Biocompatibility and Bioactivity of Set Direct Pulp Capping Materials on Human Dental Pulp Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture of Human Dental Pulp Stem Cells (hDPSCs)
2.2. Production of Experimental Pulp Capping Material Disks
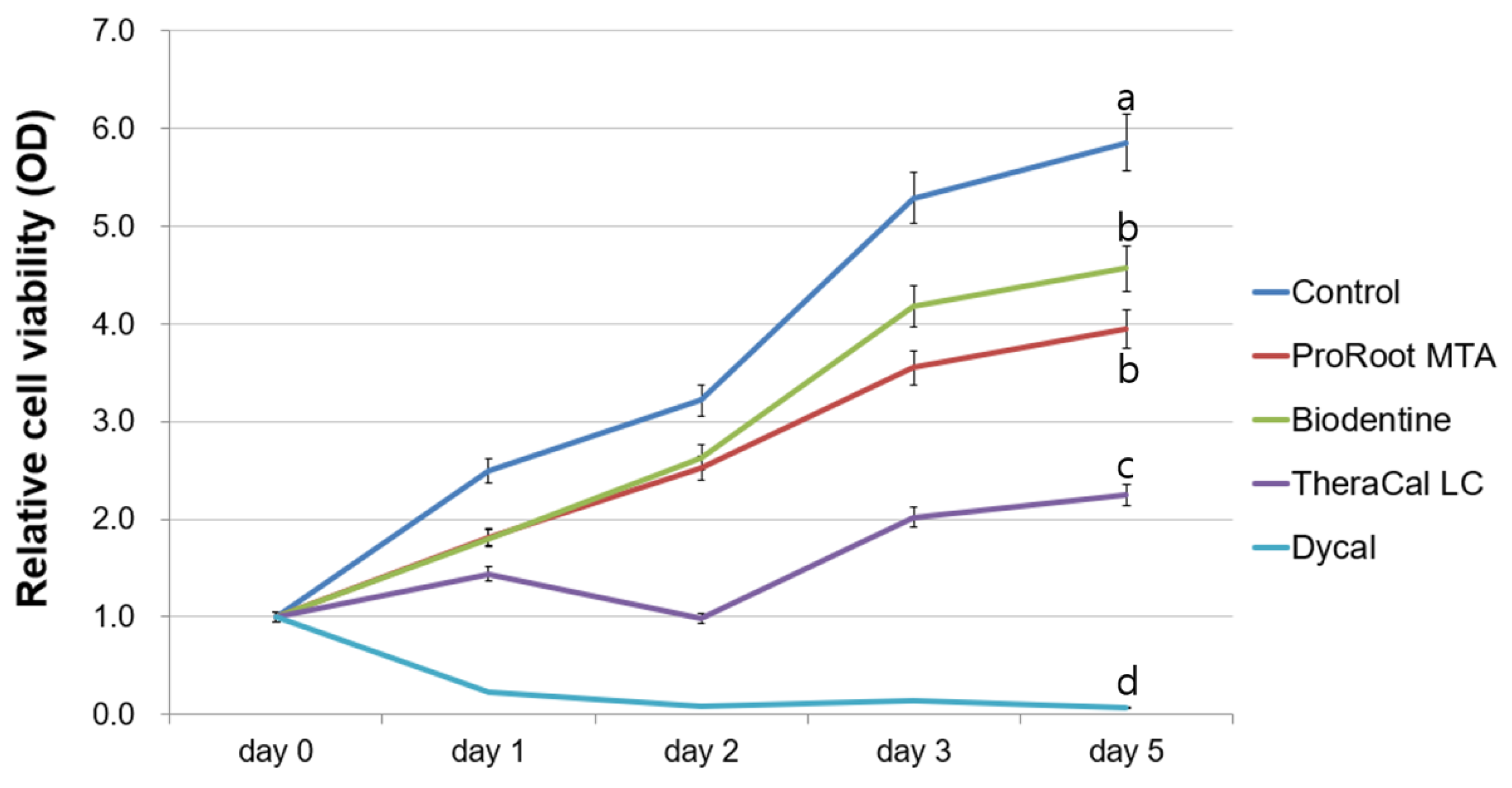
2.3. Evaluation of Cell Viability
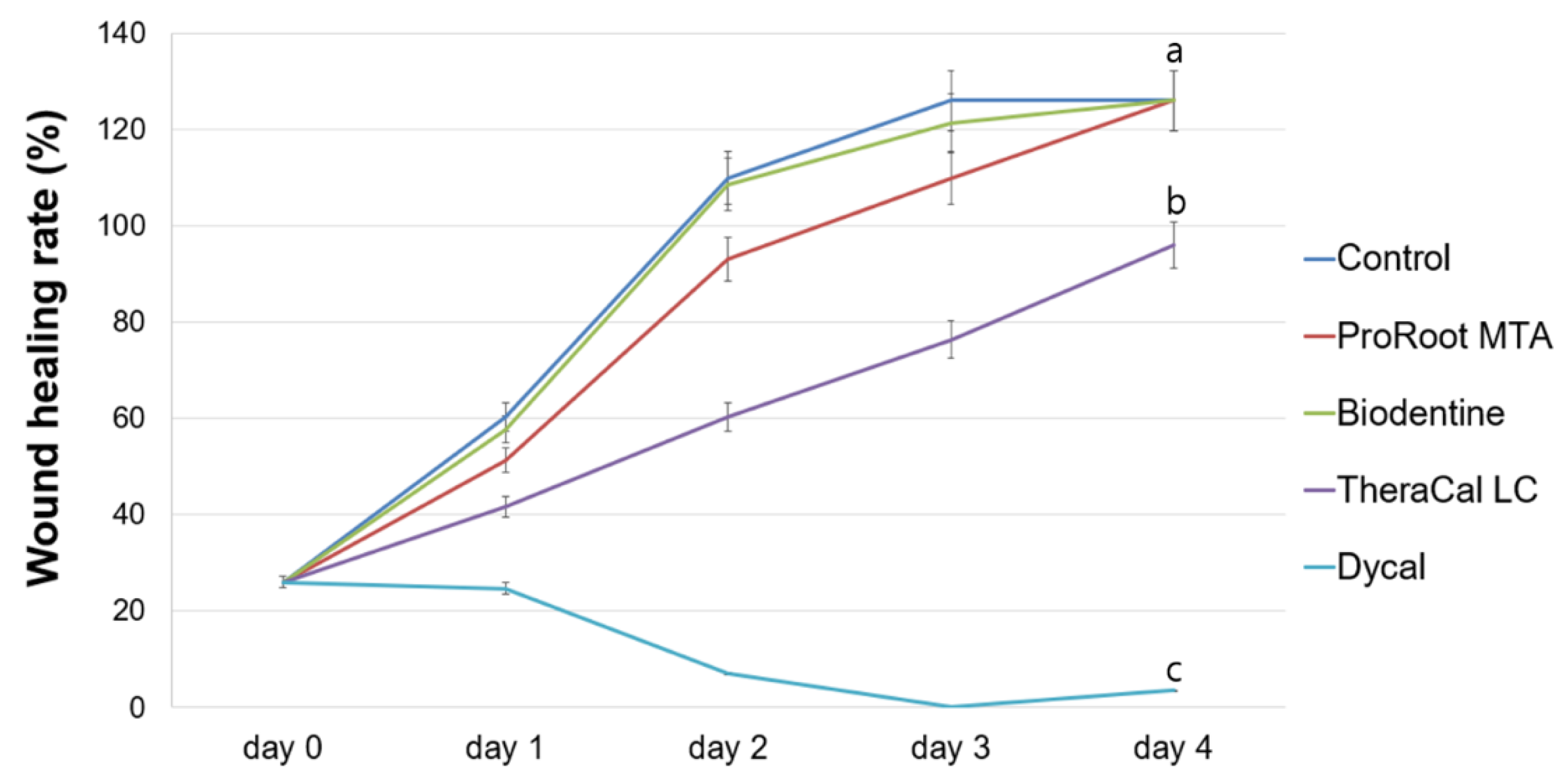
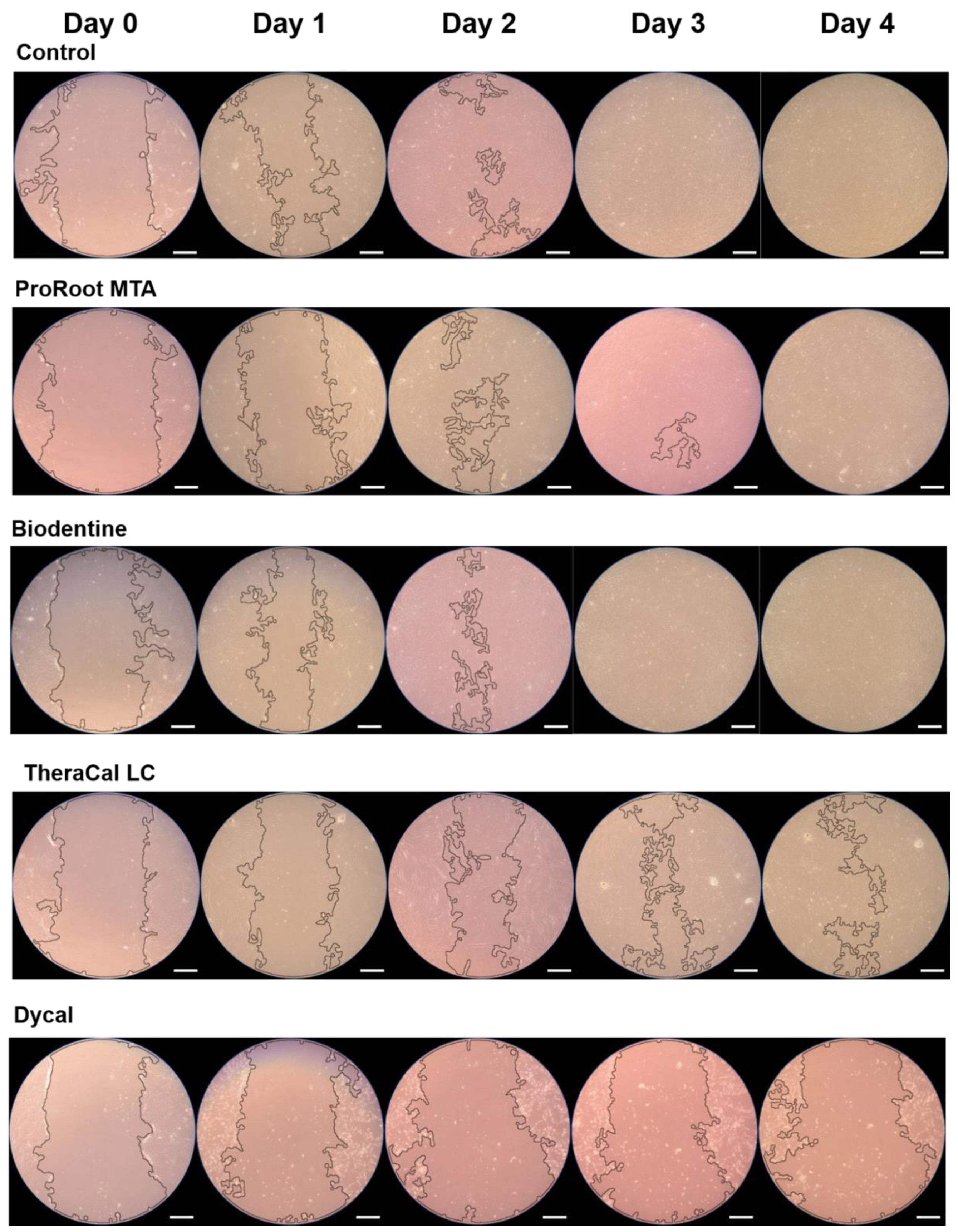
2.4. Evaluation of Cell Migration
2.5. Determination of Alkaline Phosphatase Enzyme (ALP) Activity
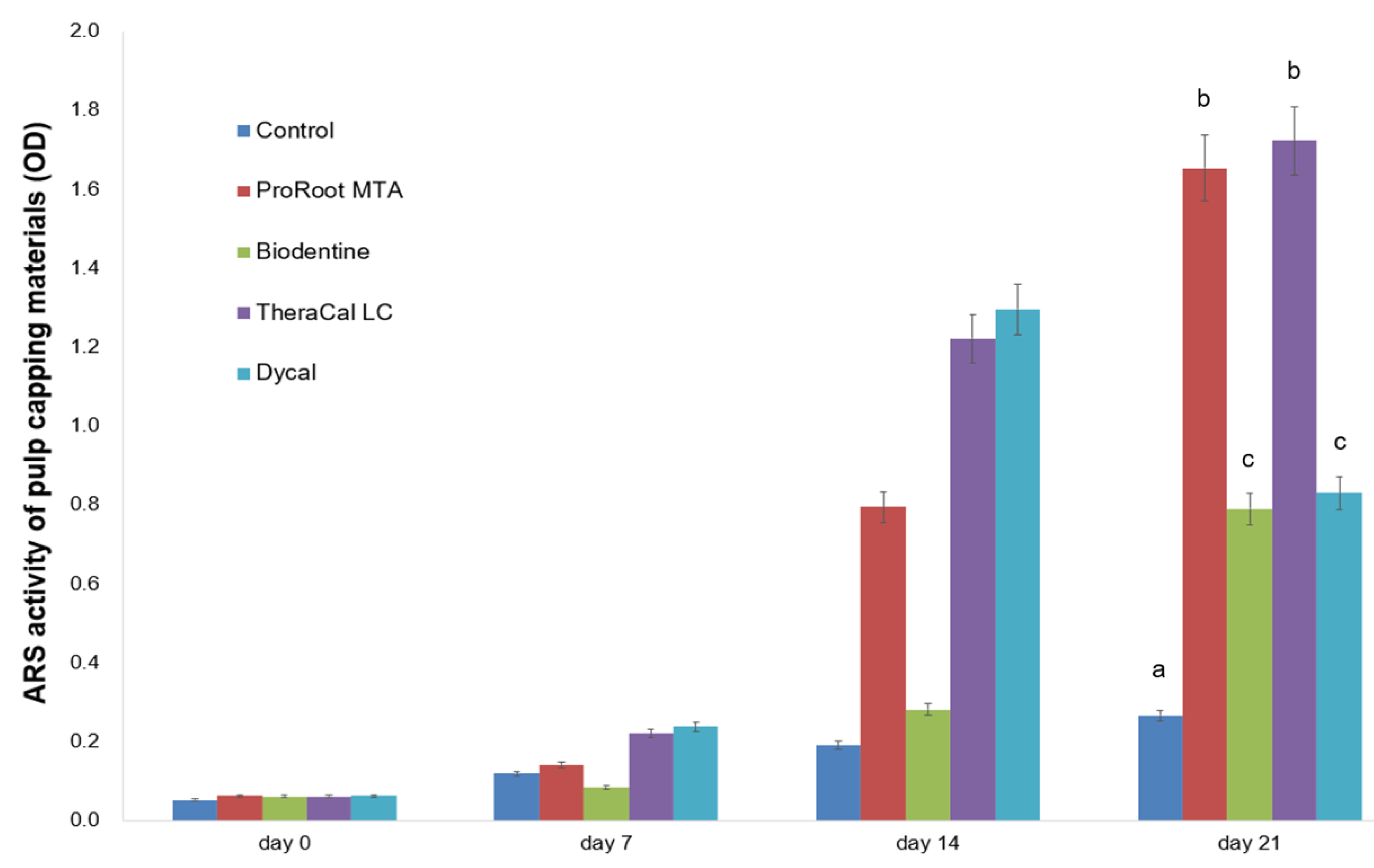
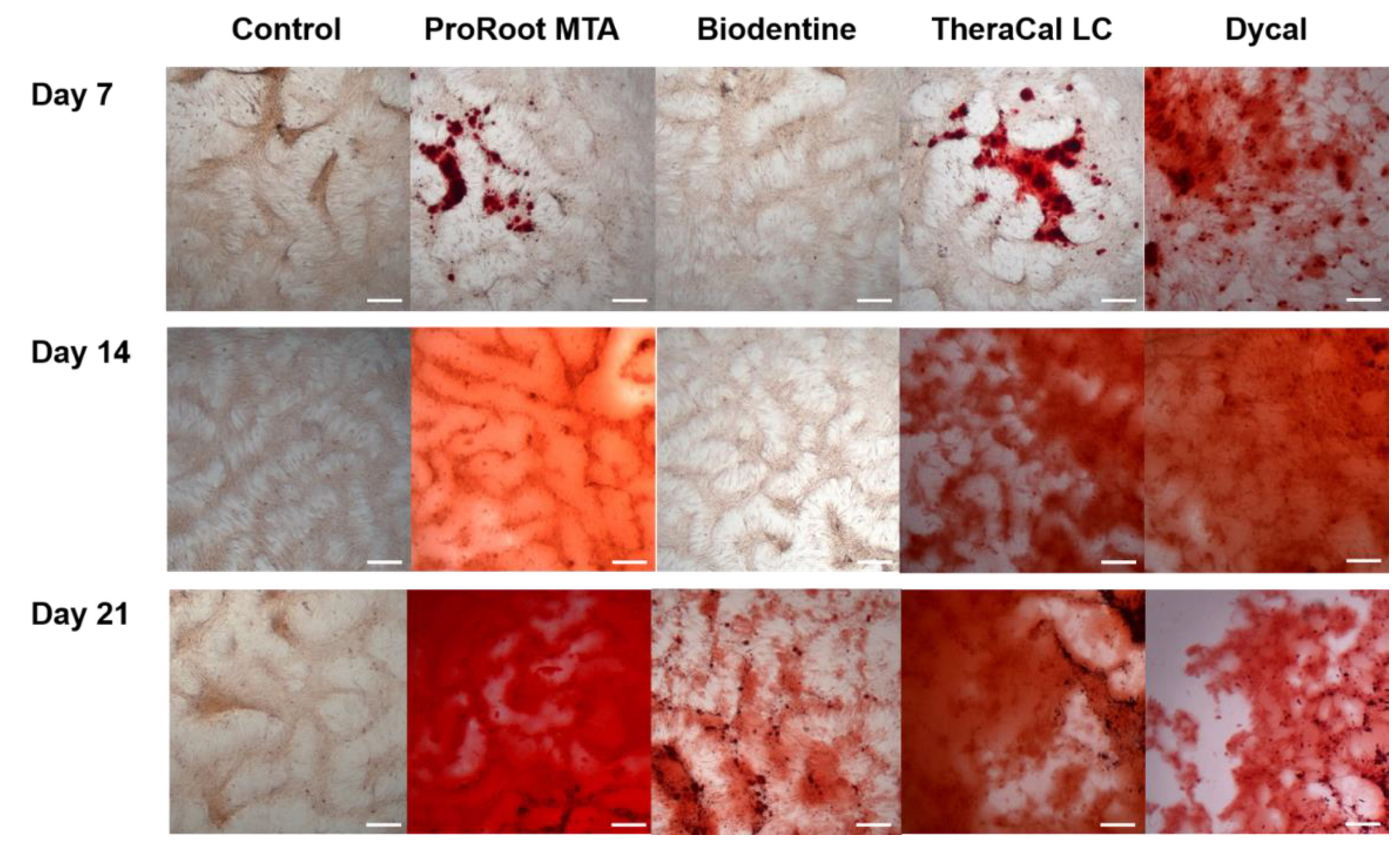
2.6. Determination of Alizarin Red S (ARS) Staining Assay
2.7. Alizarin Red S (ARS) Staining Assay of Various Biodentine Eluates
2.8. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, L.; Suh, B.I. Cytotoxicity and biocompatibility of resin-free and resin-modified direct pulp capping materials: A state-of-the-art review. Dent. Mater. J. 2017, 36, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Dong, Y.; Yang, Y.W.; Lin, P.T.; Yu, H.H.; Sun, X.; Sun, X.F.; Zhou, H.; Huang, L.; Chen, J.H. Effect of an Experimental Direct Pulp-capping Material on the Properties and Osteogenic Differentiation of Human Dental Pulp Stem Cells. Sci. Rep. 2016, 6, 34713. [Google Scholar] [CrossRef] [PubMed]
- Komabayashi, T.; Zhu, Q.; Eberhart, R.; Imai, Y. Current status of direct pulp-capping materials for permanent teeth. Dent. Mater. J. 2016, 35, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitasako, Y.; Ikeda, M.; Tagami, J. Pulpal responses to bacterial contamination following dentin bridging beneath hard-setting calcium hydroxide and self-etching adhesive resin system. Dent. Traumatol. 