A State-of-the-Art Review on the Wear of the Occlusal Surfaces of Natural Teeth and Prosthetic Crowns
Abstract
:1. Introduction
2. Wear at the Interface of Occlusal Surfaces
2.1. Tooth-Tooth
2.2. Tooth-Ceramic
2.3. Tooth-Resin-Based Material
2.4. Tooth-to-Metal
2.5. Restorative-to-Restorative Materials
2.6. Final Remarks
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.; Du, W.; Zhou, X.; Yu, H. Review of research on the mechanical properties of the human tooth. Int. J. Oral Sci. 2014, 6, 61–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.-R.; Yu, H.-Y.; Zheng, J.; Qian, L.-M.; Yan, Y. Dental Biotribology; Springer: New York, NY, USA, 2013. [Google Scholar]
- Roy, S.; Basu, B. Mechanical and tribological characterization of human tooth. Mater. Charact. 2008, 59, 747–756. [Google Scholar] [CrossRef]
- Zhou, Z.R.; Zheng, J. Tribology of dental materials: A review. J. Phys. D Appl. Phys. 2008, 41, 11301. [Google Scholar] [CrossRef]
- Chen, J. Food oral processing—A review. Food Hydrocoll. 2009, 23, 1–25. [Google Scholar] [CrossRef]
- Stokes, J.R.; Boehm, M.W.; Baier, S.K. Oral processing, texture and mouthfeel: From rheology to tribology and beyond. Curr. Opin. Colloid Interface Sci. 2013, 18, 349–359. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Huang, H.; Shi, M.Y.; Zheng, L.; Qian, L.M.; Zhou, Z.R. In vitro study on the wear behaviour of human tooth enamel in citric acid solution. Wear 2011, 271, 2313–2321. [Google Scholar] [CrossRef]
- Stumpf, A.S.G.; Bergmann, C.P.; Vicenzi, J.; Fetter, R.; Mundstock, K.S. Mechanical behavior of alumina and alumina-feldspar based ceramics in an acetic acid (4%) environment. Mater. Des. 2009, 30, 4348–4359. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Si, W.; Feng, H.; Tao, Y.; Ma, Z. Friction and wear behaviors of dental ceramics against natural tooth enamel. J. Eur. Ceram. Soc. 2012, 32, 2599–2606. [Google Scholar] [CrossRef]
- Parle, D.; Desai, D.; Bansal, A. Estimation of Individual Bite Force during Normal Occlusion using FEA. In Proceedings of the Altair Technology Conference, Pune, India, 30 June 2013; pp. 1–9. [Google Scholar]
- Gholampour, S.; Gholampour, H.; Khanmohammadi, H. Finite element analysis of occlusal splint therapy in patients with bruxism. BMC Oral Health 2019, 19, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lewis, R.; Dwyer-Joyce, R.S. Wear of human teeth: A tribological perspective. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2005, 219, 2–19. [Google Scholar] [CrossRef]
- Hutchings, I.M. Tribology: Friction and Wear of Engeneering Materials; Edward Arnold: London, UK, 1992. [Google Scholar]
- Diraçoǧlu, D.; Alptekin, K.; Çifter, E.D.; Güçlü, B.; Karan, A.; Aksoy, C. Relationship between maximal bite force and tooth wear in bruxist and non-bruxist individuals. Arch. Oral Biol. 2011, 56, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Nishigawa, K.; Bando, E.; Nakano, M. Quantitative study of bite force during sleep associated bruxism. J. Oral Rehabil. 2001, 28, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Mcauliffe, P.; Kim, J.H.; Diamond, D.; Lau, K.T.; O’Connell, B.C. A sleep bruxism detection system based on sensors in a splint—Pilot clinical data. J. Oral Rehabil. 2015, 42, 34–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osiewicz, M.A.; Werner, A.; Franciscus, J.M.; Kleverlaan, C.J.; Ma, O.; Werner, A. Wear of direct resin composites and teeth: Considerations for oral rehabilitation. Eur. J. Oral Sci. 2019, 127, 156–161. [Google Scholar] [CrossRef]
- Mair, L.H. Wear in dentistry—Current terminology. J. Dent. 1992, 20, 140–144. [Google Scholar] [CrossRef]
- Tsujimoto, A.; Barkmeier, W.W.; Fischer, N.G.; Nojiri, K.; Nagura, Y.; Takamizawa, T.; Latta, M.A.; Miazaki, M. Wear of resin composites: Current insights into underlying mechanisms, evaluation methods and influential factors. Jpn. Dent. Sci. Rev. 2018, 54, 76–87. [Google Scholar] [CrossRef]
- Mair, L.H.; Vowles, R.W.; Lloyd, C.H. Wear: Mechanisms, manifestations and measurement. Report of a workshop. J. Dent. 1996, 24, 141–148. [Google Scholar] [CrossRef]
- Buciumeanu, M.; Queiroz, J.R.C.; Martinelli, A.E.; Silva, F.S.; Henriques, B. The effect of surface treatment on the friction and wear behavior of dental Y-TZP ceramic against human enamel. Tribol. Int. 2017, 116, 192–198. [Google Scholar] [CrossRef]
- D’Incau, E.; Couture, C.; Maureille, B. Human tooth wear in the past and the present: Tribological mechanisms, scoring systems, dental and skeletal compensations. Arch. Oral Biol. 2011, 57, 214–229. [Google Scholar] [CrossRef]
- Zhou, Z.R.; Zheng, J. Oral tribology. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2006, 739–754. [Google Scholar] [CrossRef]
- Jin, Z.M.; Zheng, J.; Li, W.; Zhou, Z.R. Tribology of medical devices. Biosurf. Biotribol. 2016, 2, 173–192. [Google Scholar] [CrossRef]
