; Kozma, E.M. Propolis Modifies Collagen Types I and III Accumulation in the Matrix of Burnt Tissue. Evid. Based Complement. Altern. Med. 2013, 2013, 423809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hozzein, W.N.; Badr, G.; al Ghamdi, A.A.; Sayed, A.; Al-Waili, N.S.; Garraud, O. Topical Application of Propolis Enhances Cutaneous Wound Healing by Promoting TGF-Beta/Smad-Mediated Collagen Production in a Streptozotocin-Induced Type I Diabetic Mouse Model. Cell. Physiol. Biochem. 2015, 37, 940–954. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, E.B.; Cardoso, J.C.; de Lima, A.K.; de Oliveira, N.L.; de Pontes-Filho, N.T.; Lima, S.O.; Souza, I.C.L.; de Albuquerque-Júnior, R.L.C. The incorporation of Brazilian propolis into collagen-based dressing. J. Ethnopharmacol. 2013, 147, 419–425. [Google Scholar]
- Rassu, G.; Cossu, M.; Langasco, R.; Carta, A.; Giunchedi, R.C.; Gavini, E. Propolis as lipid bioactive nano-carrier for topical nasal drug delivery. Colloids Surf. B Biointerfaces 2015, 136, 908–917. [Google Scholar] [CrossRef]
- Mello, B.C.; Petrus, J.C.C.; Hubinger, M.D. Concentration of flavonoids and phenolic compounds in aqueous and ethanolic propolis extracts through nanofiltration. J. Food Eng. 2010, 96, 533–539. [Google Scholar] [CrossRef]
- Marcucci, M. Propolis: Chemical composition, biological properties and therapeutic activity. Apidologie 1995, 26, 83–99. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, J.; Ping, S.; Ma, Q.; Chen, X.; Xuan, H.; Shi, J.; Zhanga, C.; Hu, F. Anti-inflammatory effects of ethanol extracts of Chinese propolis and buds from poplar (Populus × canadensis). J. Ethnopharmacol. 2014, 155, 300–311. [Google Scholar] [CrossRef]
- Rajan, N.; Habermehl, J.; Cote, M.-F.; Doillon, C.J.; Mantovani, D. Preparation of ready-to-use, storable and reconstituted type I collagen from rat tail tendon for issue engineering aplications. Nat. Protoc. 2006, 6, 2753–2758. [Google Scholar] [CrossRef]
- González-Masís, J.; Cubero-Sesin, J.; Vega-Baudrit, J.R.; González-Paz, R.J. Development and characterization of biomaterials for biomimetic tissue applications. J. Eng. Med. Devices 2017, 1, 44–53. [Google Scholar]
- O’brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 17, 5421–5426. [Google Scholar] [CrossRef]
- Pobiega, K.; Kra’sniewska, K.; Derewiaka, D.; Gniewosz, M. Comparison of the antimicrobial activity of propolis extracts. J. Food Sci. Technol. 2019, 12, 5386–5395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skliar, M.; Chernyshev, V.S. Imaging of extracellular vesicles by atomic force microcopy. J. Visualized Exp. 2019, 151, 1–13. [Google Scholar]
- Yusof, N.S.M.; Ashokkumar, M. Ultrasonic Modification of Micelle Structures. In Handbook of Ultrasonics and Sonochemistry; Springer: Singapore, 2015; pp. 1–34. [Google Scholar]
- Kubiliene, L.; Laugaliene, V.; Pavilonis, A.; Maruska, A.; Majiene, D.; Barcauskaite, K.; Kubilius, R.; Kasparaviciene, G.; Savickas, A. Alternative preparation of propolis extracts: Comparison of their composition and biological activities. BMC Complement. Altern. Med. 2015, 15, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do Nascimento, T.G.; Da Silva, P.F.; Azevedo, L.F.; Da Rocha, L.G.; de Moraes Porto, I.C.; e Moura, T.F.; Basílio-Júnior, I.D.; Grillo, L.A.; Dornelas, C.B.; da Silva Fonseca, E.J.; et al. Polymeric Nanoparticles of Brazilian Red Propolis Extract: Preparation, Characterization, Antioxidant and Leishmanicidal Activity. Nanosc. Res. Lett. 2016, 1, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Dan, N.; Dan, W. Insight into the Collagen Assembly in the Presence of Lysine and Glutamic Acid: An in Vitro Study. Mater. Sci. Eng. 2017, 70, 689–700. [Google Scholar] [CrossRef]
- Ferreira, A.; González, G.; González-Paz, R.; Feijoo, J.; Lira-Olivares, J.; Noris-Suárez, K. Bone collagen role in piezoelectric mediated remineralization. Acta Microsc. 2009, 3, 278–286. [Google Scholar]