2008, 24, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Parirokh, M. Mineral trioxide aggregate: A comprehensive literature review--part II: Leakage and biocompatibility investigations. J. Endod. 2010, 36, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Parirokh, M.; Torabinejad, M. Mineral trioxide aggregate: A comprehensive literature review--Part III: Clinical applications, drawbacks, and mechanism of action. J. Endod. 2010, 36, 400–413. [Google Scholar] [CrossRef]
- Edrees, H.Y.; Abu Zeid, S.T.H.; Atta, H.M.; AlQriqri, M.A. Induction of Osteogenic Differentiation of Mesenchymal Stem Cells by Bioceramic Root Repair Material. Materials 2019, 12, 2311. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.Y.; Seo, D.G.; Lee, S.J.; Lee, J.; Lee, S.J.; Jung, I.Y. Prognostic factors for clinical outcomes according to time after direct pulp capping. J. Endod. 2013, 39, 327–331. [Google Scholar] [CrossRef]
- Mente, J.; Hufnagel, S.; Leo, M.; Michel, A.; Gehrig, H.; Panagidis, D.; Saure, D.; Pfefferle, T. Treatment outcome of mineral trioxide aggregate or calcium hydroxide direct pulp capping: Long-term results. J. Endod. 2014, 40, 1746–1751. [Google Scholar] [CrossRef]
- Caliskan, M.K.; Guneri, P. Prognostic factors in direct pulp capping with mineral trioxide aggregate or calcium hydroxide: 2- to 6-year follow-up. Clin. Oral Investig. 2017, 21, 357–367. [Google Scholar] [CrossRef]