- He, L.H.; Swain, M.V. Enamel—A ‘“metallic-like”’ deformable biocomposite. J. Dent. 2010, 35, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Yip, K.H.; Smales, R.J.; Kaidonis, J.A. Differential Wear of Teeth and Restorative Materials: Clinical Implications. Int. J. Prosthodont. 2004, 17, 350–356. [Google Scholar] [PubMed]
- Hudson, D.; Goldstein, G.R. Enamel wear caused by three different restorative materials. J. Prosthet. Dent. 1995, 74, 647–654. [Google Scholar] [CrossRef]
- Santos, F.; Branco, A.; Polido, M.; Serro, A.P. Comparative study of the wear of the pair human teeth/Vita Enamic® vs. commonly used dental ceramics through chewing simulation. J. Mech. Behav. Biomed. Mater. 2018, 88, 251–260. [Google Scholar] [CrossRef]
- Figueiredo-Pina, C.G.; Monteiro, A.; Guedes, M.; Maurício, A.; Serro, A.P.; Ramalho, A.; Santos, C. Effect of feldspar porcelain coating upon the wear behavior of zirconia dental crowns. Wear 2013, 297, 872–877. [Google Scholar] [CrossRef]
- Heintze, S.D.; Zellweger, G.; Cavalleri, A.; Ferracane, J. Influence of the antagonist material on the wear of different composites using two different wear simulation methods. Dent. Mater. 2006, 22, 166–175. [Google Scholar] [CrossRef]
- Seghi, R.R.; Rosenstiel, S.F.; Bauer, P. Abrasion of Human Enamel by Different Dental Ceramics in vitro. J. Dent. Res. 1991, 70, 221–225. [Google Scholar] [CrossRef]
- Carlsson, G.E.; Johansson, A.; Lundqvist, S. Occlusal wear: A follow-up study of 18 subjects with extensively worn dentitions. Acta Odontol. Scand. 2015, 43, 83–90. [Google Scholar] [CrossRef]
- Lambrechts, P.; Braem, M.; Vanherle, G. Quantitative in vivo Wear of Human Enamel. J. Dent. Res. 1989, 68, 1752–1754. [Google Scholar] [CrossRef]
- Mundhe, K.; Jain, V.; Pruthi, G.; Shah, N. Clinical study to evaluate the wear of natural enamel antagonist to zirconia and metal ceramic crowns. J. Prosthet. Dent. 2015, 114, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Sripetchdanond, J.; Leevailoj, C. Wear of human enamel opposing monolithic zirconia, glass ceramic, and composite resin: An in vitro study. J. Prosthet. Dent. 2014, 112, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Arsecularatne, J.A.; Hoffman, M. On the wear mechanism of human dental enamel. J. Mech. Behav. Biomed. Mater. 2010, 3, 347–356. [Google Scholar] [CrossRef]
- Arsecularatne, J.A.; Hoffman, M. Ceramic-like wear behaviour of human dental enamel. J. Mech. Behav. Biomed. Mater. 2012, 8, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhou, Z.R. Study of in vitro wear of human tooth enamel. Tribol. Lett. 2007, 26, 181–189. [Google Scholar] [CrossRef]
- Ratledge, D.K.; Smith, B.G.N.; Wilson, R.F. The effect of restorative materials on the wear of human enamel. J. Prosthet. Dent. 1994, 72, 194–203. [Google Scholar] [CrossRef]
- Eisenburger, M.; Addy, M. Erosion and attrition of human enamel in vitro Part II: Influence of time and loading. J. Dent. 2002, 30, 349–352. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Arsecularatne, J.A.; Hoffman, M. Effect of acidity upon attrition-corrosion of human dental enamel. J. Mech. Behav. Biomed. Mater. 2015, 44, 23–34. [Google Scholar] [CrossRef]
- Wu, Y.-Q.; Arsecularatne, J.A.; Hoffman, M. Attrition-corrosion of human dental enamel: A review. Biosurf. Biotribol. 2017, 3, 196–210. [Google Scholar] [CrossRef]
- Eisenburger, M.; Addy, M. Erosion and attrition of human enamel in vitro Part I: Interaction effects. J. Dent. 2002, 30, 341–347. [Google Scholar] [CrossRef]
- Zhang, Y.; Arsecularatne, J.A.; Hoffman, M. The effects of three different food acids on the attrition-corrosion wear of human dental enamel. J. Phys. D Appl. Phys. 2015, 285401, 285401. [Google Scholar] [CrossRef]
- Suputtamongkol, K.; Anusavice, K.J.; Suchatlampong, C.; Sithiamnuai, P.; Tulapornchai, C. Clinical performance and wear characteristics of veneered lithia-disilicate-based ceramic crowns. Dent. Mater. 2008, 24, 667–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aladağ, A.; Oğuz, D.; Çömlekoğlu, M.E.; Akan, E. In vivo wear determination of novel CAD/ CAM ceramic crowns by using 3D alignment. J. Adv. Prosthodont. 2019, 11, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Palaniappan, S.; Bharadwaj, D.; Mattar, D.L.; Peumans, M.; Van Meerbeek, B.; Lambrechts, P. Three-year randomized clinical trial to evaluate the clinical performance and wear of a nanocomposite versus a hybrid composite. Dent. Mater. 2009, 25, 1302–1314. [Google Scholar] [CrossRef]
- Krämer, N.; Küssner, P.; Motmaen, I.; Köhl, M.; Wöstmann, B.; Frankenberger, R. Marginal quality and wear of extended posterior resin composite restorations: Eight-year results in vivo. J. Mech. Behav. Biomed. Mater. 2015, 50, 13–22. [Google Scholar] [CrossRef]
