Effect of Structure and Composition of Non-Stoichiometry Magnesium Aluminate Spinel on Water Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Water Adsorption Calorimetry
3. Results and Discussion
3.1. Surface State Analysis
3.2. Water Adsorption Measurments
3.3. Effect of Anti-Site Defects
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gusmano, G.; Montesperelli, G.; Traversa, E.; Mattogno, G. Microstructure and electrical properties of MgAl2O4 thin films for humidity sensing. J. Am. Ceram. Soc. 1993, 76, 743–750. [Google Scholar] [CrossRef]
- Govindaraj, A.; Flahaut, E.; Laurent, C.; Peigney, A.; Rousset, A.; Rao, C.N.R. An investigation of carbon nanotubes obtained from the decomposition of methane over reduced Mg1−xMxAl2O4 spinel catalysts. J. Mater. Res. 1999, 14, 2567–2576. [Google Scholar] [CrossRef] [Green Version]
- Mei, D.; Lebarbier Dagle, V.; Xing, R.; Albrecht, K.O.; Dagle, R.A. Steam reforming of ethylene glycol over MgAl2O4 supported Rh, Ni, and Co Catalysts. ACS Catal. 2016, 6, 315–325. [Google Scholar] [CrossRef]
- Villa, A.; Gaiassi, A.; Rossetti, I.; Bianchi, C.L.; Van Benthem, K.; Veith, G.M.; Prati, L. Au on MgAl2O4 spinels: The effect of support surface properties in glycerol oxidation. J. Catal. 2010, 275, 108–116. [Google Scholar] [CrossRef]
- Mei, D.; Glezakou, V.A.; Lebarbier, V.; Kovarik, L.; Wan, H.; Albrecht, K.O.; Gerber, M.; Rousseau, R.; Dagle, R.A. Highly active and stable MgAl2O4-supported Rh and Ir catalysts for methane steam reforming: A combined experimental and theoretical study. J. Catal. 2014, 316, 11–23. [Google Scholar] [CrossRef]
- Mei, D.; Lebarbier, V.M.; Rousseau, R.; Glezakou, V.A.; Albrecht, K.O.; Kovarik, L.; Flake, M.; Dagle, R.A. Comparative investigation of benzene steam reforming over spinel supported Rh and Ir catalysts. ACS Catal. 2013, 3, 1133–1143. [Google Scholar] [CrossRef]
- Li, W.Z.; Kovarik, L.; Mei, D.; Liu, J.; Wang, Y.; Peden, C.H.F. Stable platinum nanoparticles on specific MgAl2O4 spinel facets at high temperatures in oxidizing atmospheres. Nat. Commun. 2013, 4, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, C.M.; de With, G. Computer simulation of dissociative adsorption of water on the surfaces of spinel MgAl2O4. J. Am. Ceram. Soc. 2001, 84, 1553–1558. [Google Scholar] [CrossRef]
- Hallstedt, B. Thermodynamic assessment of the system MgO-Al2O3. J. Am. Ceram. Soc. 1992, 75, 1497–1507. [Google Scholar] [CrossRef]
- Erukhimovitch, V.; Mordekoviz, Y.; Hayun, S. Spectroscopic study of ordering in non-stoichiometric magnesium aluminate spinel. Am. Mineral. 2015, 100, 1744–1751. [Google Scholar] [CrossRef]
- Mordekovitz, Y.; Hayun, S. On the effect of lithium on the energetics and thermal stability of nano-sized nonstoichiometric magnesium aluminate spinel. J. Am. Ceram. Soc. 2016, 99, 1–9. [Google Scholar] [CrossRef]
- Hilklin, T.R.; Laine, R.M. Synthesis of metastable phases in the magnesium spinel− alumina system. Chem. Mater. 2008, 20, 553–558. [Google Scholar] [CrossRef]