- Brizuela, C.; Ormeño, A.; Cabrera, C.; Cabezas, R.; Silva, C.I.; Ramírez, V.; Mercade, M. Direct Pulp Capping with Calcium Hydroxide, Mineral Trioxide Aggregate, and Biodentine in Permanent Young Teeth with Caries: A Randomized Clinical Trial. J. Endod. 2017, 43, 1776–1780. [Google Scholar] [CrossRef] [PubMed]
- Parinyaprom, N.; Nirunsittirat, A.; Chuveera, P.; Na Lampang, S.; Srisuwan, T.; Sastraruji, T.; Bua-On, P.; Simprasert, S.; Khoipanich, I.; Sutharaphan, T.; et al. Outcomes of Direct Pulp Capping by Using Either ProRoot Mineral Trioxide Aggregate or Biodentine in Permanent Teeth with Carious Pulp Exposure in 6- to 18-Year-Old Patients: A Randomized Controlled Trial. J. Endod. 2018, 44, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Katge, F.A.; Patil, D.P. Comparative Analysis of 2 Calcium Silicate-based Cements (Biodentine and Mineral Trioxide Aggregate) as Direct Pulp-capping Agent in Young Permanent Molars: A Split Mouth Study. J. Endod. 2017, 43, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Paula, A.; Carrilho, E.; Laranjo, M.; Abrantes, A.M.; Casalta-Lopes, J.; Botelho, M.F.; Marto, C.M.; Ferreira, M.M. Direct Pulp Capping: Which is the Most Effective Biomaterial? A Retrospective Clinical Study. Materials 2019, 12, 3382. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, M.J.; Casey, J.A.; VanderWeele, R.A.; Vandewalle, K.S. Mechanical properties of new dental pulp-capping materials. Gen. Dent. 2016, 64, 44–48. [Google Scholar]