- Palaniappan, S.; Bharadwaj, D.; Mattar, D.L.; Peumans, M.; Van Meerbeek, B.; Lambrechts, P. Nanofilled and microhybrid composite restorations: Five-year clinical wear performances. Dent. Mater. 2011, 27, 692–700. [Google Scholar] [CrossRef]
- Figueiredo-Pina, C.G.; Patas, N.; Canhoto, J.; Cláudio, R.; Olhero, S.M.; Serro, A.P.; Ferro, A.C.; Guedes, M. Tribological behaviour of unveneered and veneered lithium disilicate dental material. J. Mech. Behav. Biomed. Mater. 2016, 53, 226–338. [Google Scholar] [CrossRef]
- Jung, Y.-S.; Lee, J.-W.; Choi, Y.-J.; Ahn, J.-S.; Shin, S.-W.; Huh, J.-B. A study on the in-vitro wear of the natural tooth structure by opposing zirconia or dental porcelain. J. Adv. Prosthodont. 2010, 2, 111–115. [Google Scholar] [CrossRef] [Green Version]
- Bolaca, A.; Erdoğan, Y. In Vitro Evaluation of the Wear of Primary Tooth Enamel against Different Ceramic and Composite Resin Materials. Niger. J. Clin. Pract. 2019, 22, 313–319. [Google Scholar]
- Nakashima, J.; Taira, Y.; In, S.T. In vitro wear of four ceramic materials and human enamel on enamel antagonist. Eur. J. Oral Sci. 2016, 124, 295–300. [Google Scholar] [CrossRef]
- Choi, J.-W.; Bae, I.-H.; Noh, T.-H.; Ju, S.-W.; Lee, T.-K.; Ahn, J.-S.; Jeong, T.-S.; Huh, J.-B. Wear of primary teeth caused by opposed all-ceramic or stainless steel crowns. J. Adv. Prosthodont. 2016, 438, 43–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.; Swain, M.; He, L.; Lyons, K. Wear behavior of human enamel against lithium disilicate glass ceramic and type III gold. J. Prosthet. Dent. 2014, 112, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Ramp, M.H.; Suzuki, S.; Cox, C.F.; Lacefield, W.R.; Koth, D.L. Evaluation of wear: Enamel opposing three ceramic materials and a gold alloy. J. Prosthet. Dent. 1997, 77, 523–530. [Google Scholar] [CrossRef]
- Hacker, C.H.; Wagner, W.C.; Razzoog, M.E. An in vitro investigation of the wear of enamel on porcelain and gold in saliva. J. Prosthet. Dent. 1996, 75, 14–17. [Google Scholar] [CrossRef]
- Al-Hiyasat, A.S.; Saunders, W.P.; Sharkey, S.W.; Smith, G.M.R.; Gilmour, W.H. Investigation of human enamel wear against four dental ceramics and gold. J. Dent. 1998, 26, 487–495. [Google Scholar] [CrossRef]
- Pereira, G.K.R.; Dutra, D.M.; Werner, A.; Prochnow, C.; Valandro, L.F.; Kleverlaan, C.J. Effect of zirconia polycrystal and stainless steel on the wear of resin composites, dentin and enamel. J. Mech. Behav. Biomed. Mater. 2019, 91, 287–293. [Google Scholar] [CrossRef]
- Ghazal, M.; Hedderich, J.; Kern, M. Wear of feldspathic ceramic, nano-filled composite resin and acrylic resin artificial teeth when opposed to different antagonists. Eur. J. Oral Sci. 2008, 116, 585–592. [Google Scholar] [CrossRef]
- Jang, Y.S.; Nguyen, T.D.T.; Ko, Y.H.; Lee, D.W.; Baik, B.J.; Lee, M.H.; Bae, T.S. In vitro wear behavior between enamel cusp and three aesthetic restorative materials: Zirconia, porcelain, and composite resin. J. Adv. Prosthodont. 2019, 11, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Stawarczyk, B.; Özcan, M.; Schmutz, F.; Trottmann, A.; Roos, M.; Hämmerle, C.H.F. Two-body wear of monolithic, veneered and glazed zirconia and their corresponding enamel antagonists. Acta Odontol. Scand. 2013, 71, 102–112. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, S.; Suzuki, S.H.; Cox, C.F. Evaluating the antagonistic wear of restorative materials when placed against human enamel. J. Am. Dent. Assoc. 1996, 127, 74–80. [Google Scholar] [CrossRef]
- Kim, M.; Oh, S.; Kim, J.; Ju, S.; Seo, D.; Jun, S.; Ahn, J.-S.; Ryu, J.-J. Wear evaluation of the human enamel opposing different Y-TZP dental ceramics and other porcelains. J. Dent. 2012, 40, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Branco, A.C.; Silva, R.; Santos, T.; Jorge, H.; Rodrigues, A.R.; Fernandes, R.; Bandara, S.; Barahona, I.; Matos, A.P.A.; Lorenz, K.; et al. Suitability of 3D printed pieces of nanocrystalline zirconia for dental applications. Dent. Mater. 2020, 6, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, Y.; Wang, S.; Yang, X.; Sun, Y. Two-Body and Three-Body Wear Behavior of a Dental Fluorapatite Glass-Ceramic. Coatings 2019, 9, 580. [Google Scholar] [CrossRef] [Green Version]
- Janyavula, S.; Lawson, N.; Cakir, D.; Beck, P.; Ramp, L.C.; Burgess, J.O. The wear of polished and glazed zirconia against enamel. J. Prosthet. Dent. 2013, 109, 22–29. [Google Scholar] [CrossRef]
- Oh, W.S.; DeLong, R.; Anusavice, K.J. Factors affecting enamel and ceramic wear: A literature review. J. Prosthet. Dent. 2002, 87, 451–459. [Google Scholar] [CrossRef]
- Lawson, N.C.; Janyavula, S.; Syklawer, S.; McLaren, E.A.; Burgess, J.O. Wear of enamel opposing zirconia and lithium disilicate after adjustment, polishing and glazing. J. Dent. 2014, 42, 1586–1591. [Google Scholar] [CrossRef]
