Molecular Dynamics Study on Mechanical Properties of Interface between Urea-Formaldehyde Resin and Calcium-Silicate-Hydrates
Abstract
:1. Introduction
2. Computational Methodology
2.1. Forcite
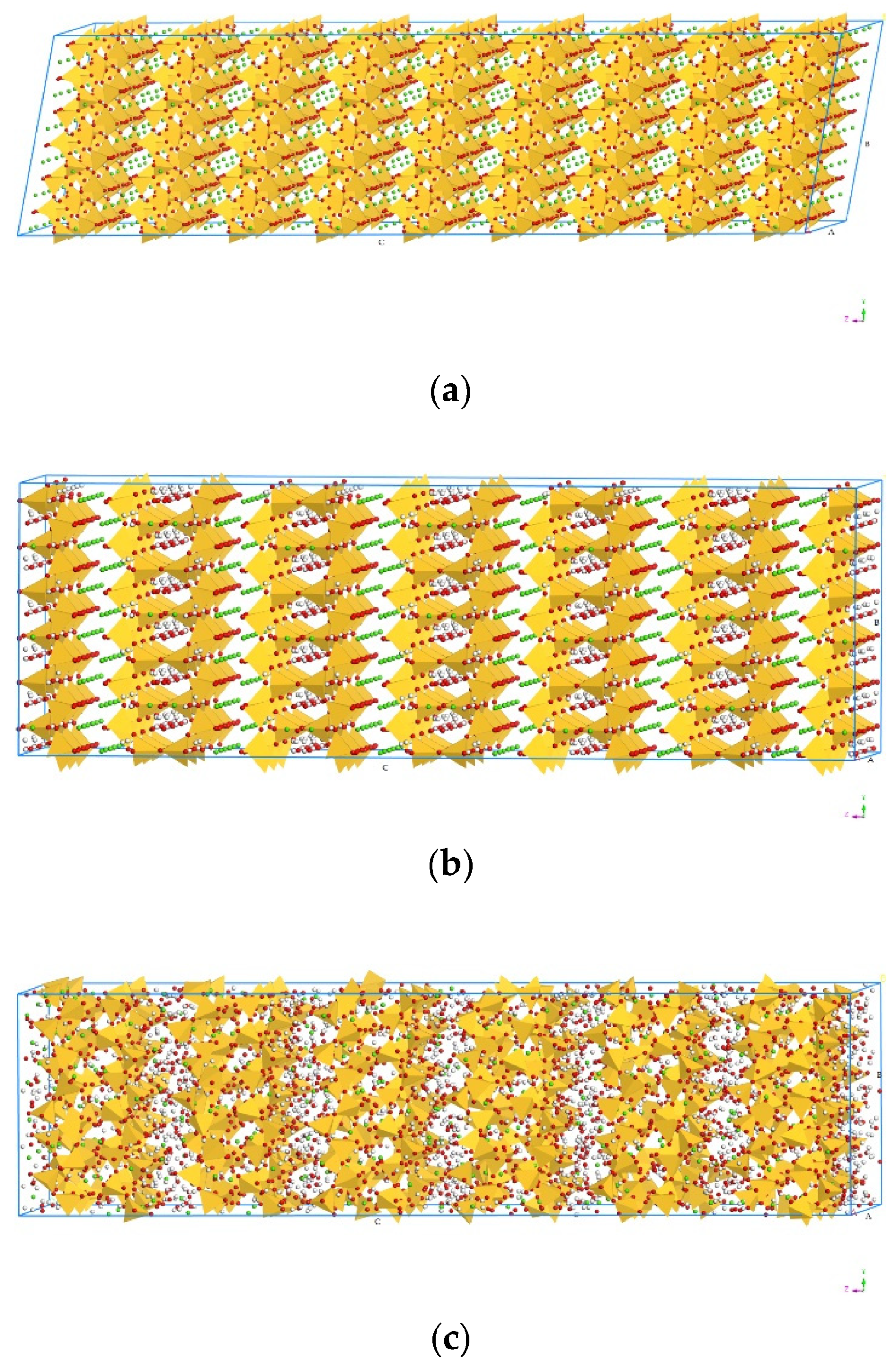
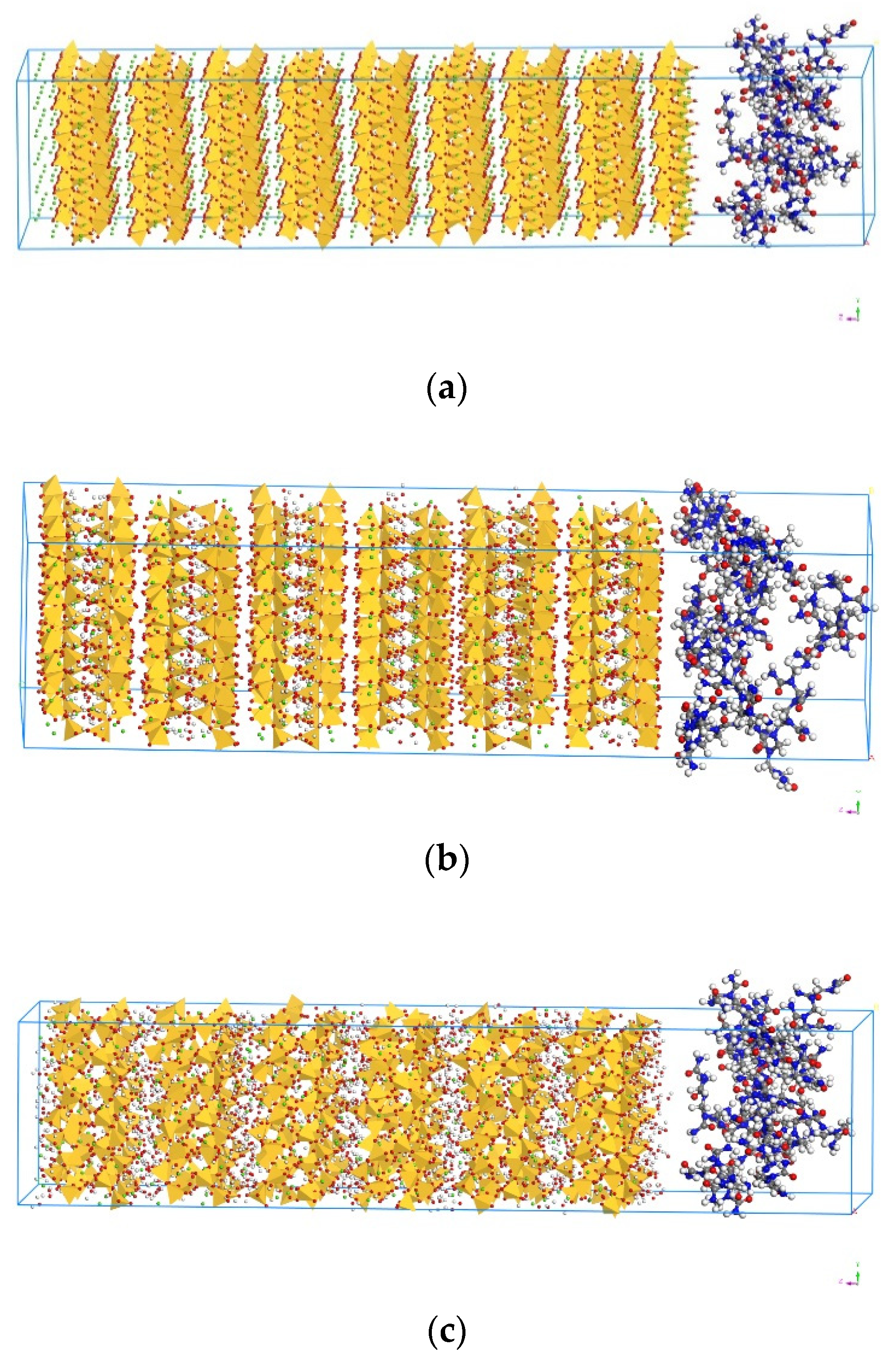
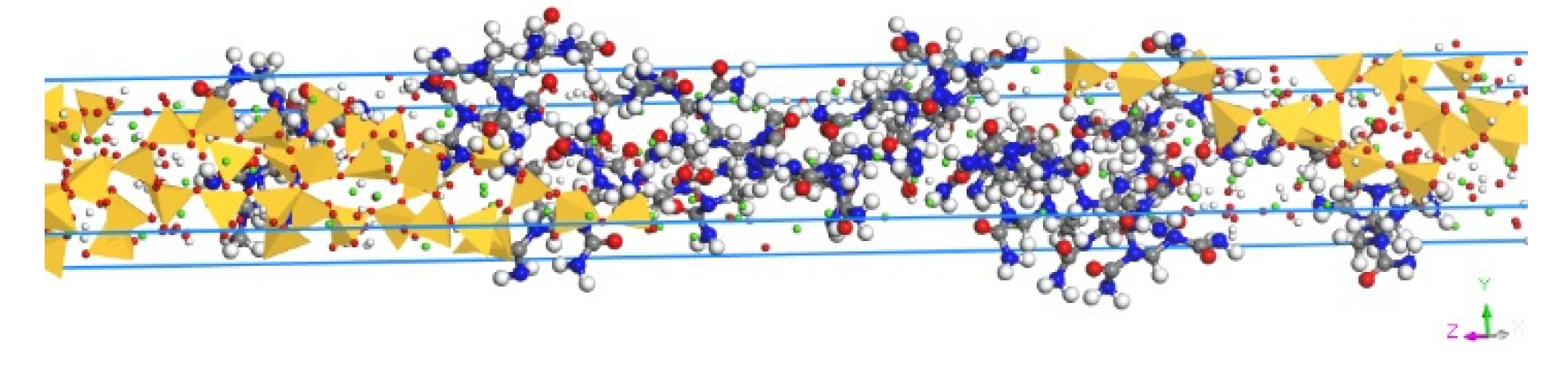
2.2. Model Construction
2.3. Molecular Dynamics Simulation
3. Results and Discussions
3.1. Mechanical Property
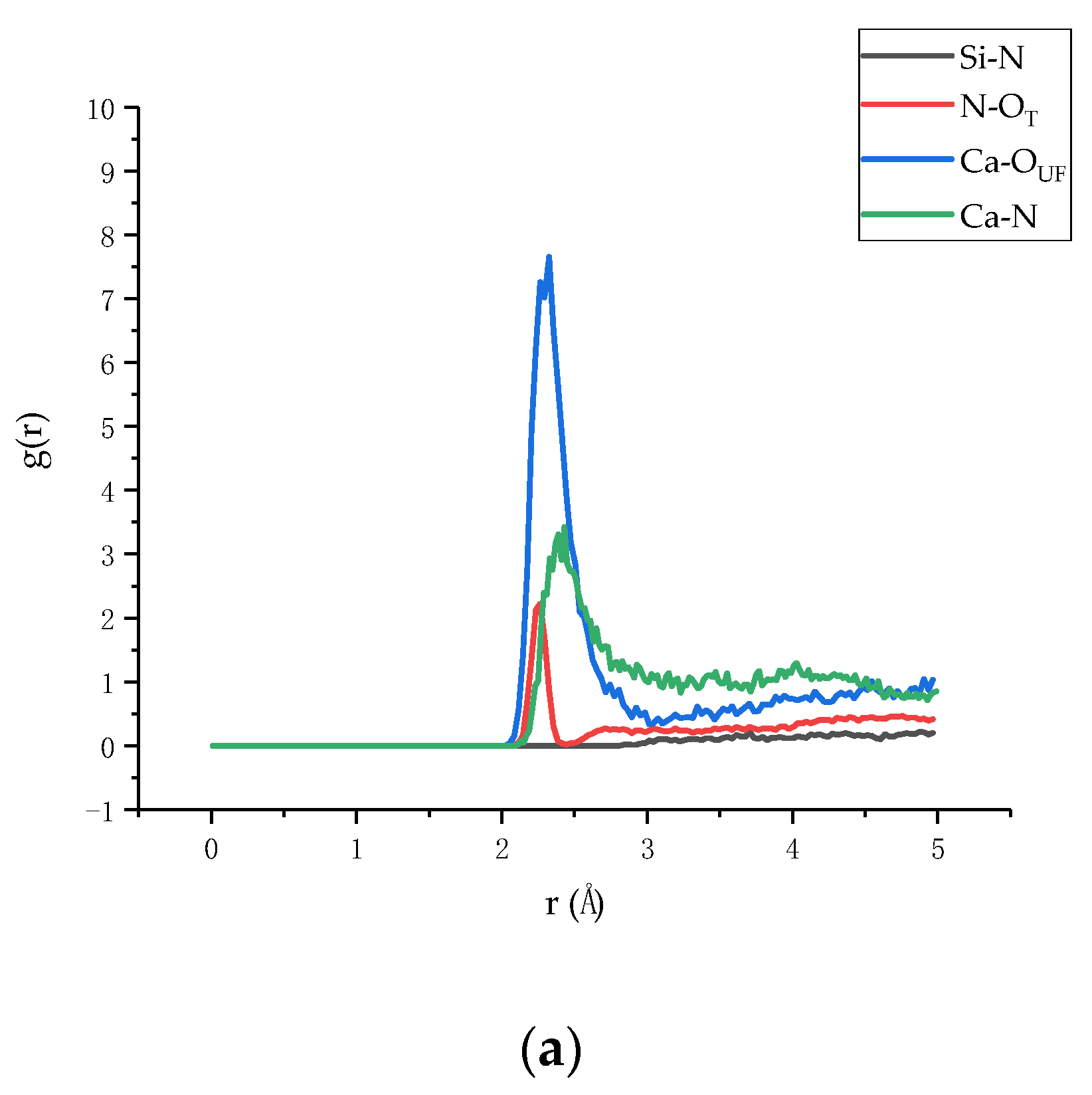
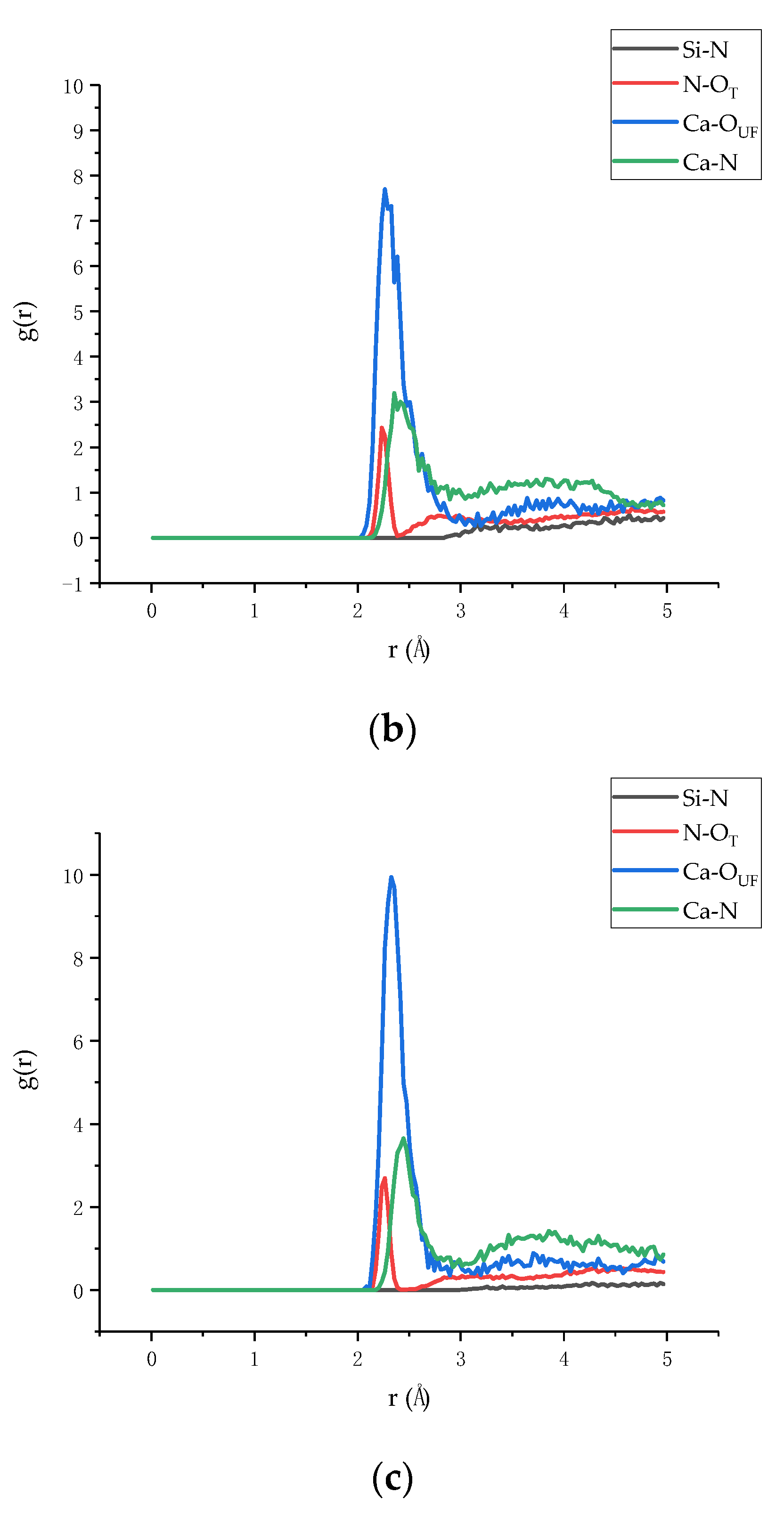