- Gandolfi, M.G.; Siboni, F.; Prati, C. Chemical-physical properties of TheraCal, a novel light-curable MTA-like material for pulp capping. Int. Endod. J. 2012, 45, 571–579. [Google Scholar] [CrossRef]
- Bortoluzzi, E.A.; Niu, L.N.; Palani, C.D.; El-Awady, A.R.; Hammond, B.D.; Pei, D.D.; Tian, F.C.; Cutler, C.W.; Pashley, D.H.; Tay, F.R. Cytotoxicity and osteogenic potential of silicate calcium cements as potential protective materials for pulpal revascularization. Dent. Mater. 2015, 31, 1510–1522. [Google Scholar] [CrossRef] [Green Version]
- Bakhtiar, H.; Nekoofar, M.H.; Aminishakib, P.; Abedi, F.; Naghi Moosavi, F.; Esnaashari, E.; Azizi, A.; Esmailian, S.; Ellini, M.R.; Mesgarzadeh, V.; et al. Human Pulp Responses to Partial Pulpotomy Treatment with TheraCal as Compared with Biodentine and ProRoot MTA: A Clinical Trial. J. Endod. 2017, 43, 1786–1791. [Google Scholar] [CrossRef]
- Seo, D.G.; Lee, D.; Kim, Y.M.; Song, D.; Kim, S.Y. Biocompatibility and Mineralization Activity of Three Calcium Silicate-Based Root Canal Sealers Compared to Conventional Resin-Based Sealer in Human Dental Pulp Stem Cells. Materials 2019, 12, 2482. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Park, J.B.; Song, D.; Kim, H.M.; Kim, S.Y. Cytotoxicity and Mineralization Potential of Four Calcium Silicate-Based Cements on Human Gingiva-Derived Stem Cells. Coatings 2020, 10, 279. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Zheng, M.; Kim, D.K.; Lee, W.P.; Yu, S.J.; Kim, B.O. Effects of 1,25-dihydroxyvitamin D3 on the differentiation of MC3T3-E1 osteoblast-like cells. J. Periodontal Implant Sci. 2018, 48, 34–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.H.; Oh, S.; Kim, S. Improvement of the osteogenic potential of ErhBMP-2-/EGCG-coated biphasic calcium phosphate bone substitute: In vitro and in vivo activity. J. Periodontal Implant Sci. 2019, 49, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Catala, C.J.; Collado-Gonzalez, M.; Garcia-Bernal, D.; Onate-Sanchez, R.E.; Forner, L.; Llena, C.; Lozano, A.; Moraleda, J.M.; Rodriguez-Lozano, F.J. Biocompatibility of New Pulp-capping Materials NeoMTA Plus, MTA Repair HP, and Biodentine on Human Dental Pulp Stem Cells. J. Endod. 2018, 44, 126–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widbiller, M.; Lindner, S.R.; Buchalla, W.; Eidt, A.; Hiller, K.A.; Schmalz, G.; Galler, K.M. Three-dimensional culture of dental pulp stem cells in direct contact to tricalcium silicate cements. Clin. Oral Investig. 2016, 20, 237–246. [Google Scholar] [CrossRef] [PubMed]
- De Rossi, A.; Silva, L.A.; Gaton-Hernandez, P.; Sousa-Neto, M.D.; Nelson-Filho, P.; Silva, R.A.; de Queiroz, A.M. Comparison of pulpal responses to pulpotomy and pulp capping with biodentine and mineral trioxide aggregate in dogs. J. Endod. 2014, 40, 1362–1369. [Google Scholar] [CrossRef]
- Valles, M.; Roig, M.; Duran-Sindreu, F.; Martinez, S.; Mercade, M. Color Stability of Teeth Restored with Biodentine: A 6-month In Vitro Study. J. Endod. 2015, 41, 1157–1160. [Google Scholar] [CrossRef]