- Chong, B.J.; Thangavel, A.K.; Rolton, S.B.; Guazzato, M.; Klineberg, I.J. Clinical and laboratory surface fi nishing procedures for zirconia on opposing human enamel wear: A laboratory study. J. Mech. Behav. Biomed. Mater. 2015, 50, 93–103. [Google Scholar] [CrossRef]
- Ghazal, M.; Kern, M. The influence of antagonistic surface roughness on the wear of human enamel and nanofilled composite resin artificial teeth. J. Prosthet. Dent. 2009, 101, 342–349. [Google Scholar] [CrossRef]
- White, S.; Miklus, V.; McLaren, E.; Lang, L.; Caputo, A. Flexural strength porcelain of a layered zirconia and dental all-ceramic system. J. Prosthet. Dent. 2005, 94, 125–131. [Google Scholar] [CrossRef]
- Kelly, J.R.; Nishimura, I.; Campbell, S.D. Ceramics in dentistry: Historical roots and current perspectives. J. Prosthet. Dent. 1996, 75, 18–32. [Google Scholar] [CrossRef]
- Zhang, Y. Overview: Damage resistance of graded ceramic restorative materials. J. Eur. Ceram. Soc. 2015, 32, 2623–2632. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo-Pina, C.G.; Rodrigues, I.; Sequeira, J.; Guedes, M.; Carneiro, C. Does the presence of a S. Salivarius biofilm influence the tooth-zirconia pair triboactivity? An in-vitro study. Wear 2019, 430–431, 50–56. [Google Scholar] [CrossRef]
- Suzuki, S. Wear resistance and antagonistic enamel wear of prosthetic resin composite. J. Dent. Mater. 1997, 16, 29–32. [Google Scholar]
- Koizumi, H.; Yoshida, T.; Garashi, T.; Saitoh, M.; Nishiyama, M. A study on toothbrush abrasion of high filler containing resin for crown and bridge. J. Prosthodont. Soc. 1999, 43, 1016–1022. [Google Scholar] [CrossRef] [Green Version]
- Shimane, T.; Endo, K.; Zheng, J.H.; Yanagi, T.; Ohno, H. Wear of opposing teeth by posterior composite resins—Evaluation of newly developed wear test methods. Dent. Mater. J. 2010, 29, 713–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, S.; Leinfelder, K.; Kawai, K.; Tsuchitani, Y. Effect of particle variation on wear rates of posterior composites. Am. J. Dent. 1995, 8, 173–178. [Google Scholar] [PubMed]
- Venhoven, B.A.M.; Gee AJDe Werner, A.; Davidson, C.L. Influence of filler parameters on the mechanical coherence of dental restorative resin composites. Biomaterials 1996, 17, 735–740. [Google Scholar] [CrossRef]
- Frankenberger, R.; Reinelt, C.; Krämer, N. Nanohybrid vs. fine hybrid composite in extended class II cavities: 8-year results. Clin. Oral Investig. 2014, 18, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Palaniappan, S.; Elsen, L.; Lijnen, I.; Peumans, M.; Van Meerbeek, B. Three-year randomised clinical trial to evaluate the clinical performance, quantitative and qualitative wear patterns of hybrid composite restorations. Clin. Oral Investig. 2010, 14, 441–458. [Google Scholar] [CrossRef] [Green Version]
- Condon, J.R.; Ferracane, J.L. Evaluation of composite wear with a new multi-mode oral wear simulator. Dent. Mater. 1996, 12, 218–226. [Google Scholar] [CrossRef]
- Suzuki, S.; Nagai, E.; Taira, Y.; Minesaki, Y. In vitro wear of indirect composite restoratives. J. Prosthet. Dent. 2002, 88, 431–436. [Google Scholar] [CrossRef]
- Correr, G.M.; Caroline, R.; Alonso, B.; Sobrinho, C.; Puppin-Rontani, R.M.; Ferracane, J.L. In Vitro Wear of Resin-Based Materials—Simultaneous Corrosive and Abrasive Wear. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 78, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.E.; Anderson, M.P.; Jahanmir, S. Influence of Fracture Toughness on the Wear Resistance of Yttria-Doped Zirconium Oxide. J. Am. Ceram. Soc. 1989, 72, 252–257. [Google Scholar] [CrossRef]
- D’Arcangelo, C.; Vanini, L.; Rondoni, G.D.; De Angelis, F. Wear properties of dental ceramics and porcelains compared with human enamel. J. Prosthet. Dent. 2016, 115, 350–355. [Google Scholar] [CrossRef]
- D’Arcangelo, C.; Vanini, L.; Rondoni, G.D.; Pirani, M.; Vadini, M.; Gattone, M. Wear properties of a novel resin composite compared to human enamel and other restorative materials. Oper. Dent. 2014, 39, 612–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Arcangelo, C.; Vanini, L.; Rondoni, G.D.; Vadini, M.; De Angelis, F. Wear evaluation of prosthetic materials opposing themselves. Oper. Dent. 2018, 43, 38–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borrero-Lopez, O.; Guiberteau, F.; Zhang, Y.; Lawn, B.R. Wear of ceramic-based dental materials. J. Mech. Behav. Biomed. Mater. 2019, 92, 144–151. [Google Scholar] [CrossRef]
- Esquivel, J.; Lawson, N.C.; Kee, E.; Bruggers, K.; Blatz, M.B. Wear of resin teeth opposing zirconia. J. Prosthet. Dent. 2020, 1–6. [Google Scholar] [CrossRef]
- Koottathape, N.; Takahashi, H.; Iwasaki, N.; Kanehira, M.; Finger, W.J. Quantitative wear and wear damage analysis of composite resins in vitro. J. Mech. Behav. Biomed. Mater. 2014, 29, 508–516. [Google Scholar] [CrossRef]