- Halabi, M.; Ezersky, V.; Kohn, A.; Hayun, S. Charge distribution in nano-scale grains of magnesium aluminate spinel. J. Am. Ceram. Soc. 2017, 100, 800–811. [Google Scholar] [CrossRef]
- Rubat du Merac, M.; Kleebe, H.-J.; Müller, M.M.; Reimanis, I.E. Fifty years of research and development coming to fruition; Unraveling the complex interactions during processing of transparent magnesium aluminate (MgAl2O4) spinel. J. Am. Ceram. Soc. 2013, 96, 3341–3365. [Google Scholar] [CrossRef]
- Reimanis, I.; Kleebe, H.-J. A review on the sintering and microstructure development of transparent spinel (MgAl2O4). J. Am. Ceram. Soc. 2009, 92, 1472–1480. [Google Scholar] [CrossRef]
- Ball, J.A.; Murphy, S.T.; Grimes, R.W.; Bacorisen, D.; Smith, R.; Uberuaga, B.P.; Sickafus, K.E. Defect processes in MgAl2O4 spinel. Solid State Sci. 2008, 10, 717–724. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Foster, A.S.; Hinnemann, B.; Canova, F.F.; Helveg, S.; Meinander, K.; Martin, N.M.; Knudsen, J.; Vlad, A.; Lundgren, E.; et al. Stable cation inversion at the MgAl2O4(100) surface. Phys. Rev. Lett. 2011, 107, 2–5. [Google Scholar] [CrossRef] [Green Version]
- Simeone, D.; Dondane-Thiriiet, C.; Gosset, D.; Daniel, P.; Beauvy, M. Comment—Disorder phase transition induced by swift ions in MgAl2O4 and ZnAl2O4 spinels. J. Nucl. Mater. 2002, 300, 151–160. [Google Scholar] [CrossRef]
- Méducin, F.; Redfern, S.A.T.; Le Godec, Y.; Stone, H.J.; Tucker, M.G.; Dove, M.T.; Marshall, W.G. Study of cation order-disorder in MgAl2O4 spinel by in situ neutron diffraction up to 1600 K and 3.2 GPa. Am. Mineral. 2004, 89, 981–986. [Google Scholar] [CrossRef]
- Wood, B.J.; Kirkpatrick, R.J.; Montez, B. Order-disorder phenomena in MgAl2O4 spinel. Am. Mineral. 1986, 71, 999–1006. [Google Scholar]
- O’Neill, H.S.C.; Navrotsky, A. Simple spinels: Crystallographic parameters, cation radii, lattice energies, and cation distribution. Am. Mineral. 1983, 68, 181–194. [Google Scholar]
- Sickafus, K.E.; Yu, N.; Nastasi, M. Radiation resistance of the oxide spinel: The role of stoichiometry on damage response. Nucl. Imstrum. Meth. B 1996, 116, 85–91. [Google Scholar] [CrossRef]
- Ting, C.J.; Lu, H.Y. Defect reactions and the controlling mechanism in the sintering of magnesium aluminate spinel. J. Am. Ceram. Soc. 1999, 82, 841–848. [Google Scholar] [CrossRef]
- Chiang, Y.-M. Grain Boundary Mobility and Segregation in Non-Stoichiometric Solid Solutions of Magnesium Aluminate Spinel; Massachusetts Institute of Technology: Cambridge, MA, USA, 1980. [Google Scholar]
- Chiang, Y.-M.; Kingery, W.D. Grain-boundary migration in nonstoichiometric solid solutions of magnesium aluminate spinel: I, grain growth studies. J. Am. Ceram. Soc. 1989, 72, 271–277. [Google Scholar] [CrossRef]
- Sutorik, A.C.; Gilde, G.; Swab, J.J.; Cooper, C.; Gamble, R.; Shanholtz, E. Transparent solid solution magnesium aluminate spinel polycrystalline ceramic with the alumina-rich composition MgO·1.2 Al2O3. J. Am. Ceram. Soc. 2012, 95, 636–643. [Google Scholar] [CrossRef]