- Camilleri, J. Hydration characteristics of Biodentine and Theracal used as pulp capping materials. Dent. Mater. 2014, 30, 709–715. [Google Scholar] [CrossRef]
- Hebling, J.; Lessa, F.C.; Nogueira, I.; Carvalho, R.M.; Costa, C.A. Cytotoxicity of resin-based light-cured liners. Am. J. Dent. 2009, 22, 137–142. [Google Scholar]
- Jeanneau, C.; Laurent, P.; Rombouts, C.; Giraud, T.; About, I. Light-cured Tricalcium Silicate Toxicity to the Dental Pulp. J. Endod. 2017, 43, 2074–2080. [Google Scholar] [CrossRef]
- Ranjkesh, B.; Isidor, F.; Kraft, D.C.E.; Lovschall, H. In vitro cytotoxic evaluation of novel fast-setting calcium silicate cement compositions and dental materials using colorimetric methyl-thiazolyl-tetrazolium assay. J. Oral Sci. 2018, 60, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Hirschman, W.R.; Wheater, M.A.; Bringas, J.S.; Hoen, M.M. Cytotoxicity comparison of three current direct pulp-capping agents with a new bioceramic root repair putty. J. Endod. 2012, 38, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Singh, M.; Nawal, R.R.; Chaudhry, S.; Yadav, S.; Mohanty, S.; Talwar, S. Evaluation of Biocompatibility and Osteogenic Potential of Tricalcium Silicate-based Cements Using Human Bone Marrow-derived Mesenchymal Stem Cells. J. Endod. 2018, 44, 446–451. [Google Scholar] [CrossRef]
- Margunato, S.; Tasli, P.N.; Aydin, S.; Karapinar Kazandag, M.; Sahin, F. In Vitro Evaluation of ProRoot MTA, Biodentine, and MM-MTA on Human Alveolar Bone Marrow Stem Cells in Terms of Biocompatibility and Mineralization. J. Endod. 2015, 41, 1646–1652. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, A.; Lipski, M.; Parafiniuk, M.; Sporniak-Tutak, K.; Lichota, D.; Kosierkiewicz, A.; Kaczmarek, W.; Buczkowska-Radlinska, J. Response of human dental pulp capped with biodentine and mineral trioxide aggregate. J. Endod. 2013, 39, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J.; Laurent, P.; About, I. Hydration of Biodentine, Theracal LC, and a prototype tricalcium silicate-based dentin replacement material after pulp capping in entire tooth cultures. J. Endod. 2014, 40, 1846–1854. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.I.; Ko, Y.; Park, J.B. Evaluation of the secretion and release of vascular endothelial growth factor from two-dimensional culture and three-dimensional cell spheroids formed with stem cells and osteoprecursor cells. Adv. Clin. Exp. Med. 2018, 27, 971–977. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, T.; Xu, A.; Zhang, L. 3D spheroid culture enhances survival and therapeutic capacities of MSCs injected into ischemic kidney. J. Cell. Mol. Med. 2016, 20, 1203–1213. [Google Scholar] [CrossRef] [Green Version]


| Product | Manufacturer | Composition | Batch Number |
|---|---|---|---|
| ProRoot MTA | Dentsply Tulsa Dental Specialties, Tulsa, OK, USA | Portland cement (tricalcium silicate, dicalcium silicate, and tricalcium aluminate), calcium sulfate dihydrate (gypsum), and bismuth oxide | 0000186484 |
| Biodentine | Septodont, Saint-Maur-des-Fossés, France | Tricalcium silicate, dicalcium silicate, calcium carbonate, calcium oxide, and zirconium oxide in its powder form Water, calcium chloride, and soluble polymer as an aqueous liquid | B24553 |