- Ghazal, M.; Albashaireh, Z.S.; Kern, M. Wear resistance of nanofilled composite resin and feldspathic ceramic artificial teeth. J. Prosthet. Dent. 2008, 100, 441–448. [Google Scholar] [CrossRef]
- Silva, C.S.; Henriques, B.; Novaes de Oliveira, A.P.; Silva, F.; Gomes, J.R.; Souza, J.C.M. Micro-scale abrasion and sliding wear of zirconium-lithium silicate glass-ceramic and polymer-infiltrated ceramic network used in dentistry. Wear 2020, 448–449, 203214. [Google Scholar] [CrossRef]
- Yilmaz, E.Ç.; Sadeler, R.; Duymuş, Z.Y.; Öcal, M. Effects of Two-body Wear on Microfill, Nanofill, and Nanohybrid Restorative. Biomed. Biotechnol. Res. J. 2017, 50, 553–558. [Google Scholar] [CrossRef]
- Barkmeier, W.W.; Erickson, R.L.; Latta, M.A.; Wilwerding, T.M. Wear rates of resin composites. Oper. Dent. 2013, 38, 226–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alarcon, J.V.; Engelmeier, R.L.; Powers, J.M.; Triolo, P.T. Wear testing of composite, gold, porcelain, and enamel opposing a removable cobalt-chromium partial denture alloy. J. Prosthodont. 2009, 18, 421–426. [Google Scholar] [CrossRef]
- Yap, A.U.J.; Teoh, S.H.; Tan, K.B. Influence of water exposure on three-body wear of composite restoratives. J. Biomed. Mater. Res. 2000, 53, 547–553. [Google Scholar] [CrossRef]
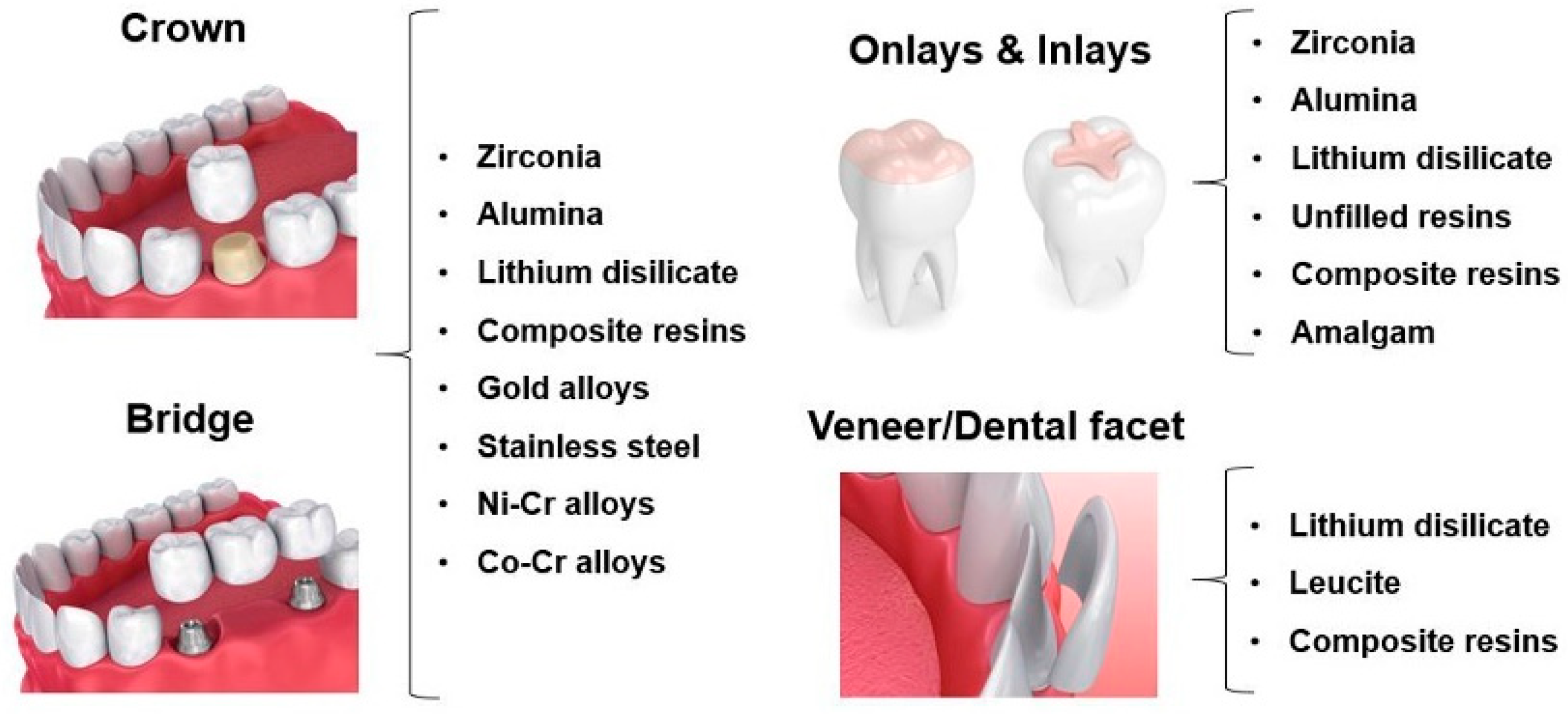
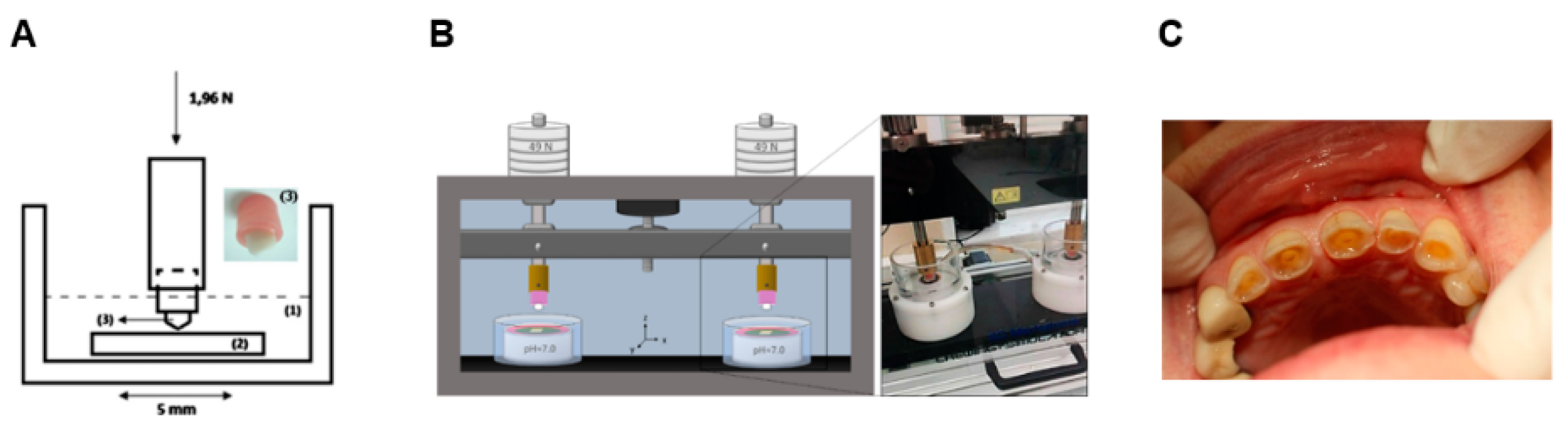
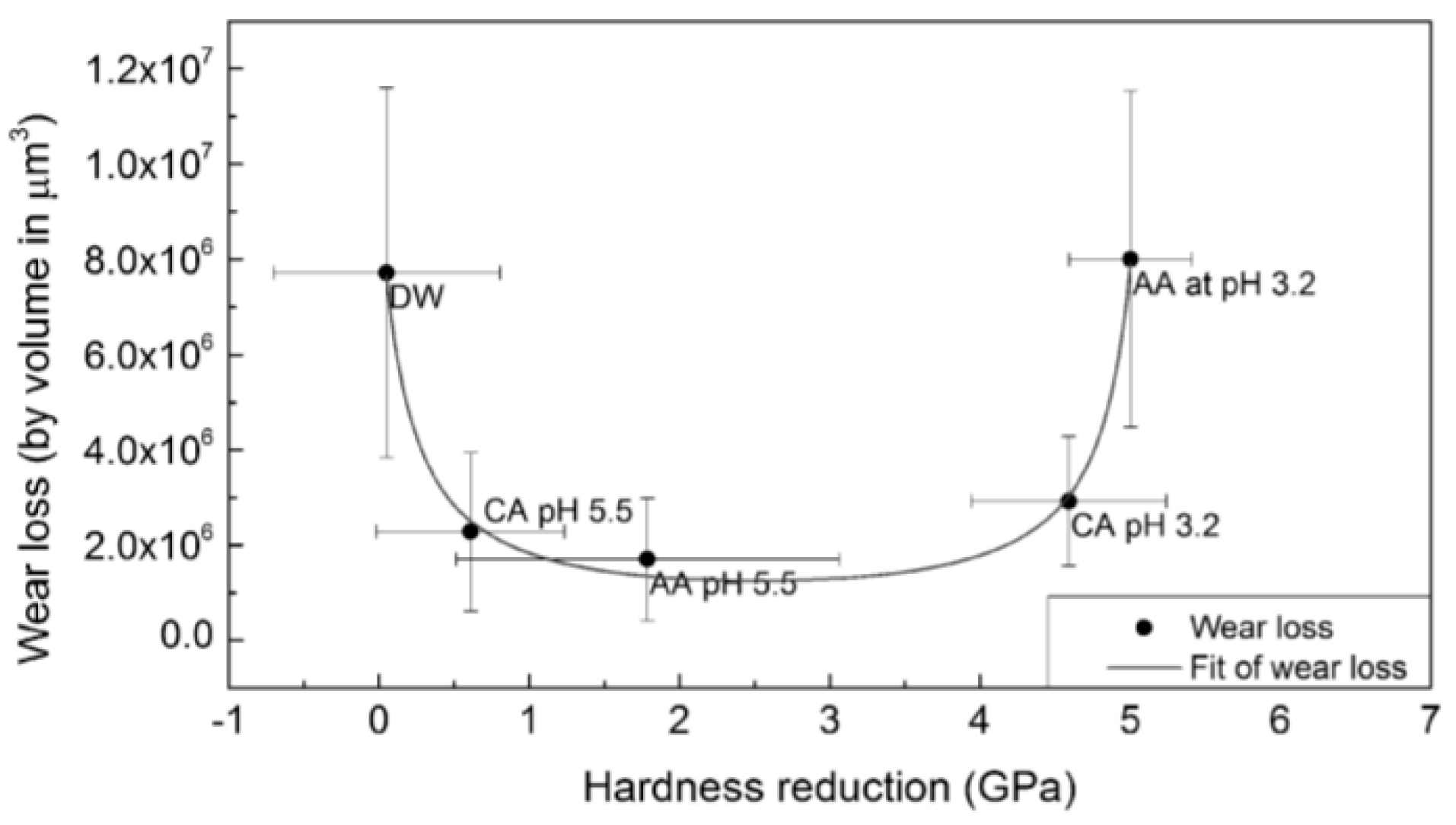

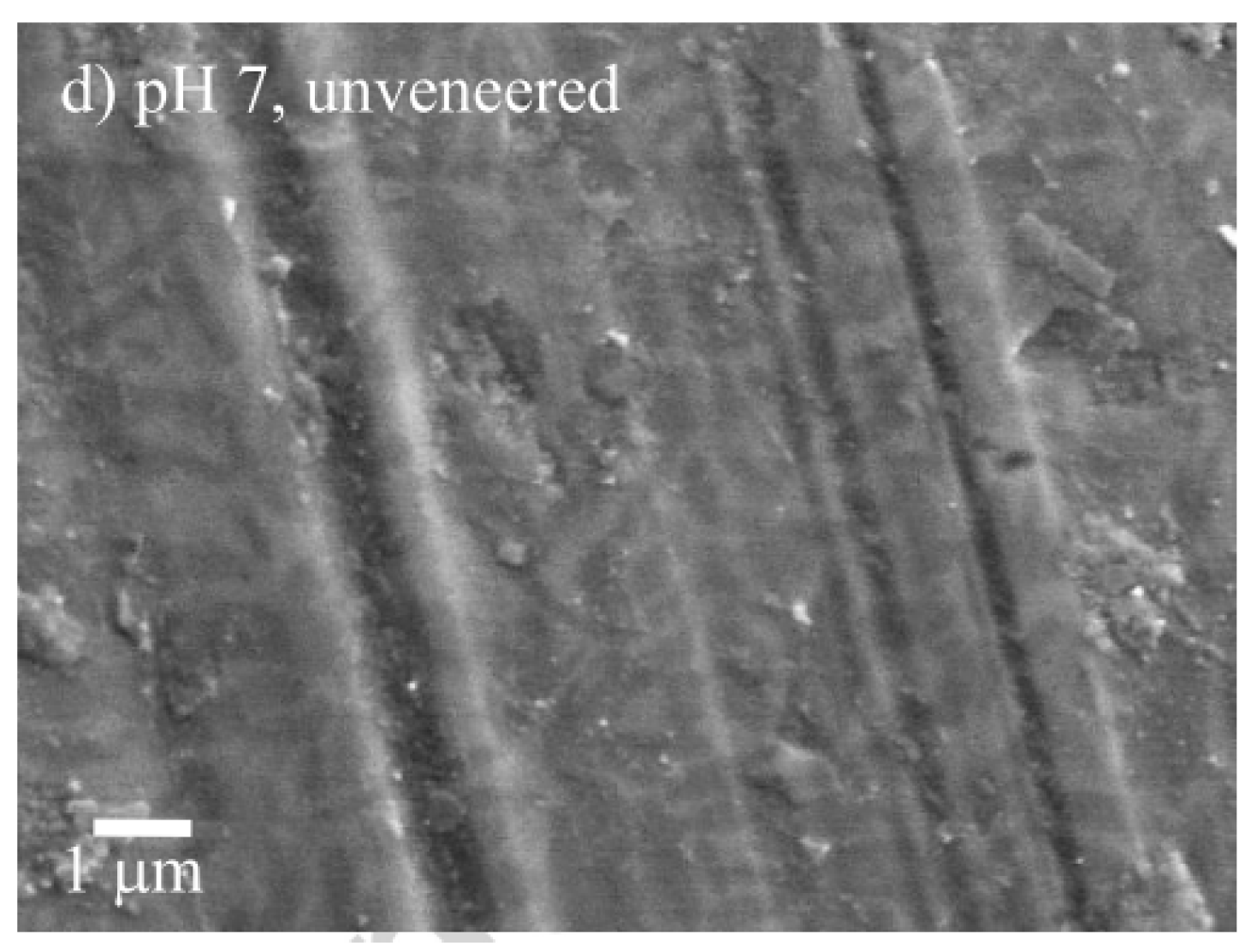
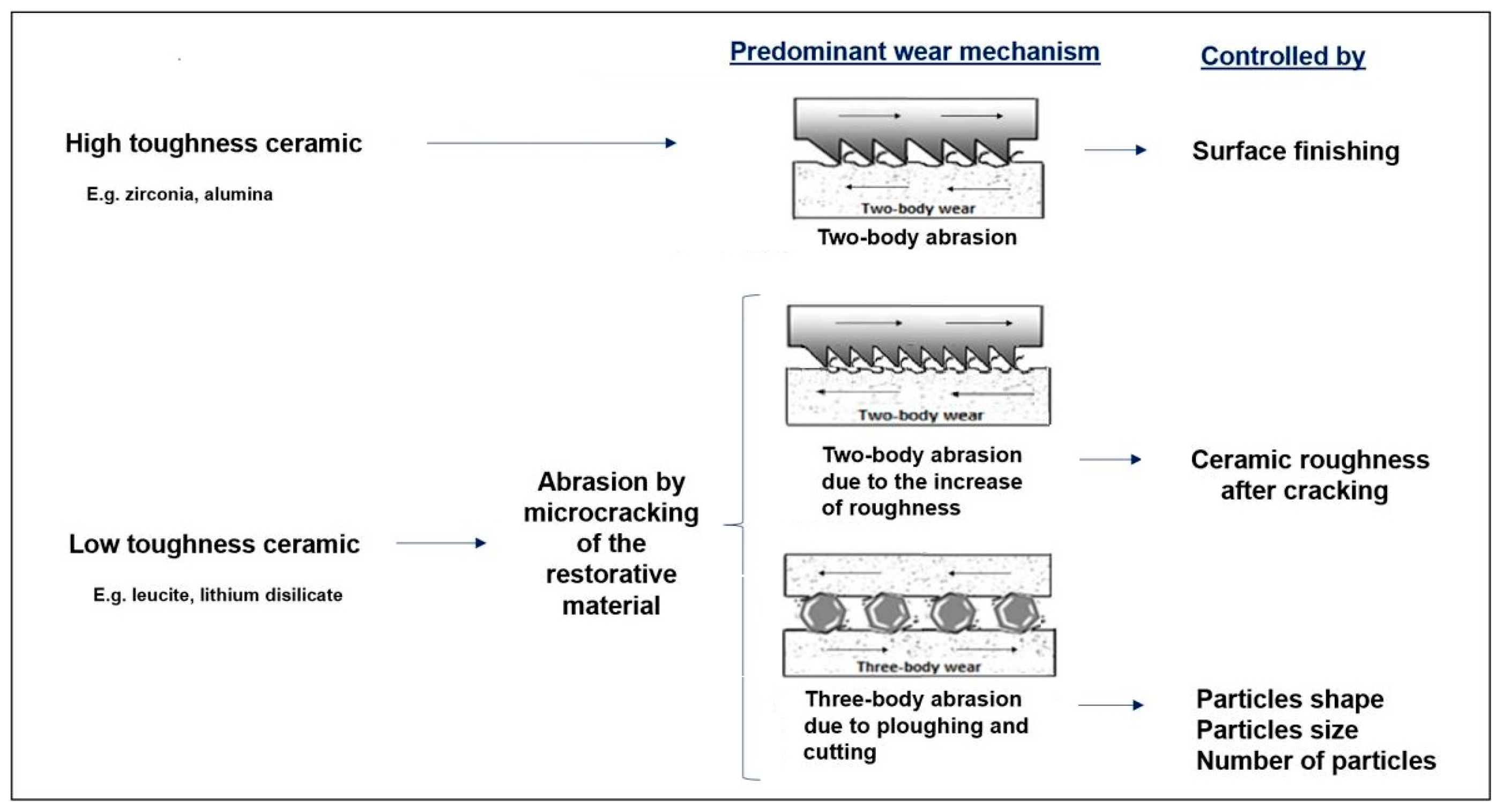
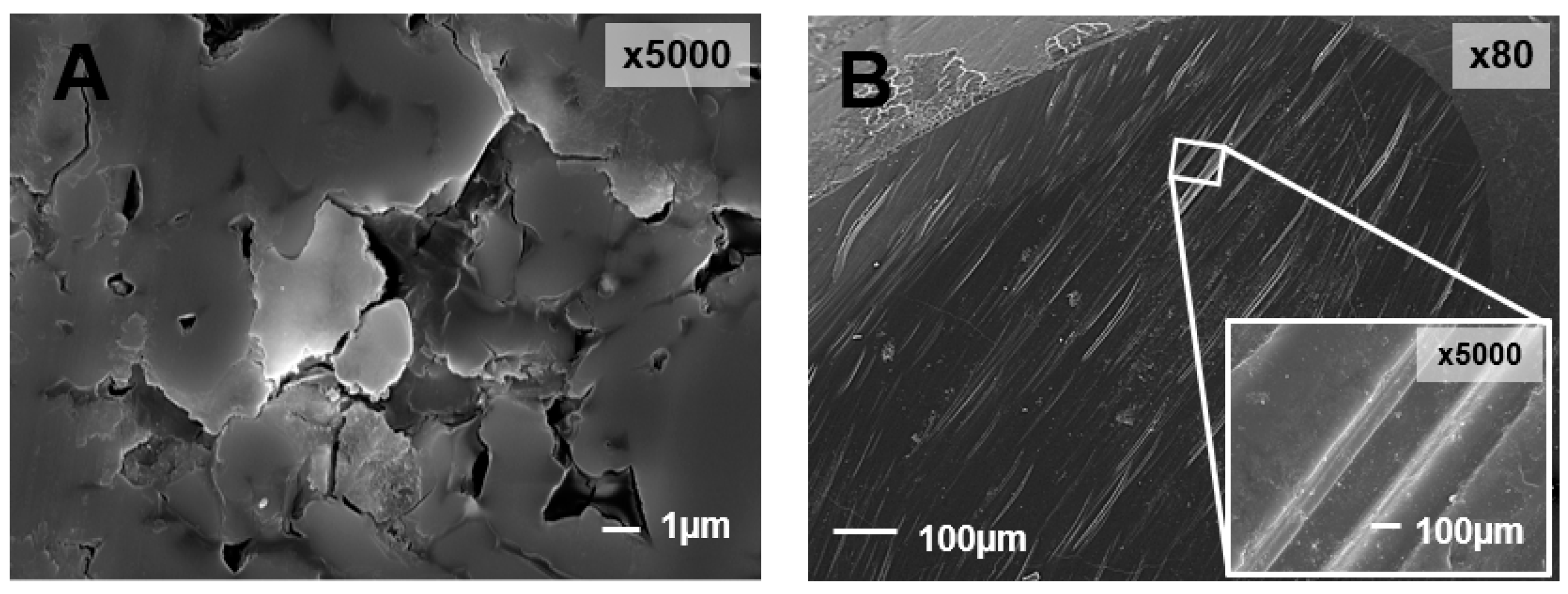
| Ref. | Tribological Pair | Enamel Wear | Restorative Material Wear |
|---|---|---|---|
| [33] | enamel/enamel | Premolars: 20 µm/year Molars: 40 µm/year | - |
| [34] | PFM ceramic/enamel | Premolars: 69.20 ± 4.10 µm/year Molars: 179.70 ± 8.09 µm/enamel | - |
| zirconia/enamel | Premolars: 42.10 ± 4.30 µm/year Molars: 127.00 ± 5.03 µm/enamel | - | |
| enamel/enamel | Premolars: 17.30 ± 1.88 µm/year Molars: 35.10 ± 2.60 µm/enamel | - | |
| [45] | lithium disilicate/enamel | Premolars: 0.21 ± 0.06 mm3/year Molars: 0.50 ± 0.22 mm3/year | Premolars: 0.19 ± 0.065 mm3/year Molars: 0.34 ± 0.08 mm3/year |
| [46] | lithium disilicate/enamel | 0.26 ± 0.17 mm3/6 months | 0.27 ± 0.16 mm3/6 months |
| zirconia reinforced lithium silicate/enamel | 0.28 ± 0.14 mm3/6 months | 0.14 ± 0.14 mm3/6 months | |
| [47] | microhybrid composite resin (Z100)/enamel | 0.2 ± 0.1 mm3/3 years | 0.4 ± 0.2 mm3/3 years |
| nanocomposite resin (Filtek Supreme)/enamel | 0.1 ± 0.1 mm3/3 years | 0.3 ± 0.1 mm3/3 years | |
| [48] | nanohybrid composite resin (Grandio)/enamel | 135 ± 104 µm | 108 ± 88 µm |
| fine hybrid resin composite (Tetric Ceram) /enamel | 110 ± 58 µm | 98 ± 53 µm | |
| [49] | nanofilled restoration (Filtek Supreme)/enamel | 0.31 ± 0.1 mm3/5 years | 0.82 ± 0.2 mm3/5 years |
| microhybrid restoration (Z100) | 0.47 ± 0.2 mm3/5 years | 1.04 ± 0.9 mm3/5 years |
| Ref. | Type of Test | Operational Conditions | Lubricant | Tribological Pair | Enamel Wear | Restorative Material Wear | Wear Mechanisms and Features | |
|---|---|---|---|---|---|---|---|---|
| Enamel | Restorative Material | |||||||
| [35] | Pin-on-disc | 25 N 4800 cycles 20 rpm | Distilled water | enamel/enamel | 8.81 ± 5.16 µm | - | Delamination | Delamination |
| enamel/zirconia | 1.83 ± 0.75 µm | - | Delamination | Few scratches | ||||
| enamel/lithium disilicate | 7.32 ± 2.06 µm | - | Abrasion | Massive fracture | ||||
| enamel/composite resin | 1.37 ± 0.81 µm | - | Polishing | Abrasion (?) | ||||
| [50] | Pin-on-plate | 1.96 N 21,600 cycles 1 Hz Stroke: 3 mm | Artificial saliva (pH = 3 & 7) | unveneered lithium disilicate/enamel (pH = 3) | 1.3 × 10−3 mm3/Nm | 0.2 × 10−3 mm3/Nm | Abrasion/ polishing | Microfracture |
| unveneered lithium disilicate/enamel (pH = 7) | 2.5 × 10−3 mm3/Nm | 0.1 × 10−3 mm3/Nm | Abrasion and tribolayer formation | Microfracture and dental transfer | ||||
| veneered lithium disilicate/enamel (pH = 3) | 1.75 × 10−3 mm3/Nm | 0.3 × 10−3 mm3/Nm | Abrasion/ polishing | Microfracture | ||||
| veneered lithium disilicate/enamel (ph = 7) | 2.9 × 10−3 mm3/Nm | 0.5 × 10−3 mm3/Nm | Abrasion and tribolayer formation | Microfracture and dental transfer | ||||
| [39] | Cycling machine | 40 N 25,000 cycles | A: Citric acid (pH = 4) W: control group | enamel/enamel | W:0.6 ± 0.4 mm2 | - | Chipping | Chipping |
| A: 1.2 ± 0.6 mm2 | ||||||||
| enamel/amalgam | W:0.3 ± 0.3 mm2 | - | - | Dental transfer | ||||
| A: 0.5 ± 0.4 mm2 | ||||||||
| enamel/conventional composite | W:0.7 ± 0.5 mm2 | - | - | Scratching and pull-out (?) | ||||
| A: 1.2 ± 0.7 mm2 | ||||||||
| enamel/microfilled composite | W:0.4 ± 0.4 mm2 | - | - | Scratching (?) | ||||
| A: 0.5 ± 0.6 mm2 | ||||||||
| enamel/glazed porcelain | W:1.2 ± 0.6 mm2 | - | - | Abrasion (?) | ||||
| A: 1.5 ± 0.4 mm2 | ||||||||