- Barzilai, S.; Aizenshtein, M.; Mintz, M.H.; Hayun, S. Effect of adsorbed oxygen on the dissociation of water over gadolinium oxide surfaces: Density functional theory calculations and experimental results. J. Phys. Chem. C 2020. [Google Scholar] [CrossRef]
- Ianoş, R.; Lazǎu, I.; Pǎcurariu, C.; Barvinschi, P. Solution combustion synthesis of MgAl2O4 using fuel mixtures. Mater. Res. Bull. 2008, 43, 3408–3415. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Ushakov, S.V.; Navrotsky, A. Direct measurements of water adsorption enthalpy on hafnia and zirconia. Appl. Phys. Lett. 2005, 87, 1–3. [Google Scholar] [CrossRef]
- Jing, S.-Y.; Lin, L.-B.; Houng, N.-K.; Zhang, J.; Lu, Y. Investigation on lattice constants of Mg-Al spinels. J. Mater. Sci. Lett. 2000, 19, 225–227. [Google Scholar] [CrossRef]
- Navrotsky, A.; Wechsler, B.A.; Geisinger, K.; Seifert, F. Thermochemistry of MgAl2O4-Al8/3O4 defect spinels. J. Am. Cerum. Soc 1986, 69, 418–422. [Google Scholar] [CrossRef]
- Corsi, J.S.; Fu, J.; Wang, Z.; Lee, T.; Ng, A.K.; Detsi, E. Hierarchical bulk nanoporous aluminum for on-site generation of hydrogen by hydrolysis in pure water and combustion of solid fuels. ACS Sustain. Chem. Eng. 2019, 7, 11194–11204. [Google Scholar] [CrossRef]
- Hinnen, C.; Imbert, D.; Siffre, J.M.; Marcus, P. An in situ XPS study of sputter-deposited aluminium thin films on graphite. Appl. Surf. Sci. 1994, 78, 219–231. [Google Scholar] [CrossRef]
- He, H.; Alberti, K.; Barr, T.L.; Klinowski, J. ESCA studies of aluminophosphate molecular sieves. J. Phys. Chem. 1993, 97, 13703–13707. [Google Scholar] [CrossRef]
- Grigorova, E.; Khristov, M.; Peshev, P.; Nihtianova, D.; Velichkova, N.; Atanasova, G. Hydrogen sorption properties of a MgH2–V2O5 composite prepared by ball milling. Bulg. Chem. Commun. 2013, 45, 280–287. [Google Scholar]
- Shelly, L.; Schweke, D.; Zalkind, S.; Shamir, N.; Barzilai, S.; Gouder, T.; Hayun, S. Effect of U content on the activation of H2O on Ce1-xUxO2+δ surfaces. Chem. Mater. 2018, 30, 8650–8660. [Google Scholar] [CrossRef]
- Hayun, S.; Shvareva, T.Y.; Navrotsky, A. Nanoceria—Energetics of surfaces, interfaces and water adsorption. J. Am. Ceram. Soc. 2011, 94, 3992–3999. [Google Scholar] [CrossRef]
- Uner, D.; Uner, M. Adsorption calorimetry in supported catalyst characterization: Adsorption structure sensitivity on Pt/γ-Al2O3. Thermochim. Acta 2005, 434, 107–112. [Google Scholar] [CrossRef]
- Garcia-Cuello, V.; Moreno-Piraján, J.C.; Giraldo-Gutiérrez, L.; Sapag, K.; Zgrablich, G. Determination of differential enthalpy and isotherm by adsorption calorimetry. Res. Lett. Phys. Chem. 2008, 2008, 127328. [Google Scholar] [CrossRef] [Green Version]
- Hayun, S.; Tran, T.; Ushakov, S.V.; Thron, A.M.; Van Benthem, K.; Navrotsky, A.; Castro, R.H.R. Experimental methodologies for assessing the surface energy of highly hygroscopic materials: The case of nanocrystalline magnesia. J. Phys. Chem. C 2011, 115, 23929–23935. [Google Scholar] [CrossRef]