- Kim, H.G.; Kim, J.H. Preparation and Properties of Antibacterial Poly (vinyl alcohol). Fibers Polym. 2011, 5, 602–609. [Google Scholar] [CrossRef]
- Miles, C.A.; Burjanadze, T.V.; Bailey, A.J. The Kinetics of the Thermal Denaturation of Collagen in Unrestrained Rat Tail Tendon Determined by Differential Scanning Calorimetry. J. Mol. Biol. 1995, 4, 437–446. [Google Scholar] [CrossRef]
- M, F.; Banaszak, M. Variation in type I collagen fibril nanomorphology: The significance and origin. Bone Key Rep. 2013, 394, 1–7. [Google Scholar]
- Krell, R. Value-Added Products from Beekeeping; Food and Agriculture Organization of the United Nations: Rome, Italy, 1996. [Google Scholar]
- Yousefi, M.; Ariffin, F.A.; Huda, N. An alternative source of type I collagen based on by-product with higer thermal stability. Food Hydrocolloids 2016, 63, 372–382. [Google Scholar] [CrossRef]
- Tyszka-Czochara, M.; Paśko, P.; Reczyński, W.; Szlósarczyk, M.; Bystrowska, B.; Opoka, W. Zinc and Propolis Reduces Cytotoxicity and Proliferation in Skin Fibroblast Cell Culture: Total Polyphenol Content and Antioxidant Capacity of Propolis. Biol. Trace Elem. Res. 2014, 160, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Elkhenany, H.; El-Badri, N.; Dhar, M. Green propolis extract promotes in vitro proliferation, differentiation, and migration of bone marrow stromal cells. Biomed. Pharmacother. 2019, 115, 108861. [Google Scholar] [CrossRef] [PubMed]
- Fung, C.S.; Mohamad, H.; Hashim, S.N.; Htun, A.T.; Ahmad, A. Proliferative Effect of Malaysian Propolis on Stem Cells from Human Exfoliated Deciduous Teeth: An in vitroStudy. Br. J. Pharm. Res. 2015, 1, 1–8. [Google Scholar] [CrossRef]
- Gjertsen, A.W.; Stothz, K.A.; Neiva, K.G.; Pileggi, R. Effect of propolis on proliferation and apoptosis of periodontal ligament fibroblasts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 6, 843–848. [Google Scholar] [CrossRef]
- Grenho, L.; Barro, J.; Ferreira, C.; Santos, V.R.; Monteiro, F.J.; Ferraz, M.; Cortes, M.E. In vitro antimicrobial activity and biocompatibility of propolis containing nanohydroxyapatite. Biomed. Mater. 2015, 10, 025004. [Google Scholar] [CrossRef]
- Grunberger, D.; Banerjee, R.; Eisinger, K.; Oltz, E.M.; Efros, L.; Caldwell, M.; Estevez, V.; Nakanishi, K. Preferential cytotoxicity on tumor cells by caffeic acid phenethyl ester isolated from propolis. Experientia 1988, 44, 230–232. [Google Scholar] [CrossRef]
- Frenkel, K.; Wei, H.; Bhimani, R.; Ye, J.; Zadunaisky, J.; Huang, M.T.; Ferraro, T.; Conney, A.H.; Grunberger, D. Inhibition of Tumor Promoter-Mediated Processes in Mouse Skin and Bovine Lens by Caffeic Acid Phenethyl Ester. Cancer Res. 1993, 53, 1255–1261. [Google Scholar]







| Samples | Denaturation Temperature Td (°C) | Enthalpy ΔH (J/g) |
|---|---|---|
| Clg + propolis NPs 10 μg/mL | 77.35 | 326.2 |
| Clg + propolis NPs 100 μg/mL | 63.10 | 209.8 |
| Clg + propolis NPs 1000 μg/mL | 63.50 | 213.0 |
| Self-assembled Clg | 81.52 | 446.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Masís, J.; Cubero-Sesin, J.M.; Corrales-Ureña, Y.R.; González-Camacho, S.; Mora-Ugalde, N.; Baizán-Rojas, M.; Loaiza, R.; Vega-Baudrit, J.R.; Gonzalez-Paz, R.J. Increased Fibroblast Metabolic Activity of Collagen Scaffolds via the Addition of Propolis Nanoparticles. Materials 2020, 13, 3118. https://doi.org/10.3390/ma13143118
González-Masís J, Cubero-Sesin JM, Corrales-Ureña YR, González-Camacho S, Mora-Ugalde N, Baizán-Rojas M, Loaiza R, Vega-Baudrit JR, Gonzalez-Paz RJ. Increased Fibroblast Metabolic Activity of Collagen Scaffolds via the Addition of Propolis Nanoparticles. Materials. 2020; 13(14):3118. https://doi.org/10.3390/ma13143118
Chicago/Turabian StyleGonzález-Masís, Jeimmy, Jorge M. Cubero-Sesin, Yendry R. Corrales-Ureña, Sara González-Camacho, Nohelia Mora-Ugalde, Mónica Baizán-Rojas, Randall Loaiza, José Roberto Vega-Baudrit, and Rodolfo J. Gonzalez-Paz. 2020. "Increased Fibroblast Metabolic Activity of Collagen Scaffolds via the Addition of Propolis Nanoparticles" Materials 13, no. 14: 3118. https://doi.org/10.3390/ma13143118