| enamel/unglazed metal-free ceramic | W:0.8 ± 0.6 mm2 | - | - | Abrasion (?) | ||||
| A: 1.3 ± 0.9 mm2 | ||||||||
| [28] | Chewing simulator | 49 N 3600 cycles 1 Hz Stroke: 0.7 mm | Artificial saliva (pH = 7) | Vita Enamic®/enamel | 0.09 ± 0.01 mm3 | 0.24 ± 0.04 mm3 | Abrasion, delamination | Abrasive wear and pull-out |
| zirconia/enamel | 0.08 ± 0.01 mm3 | 0 | Polishing wear | No abrasion | ||||
| leucite/enamel | 0.19 ± 0.01 mm3 | 0.14 ± 0.02 mm3 | Abrasion | Microfracture | ||||
| zirconia veneered/enamel | 0.21 ± 0.02 mm3 | 0.19 ± 0.09 mm3 | Abrasion | Microfracture | ||||
| [51] | Chewing simulator | 5 kg 240,000 cycles 0.8 Hz Stroke: 0.3 mm | Water (5 °C/55 °C) | polished feldspathic porcelain/enamel | 0.119 ± 0.059 mm3 | - | - | - |
| polished zirconia/enamel | 0.031 ± 0.033 mm3 | - | - | - | ||||
| polished glazed zirconia/enamel | 0.078 ± 0.063 mm3 | - | - | - | ||||
| [9] | Chewing simulator | 4 N 5000 cycles 2 Hz Stroke: 1 mm | Artificial saliva | polished zirconia/enamel | 200–300 µm | - | Fatigue and adhesive wear | - |
| rough zirconia/enamel | 200–300 µm | - | Abrasive and fatigue wear | - | ||||
| lithium disilicate/enamel | 600 µm | - | Abrasive wear | - | ||||
| porcelain/enamel | 500 µm | - | Abrasive wear | - | ||||
| Au-Pd/enamel | 100 µm | - | Adhesive wear | - | ||||
| Ni-Cr/enamel | 100 µm | - | Fatigue and adhesive wear | - | ||||
| [52] | Chewing simulator | 50 N 100,000 1.6 Hz Stroke: 2 mm | Artificial saliva (5 °C/55 °C) | zirconia/enamel | 2.66 ± 0.65 mm3 | - | - | - |
| lithium disilicate/enamel | 3.84 ± 0.7 mm3 | - | - | - | ||||
| resin nanoceramic/ enamel | 3.48 ± 0.71 mm3 | - | - | - | ||||
| nanohybrid composite resin/enamel | 3.68 ± 0.76 mm3 | - | - | - | ||||
| primary tooth enamel/enamel | 1.66 ± 0.42 mm3 | - | - | - | ||||
| [53] | Two-body wear testing device | 75 N 100,000 cycles 1.2 Hz | Water | lithium disilicate glass/enamel | 0.33 ± 0.12 mm3 | 0.10 ± 0.03 mm3 | - | - |
| leucite-reinforced glass/enamel | 0.42 ± 0.09 mm3 | 0.11 ± 0.02 mm3 | - | - | ||||
| zirconia/enamel | 0.07 ± 0.03 mm3 | 0.23 × 10-3 ± 0.18 × 10−3 mm3 | - | - | ||||
| feldspathic porcelain/enamel | 0.62 ± 0.27 mm3 | 0.05 ± 0.03 mm3 | - | - | ||||
| enamel/enamel | 0.40 ± 0.16 mm3 | 0.08 ± 0.08 mm3 | - | - | ||||
| [54] | Chewing simulator | 50 N 100,000 cycles 0.8 Hz Stroke: 2 mm | Water (5 °C/55 °C) | zirconia/primary enamel | 1.426 ± 0.477 mm3 | 0.002 ± 0.001 mm3 | Mild abrasion | No considerable features |
| lithium disilicate/primary enamel | 2.042 ± 0.696 mm3 | 0.006 ± 0.002 mm3 | Abrasion | Microcracking/ abrasion | ||||
| leucite/primary enamel | 2.670 ± 1.471 mm3 | 0.003 ± 0.002 mm3 | Abrasion | Microcracking/ abrasion | ||||
| stainless steel/primary enamel | 0.397 ± 0.192 mm3 | 0.002 ± 0.001 mm3 | Abrasion | Plastic deformation and abrasion by ploughing | ||||
| [55] | Pin-on-plate | 9.8 N 1100 cycles 1.6 Hz Stroke: 0.2 mm | Distilled water | lithium disilicate/enamel | - | - | Abrasive wear | Adhered enamel layer |
| type III gold/enamel | - | - | Adhesive wear | Polished surface | ||||
| [56] | Chewing simulator | 75 N 100,000 cycles 1.2 Hz | Distilled water | Dicor MGC Light /enamel | 0.024 ± 0.014 mm2 | 0.153± 0.049 mm2 | - | - |
| Vita Mark II/enamel | 0.078 ± 0.041 mm2 | 0.140 ± 0.02 mm2 | - | - | ||||
| IPS Empress/enamel | 0.089 ± 0.045 mm2 | 0.116 ± 0.038 mm2 | - | - | ||||
| cast type III gold/enamel | 0.019 ± 0.025 mm2 | 0.067 ± 0.036 mm2 | - | - | ||||
| [57] | Pin-on-disc | 5 N 10,000 cycles | Human saliva | Olympia gold/enamel | 9 ± 13 µm | 0.32 ± 0.1 µm | - | - |
| Procera All-Ceramic/enamel | 60 ± 28 µm | 4.3 ± 2.3 µm | - | - | ||||
| Ceramco feldspathic porcelain/enamel | 230 ± 38 µm | 3.7 ± 0.6 µm | - | - | ||||
| [58] | Pin-on-disc | 40 N 25,000 cycles Stroke: 10 mm | Distilled water | Alpha porcelain/enamel | 0.93 ± 0.15 mm | 76.04 ± 12.39 mm | - | - |
| Omega porcelain/enamel | 0.96 ± 0.20 mm | 62.02 ± 20.85 mm | - | - | ||||
| Duceram-LFC/enamel | 0.54 ± 0.15 mm | 41.88 ± 17.36 mm | - | - | ||||
| Vita Mark II/enamel | 0.65 ± 0.16 mm | 25.86 ± 10.52 mm | - | - | ||||
| gold/enamel | 0.09 ± 0.03 mm | 16.28 ± 5.59 mm | - | - | ||||
| [59] | Pin-on-plate | 15 N 200,000 cycles 1 Hz | Water | zirconia/enamel | 1 ± 0.2 µm | - | - | Adhesion of enamel particles |
| stainless steel/enamel | 0.6 ± 0.4 µm | - | - | Adhesion of enamel particles | ||||
| [60] | Chewing simulator | 49 N 200,000 cycles Stroke: 0.3 mm | Water | feldspathic ceramic/enamel | 0.067 ± 0.018 mm3 | - | - | Abrasion/ delamination |
| nano-filled composite resin/enamel | 0.016 ± 0.006 mm3 | - | - | Pull-out | ||||
| acrylic resin/enamel | 0.093 ± 0.021 mm3 | - | - | Pull-out and fatigue wear | ||||
| [61] | Pin-on-disc | 9.8 N 100 rpm Stroke: 100 m | Distilled water | Lava Zirconia/enamel | ~51 µm | - | - | No features |