- Jia, C.; Fan, W.; Yang, F.; Zhao, X.; Sun, H.; Li, P.; Liu, L. A theoretical study of water adsorption and decomposition on low-index spinel ZnGa2O4 surfaces: Correlation between surface structure and photocatalytic properties. Langmuir 2013, 29, 7025–7037. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Wang, J.G.; Wang, Y.; Mei, D. First-principles hermodynamics study of spinel MgAl2O4 surface stability. J. Phys. Chem. C 2016, 120, 19087–19096. [Google Scholar] [CrossRef]











| n | (MgxAlyO4) | Lattice Parameter, Å | Crystallite Size, nm | Surface Area, m2/g | ||
|---|---|---|---|---|---|---|
| (x) Mg | (y) Al | XRD | BET | |||
| 0.95 | 1.04 | 1.97 | 8.089(2) | 14.0 ± 0.2 | 117.7 ± 1.6 | 32.1 ± 0.2 |
| 1.07 | 0.95 | 2.03 | 8.078(2) | 13.3 ± 0.2 | 123.9 ± 1.7 | 37.6 ± 0.2 |
| 1.15 | 0.72 | 2.18 | 8.065(6) | 10.2 ± 0.3 | 161.6 ± 4.9 | 56.4 ± 0.2 |
| 2.45 | 0.48 | 2.35 | 7.989(4) | 15.5 ± 0.8 | 106.3 ± 5.2 | 41.2 ± 0.2 |
| n | Heat of Adsorption, kJ/mol | Hydroxides, mol. % | H2O Coverage, Molecules/nm2 | |||
|---|---|---|---|---|---|---|
| Al–OH | Mg–OH | Total | ||||
| Clean | 0.95 | −75.1 ± 0.2 | 6.2 ± 0.3 | 13.7 ± 0.7 | 20.0 ± 1.0 | 12.2 ± 1.0 |
| 1.07 | −73.3 ± 0.4 | 34.0 ± 1.7 | 25.5 ± 1.3 | 59.4 ± 3.0 | 12.9 ± 0.1 | |
| 1.15 | −71.0 ± 0.7 | 35.4 ± 1.8 | 12.4 ± 0.6 | 47.0 ± 2.4 | 11.5 ± 0.1 | |
| 2.45 | −67.2 ± 0.2 | 32.1 ± 1.0 | 2.2 ± 0.1 | 34.3 ± 1.7 | 10.1 ± 0.1 | |
| Reduced | 0.95 | −71.0 ± 1.0 | 8.3 ± 0.4 | 16.9 ± 0.8 | 25.1 ± 1.2 | 15.3 ± 0.3 |
| 1.07 | −75.6 ± 0.9 | 30.3 ± 1.5 | 18.9 ± 0.9 | 48.2 ± 2.5 | 11.3 ± 0.2 | |
| 1.15 | −68.5 ± 0.4 | 37.5 ± 1.9 | 11.5 ± 0.6 | 49.0 ± 2.4 | 13.4 ± 0.1 | |
| 2.45 | −66.3 ± 0.3 | 41.3 ± 2.1 | 7.8 ± 0.4 | 49.0 ± 2.4 | 13.5 ± 0.2 | |
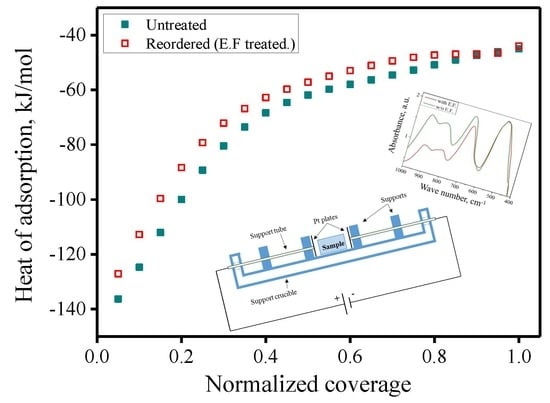
| n | Inversion (i) | Heat of Adsorption, kJ/mol | Extent of Hydroxides Formed, mol. % | H2O Coverage, Molecules/nm2 | |||
|---|---|---|---|---|---|---|---|
| Al–OH | Mg–OH | Total | |||||
| 2.45 | Untreated | 0.44 | −67.2 ± 0.3 | 32.1 ± 1.6 | 2.2 ± 0.1 | 34.3 ± 1.7 | 10.1 ± 0.1 |
| Treated/(EF) | 0.33 | −62.5 ± 0.5 | 25.0 ± 1.3 | 1.8 ± 0.1 | 26.8 ± 1.4 | 8.8 ± 0.1 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mordekovitz, Y.; Shoval, Y.; Froumin, N.; Hayun, S. Effect of Structure and Composition of Non-Stoichiometry Magnesium Aluminate Spinel on Water Adsorption. Materials 2020, 13, 3195. https://doi.org/10.3390/ma13143195
Mordekovitz Y, Shoval Y, Froumin N, Hayun S. Effect of Structure and Composition of Non-Stoichiometry Magnesium Aluminate Spinel on Water Adsorption. Materials. 2020; 13(14):3195. https://doi.org/10.3390/ma13143195
Chicago/Turabian StyleMordekovitz, Yuval, Yael Shoval, Natali Froumin, and Shmuel Hayun. 2020. "Effect of Structure and Composition of Non-Stoichiometry Magnesium Aluminate Spinel on Water Adsorption" Materials 13, no. 14: 3195. https://doi.org/10.3390/ma13143195