| TheraCal LC | Bisco, Schamberg, IL, USA, | Portland cement (calcium silicates), fumed silica, Bis-GMA, and polyglycol dimethacrylate | 1900004558 |
| Dycal | Dentsply Caulk, Midford, DE, USA | Base paste: calcium phosphate, calcium tungstate, zinc oxide, iron oxide pigments, and 1,3-butylene glycol disalicylate Catalyst paste: calcium hydroxide, zinc oxide, zinc stearate, titanium oxide, iron oxide pigments, and N-ethyl-o/p-toluene sulphonamide | 160801 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Lee, D.; Song, D.; Kim, H.-M.; Kim, S.-Y. Biocompatibility and Bioactivity of Set Direct Pulp Capping Materials on Human Dental Pulp Stem Cells. Materials 2020, 13, 3925. https://doi.org/10.3390/ma13183925
Kim Y, Lee D, Song D, Kim H-M, Kim S-Y. Biocompatibility and Bioactivity of Set Direct Pulp Capping Materials on Human Dental Pulp Stem Cells. Materials. 2020; 13(18):3925. https://doi.org/10.3390/ma13183925
Chicago/Turabian StyleKim, Yemi, Donghee Lee, Dani Song, Hye-Min Kim, and Sin-Young Kim. 2020. "Biocompatibility and Bioactivity of Set Direct Pulp Capping Materials on Human Dental Pulp Stem Cells" Materials 13, no. 18: 3925. https://doi.org/10.3390/ma13183925
APA StyleKim, Y., Lee, D., Song, D., Kim, H.-M., & Kim, S.-Y. (2020). Biocompatibility and Bioactivity of Set Direct Pulp Capping Materials on Human Dental Pulp Stem Cells. Materials, 13(18), 3925. https://doi.org/10.3390/ma13183925