| Vintage MP veneering porcelain/enamel | ~425 µm | - | - | Delamination | ||||
| Cerabien ZR veneering porcelain/enamel | ~450 µm | - | - | Delamination | ||||
| Gradia Direct microhybrid composite resin/enamel | ~85 µm | - | - | Microcracking (low extent) and scratching | ||||
| Filtek Z250 microhybrid composite resin/enamel | ~165 µm | - | - | Microcracking between the filler and the matrix; particles pull-out | ||||
| Filtek Z350 nanocomposite/ enamel | ~100 µm | - | - | Intensive plastic deformation with accumulation of resin particles | ||||
| [62] | Chewing simulator | 49 N 1,200,000 cycles 1.7 Hz | Water (5 °C/50 °C) | veneered zirconia (VZ)/enamel | 73.5 ± 32.8 µm | 66.8 ± 47.5 µm | Delamination | Delamination of the coating |
| glazed zirconia (GZC)/enamel | 118 ± 30.9 µm | 49.5 ± 10.3 µm | - | Spalling of the coating | ||||
| glazed zirconia with glaze spray (GZS)/enamel | 62.2 ± 16.6 µm | 91.3 ± 38.6 µm | - | Spalling of the coating | ||||
| manually polished zirconia (MAZ)/enamel | 27.3 ± 15.2 µm | 0.8 ± 0.8 µm | - | No features | ||||
| mechanically polished zirconia (MEZ)/enamel | 28 ± 11.1 µm | 0.8 ± 0.8 µm | - | Abrasion | ||||
| monolithic base alloy (MA)/enamel | 55.3 ± 38.5 µm | 13.2 ± 8.3 µm | Polished surface | - | ||||
| [63] | Chewing simulator | 75 N 100,000 cycles 1.2 Hz | Water | microfilled composite (Epic-TMPT (Parkell)) | 0.5 × 10−2 mm2 | 4.5 × 10−2 mm3 | - | - |
| hybrid composite resin (Superlux Universal Hybrid (DMG)) | 0.8 × 10−2 mm2 | 4.5 × 10−2 mm3 | - | - | ||||
| Clearfil AP-X (Kuraray Co.) | 1.05 × 10−2 mm2 | 10 × 10−2 mm3 | - | - | ||||
| Charisma (Kulzer Co.) | 1.1 × 10−2 mm2 | 7 × 10−2 mm3 | - | - | ||||
| Conquest Crystal (Jeneric/ Pentron Inc.) | 1.1 × 10−2 mm2 | 6 × 10−2 mm3 | - | - | ||||
| Estio LC (GC Co.) | 1.2 × 10−2 mm2 | 7.5 × 10−2 mm3 | ||||||
| Prisma TPH (L.D. Caulk Co.) | 1 × 10−2 mm2 | 4.5 × 10−2 mm3 | - | - | ||||
| Quartz-filled composite resin (Clearfil Photo Posterior (KurarayCo.) | 4.05 × 10−2 mm2 | 11 × 10−2 mm3 | - | - | ||||
| Zirconium silicate filled composite (Z100) | 3.2 × 10−2 mm2 | 13.5 × 10−2 mm3 | - | - | ||||
| Zirconium silicate filled composite (P-50) | 5.1 × 10−2 mm2 | 17.5 × 10−2 mm3 | - | - | ||||
| gold alloy | 1.6 × 10−2 mm2 | 3 × 10−2 mm3 | - | - | ||||
| [64] | Chewing simulator | 49 N | Water (5 °C–55 °C) | zirconia (Prettau)/enamel | 0.04 ± 0.02 mm3 | 0.04 mm3 | - | Dental particles transfer (?) |
| zirconia (Lava)/enamel | 0.04 ± 0.02 mm3 | 0.042 mm3 | - | Dental particles transfer (?) | ||||
| zirconia (Rainbow)/enamel | 0.04 ± 0.02 mm3 | 0.04 mm3 | - | Dental particles transfer (?) | ||||
| lithium disilicate (e.max Press)/enamel | 0.06 ± 0.03 mm3 | 0.08 mm3 | - | Microfracture Dental particles transfer (?) | ||||
| low fusing porcelain (Vita-Omega 900)/enamel | 0.11 ± 0.03 mm3 | 0.013 mm3 | - | Microfracture Dental particles transfer (?) | ||||
| [65] | Chewing simulator | 50 N 360,000 cycles 1 Hz Stroke: 0.7 mm | Artificial saliva | zirconia (zirkonzahn)/enamel | 6.4 ± 1.5 (×10−5) mm3/Nm | - | Abrasion, adhesive wear | Abrasion, adhesive wear |
| glazed zirconia (zirkonzahn)/enamel | 8.3 ± 1.2 (×10−5) mm3/Nm | 0.5 ± 0.05 (×10−5) mm3/Nm | Abrasion, adhesive wear | Abrasion, adhesive wear, microfracture | ||||
| [66] | Pin-on-disc | 40 N 1500 revolutions 150 r/min | Natural Saliva (S) Food slurry (F) | fluorapatite/enamel | S: ~1.2 mm3 | S: ~0.8 mm3 | Abrasion, delamination, adhesive wear | Abrasion, delamination, adhesive wear |
| F: ~0.01 mm3 | F: ~0.01 mm3 | Abrasion | Abrasion | |||||
| feldspar/enamel | S: ~1.25 mm3 | S: ~1 mm3 | Abrasion, delamination, adhesive wear, | Abrasion, delamination, adhesive wear | ||||
| F: ~0.01 mm3 | F: ~0.01 mm3 | Microcracking, abrasion | Abrasion | |||||
| Ref. | Type of Test | Operational Conditions | Lubricant | Restorative Material | Counterbody | Restorative Material Wear | Restorative Material Wear Mechanisms |
|---|---|---|---|---|---|---|---|
| [90] | Ball-on-3-flat tribometer | 30 N 25 rpm 1 h testing Stroke: 37 m | Artificial saliva | zirconia-Zpex (3Y-PSZ) | zirconia (3Y-TZP) ball | 2.7 × 10−6 mm3/N·m | Abrasive wear |
| zirconia-Zpex Smile (5Y-PSZ) | 3.1 × 10−6 mm3/N·m | - | |||||
| zirconia-Zpex (graded) | 3.3 × 10−6 mm3/N·m | - | |||||
| lithium disilicate (IPS e.max CAD) | 1.2 × 10−4 mm3/N·m | Abrasive wear, microfracture | |||||
| feldspathic ceramic (Vitablocs) | 5.5 × 10−5 mm3/N·m | - | |||||
| ceramic–polymer composites—Enamic | 3.7 × 10−5 mm3/N·m | Abrasive wear, pull-out, fatigue | |||||
| ceramic–polymer composites—Lava Ultimate | 7.7 × 10−5 mm3/N·m | - | |||||
| [87] | Chewing simulator | 49 N 120,000 cycles 1.6 Hz Stroke: 0.7 mm | Water | type III gold alloy | zirconia cusp | 0.331 ± 0.138 mm3 | - |
| hot pressed ceramic (Imagine PressX) | 0.508 ± 0.150 mm3 | - | |||||
| hot pressed ceramic (IPS e.max Press) | 0.459 ± 0.137 mm3 | - | |||||
| CAD/CAM ceramic (IPS e.max CAD) | 0.355 ± 0.133 mm3 | - | |||||
| CAD/CAM ceramic (Celtra Duo) | 0.542 ± 0.115 mm3 | - | |||||
| CAD/CAM feldspathic porcelain (Vitablocs Mark II) | 0.472 ± 0.133 mm3 | - | |||||
| [91] | Chewing simulator | 200 N 200,000 cycles 1 Hz Stroke: 2 mm | 33% glycerin solution | cross-linked PMMA (DCL) | zirconia cusp | 17.3 ± 1.0 mm3 | Abrasion, microfatigue (?) |
| cross-linked acrylate polymer (ZCAD) | 14.3 ± 0.8 mm3 | Abrasion, microfatigue (?) | |||||
| cross-linked PMMA (TEL) | 11.9 ± 2.0 mm3 | Abrasion, microfatigue (?) | |||||
| nano-hybrid composite resin (PHO) | 4.3 ± 1.0 mm3 | Abrasion | |||||
| [88] | Chewing simulator | 49 N 120,000 cycles 1.6 Hz Stroke: 0.7 mm | Water | type III gold alloy (Aurocast8) | zirconia cusp | 0.328 ± 0.140 mm3 | - |
| resin composite (Enamel plus HRi) light (L) and heat (H) cured | L: 1.452 ± 0.245 mm3 H: 1.016 ± 0.198 mm3 | - | |||||
| resin composite (Filtek Supreme XTE) light (L) and heat (H) cured | L: 0.972 ± 0.247 mm3 H: 1.017 ± 0.239 mm3 | - | |||||
| resin composite (Ceram.X duo) light (L) and heat (H) cured | L: 0.894 ± 0.259 mm3 H: 0.806 ± 0.397 mm3 | - | |||||
| microhybrid resin composite (Enamel plus HRi-Function) light (L) and heat (H) cured | L: 0.529 ± 0.139 mm3 H: 0.464 ± 0.191 mm3 | - | |||||
| [93] | Chewing simulator | 49 N 600,000 cycles 1.3 Hz Stroke: 0.3 mm | Water (5 °C–55 °C) | nanofilled composite resin | zirconia cusp | 0.048 ± 0.017 mm3 | Abrasion, delamination |
| feldspathic ceramic | 0.056 ± 0.008 mm3 | Abrasion, microcracking | |||||
| nanofilled composite resin | alumina cusp | 0.033 ± 0.013 mm3 | Abrasion, delamination | ||||
| feldspathic ceramic | 0.050 ± 0.018 mm3 | Abrasion, microcracking | |||||
| [94] | Ball-on-plate | 30 N 1 Hz Stroke: 2 mm | Artificial saliva | zirconium-lithium silicate glass-ceramic | alumina ball | 3.17 × 10−5 mm3/N·m | Abrasion, microcracking, thin and almost absent layer of debris |
| polymer-infiltrated ceramic network | 5.33 × 10−5 mm3/N·m | Thick and unstable tribolayer | |||||
| [95] | Chewing simulator | 50 N 360,000 cycles 1.2 Hz | Water | nanofilled composite resin (Filtek silorane) | alumina cusp | 6.4 µm3 | - |
| microfilled composite resin (Ivoclar heliomolar) | 3.1 µm3 | - | |||||
| nanohybrid composite resin (Voco Grandio) | 3.7 µm3 | - | |||||
| [64] | Chewing simulator | 49 N | Water (5 °C–55 °C) | zirconia (Lava) | feldspathic porcelain cusp | 0.027 mm3 | - |
| zirconia (Rainbow) | 0.02 mm3 | - | |||||
| lithium disilicate (e.max Press) | 0.055 mm3 | - | |||||
| low fusing porcelain (Vita-Omega 900) | 0.028 mm3 | - | |||||
| [96] | Wear simulation device | 78.5 N 1,200,000 cycles 2 Hz | Esthet X (EX) | stainless-steel cylinder | 1.162 ± 0.139 mm3 | - | |
| Filtek Supreme Plus (SP) | 0.541 ± 0.072 mm3 | - | |||||
| Filtek Z250 (Z2) | 0.477 ± 0.044 mm3 | - | |||||
| Tetric EvoCeram (EC) | 0.584 ± 0.037 mm3 | - | |||||
| Z100 Restorative (Z1) | 0.248 ± 0.036 mm3 | - | |||||
| [97] | Wear simulator | 250 000 cycles | Water | microhybrid composite (Filtek Z250) | CoCr alloy cusp | 0.110 mm3 | - |
| type III gold alloy | 0.021 mm3 | - | |||||
| porcelain | 0.006 mm3 | - | |||||
| [92] | Pin-on-disc | 50 N 10,000 cycles 1.2 Hz Stroke: 3.7 mm | Water (W) 33% mass Poppy seeds (P) 30% mass PMMA beads (PMMA) | microfilled composite (Durafill) | zirconia ball | W: 0.1 mm3 P: 1.6 mm3 PMMA: 0.55 mm3 | Abrasive wear, Microfatigue |
| hybrid composite (Clearfil AP-X) | W: 1.25 mm3 P: 0.2 mm3 PMMA: 1.4 mm3 | ||||||
| microhybrid composite (Filtek Z250) | W: 2.05 mm3 P: 0.15 mm3 PMMA: 0.5 mm3 | ||||||
| nanofilled composite (Filtek Supreme XT) | W: 2.1 mm3 P: 0.15 mm3 PMMA: 0.4 mm3 | ||||||
| nanohybrid composite (GC Kalore) | W: 0.15 mm3 P: 0.4 mm3 PMMA: 1.45 mm3 | ||||||
| nanohybrid composite (MI flow) | W: 0.15 mm3 P: 0.5 mm3 PMMA: 1.2 mm3 | ||||||
| nanohybrid composite (Venus Diamond) | W: 0.95 mm3 P: 0.35 mm3 PMMA: 2.05 mm3 | ||||||
| nanohybrid composite (Venus Pearl) | W: 0.7 mm3 P: 0.15 mm3 PMMA: 2 mm3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branco, A.C.; Colaço, R.; Figueiredo-Pina, C.G.; Serro, A.P. A State-of-the-Art Review on the Wear of the Occlusal Surfaces of Natural Teeth and Prosthetic Crowns. Materials 2020, 13, 3525. https://doi.org/10.3390/ma13163525
Branco AC, Colaço R, Figueiredo-Pina CG, Serro AP. A State-of-the-Art Review on the Wear of the Occlusal Surfaces of Natural Teeth and Prosthetic Crowns. Materials. 2020; 13(16):3525. https://doi.org/10.3390/ma13163525
Chicago/Turabian StyleBranco, Ana Catarina, Rogério Colaço, Célio Gabriel Figueiredo-Pina, and Ana Paula Serro. 2020. "A State-of-the-Art Review on the Wear of the Occlusal Surfaces of Natural Teeth and Prosthetic Crowns" Materials 13, no. 16: 3525. https://doi.org/10.3390/ma13163525