3.2. Radial Distribution Function
3.3. Stress–Strain Curve
3.4. Binding Energy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Beushausen, H.; Bester, N. The influence of curing on restrained shrinkage cracking of bonded concrete overlays. Cement Concr. Res. 2016, 87, 87–96. [Google Scholar] [CrossRef]
- Li, W.; Dong, B.; Yang, Z.; Xu, J.; Chen, Q.; Li, H.; Xing, F.; Jiang, Z. Recent Advances in Intrinsic Self-Healing Cementitious Materials. Adv. Mater. 2018, 30, 1705679. [Google Scholar] [CrossRef]
- Ryu, J.S.; Otsuki, N. Crack closure of reinforced concrete by electrodeposition technique. Cement Concr. Res. 2002, 32, 159–164. [Google Scholar] [CrossRef]
- Banthia, N.; Gupta, R. Influence of polypropylene fiber geometry on plastic shrinkage cracking in concrete. Cement Concr. Res. 2006, 36, 1263–1267. [Google Scholar] [CrossRef]
- Ray, I.; Gong, Z.; Davalos, J.F.; Kar, A. Shrinkage and cracking studies of high performance concrete for bridge decks. Constr. Build. Mater. 2012, 28, 244–254. [Google Scholar] [CrossRef]
- Han, N.; Xing, F. A Comprehensive Review of the Study and Development of Microcapsule Based Self-Resilience Systems for Concrete Structures at Shenzhen University. Materials 2016, 10, 2. [Google Scholar] [CrossRef]
- Dong, B.; Han, N.; Zhang, M.; Wang, X.; Cui, H.; Xing, F. A microcapsule technology based self-healing system for concrete structures. J. Earthq. Tsunami 2013, 7, 1350014. [Google Scholar] [CrossRef]
- Tang, W.; Kardani, O.; Cui, H. Robust evaluation of self-healing efficiency in cementitious materials—A review. Constr. Build. Mater. 2015, 81, 233–247. [Google Scholar] [CrossRef]
- Tittelboom, K.V.; Belie, N.D. Self-Healing in Cementitious Materials—A Review. Materials 2013, 6, 2182–2217. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Sun, P.; Han, N.; Xing, F. Experimental Study on Mechanical Properties and Porosity of Organic Microcapsules Based Self-Healing Cementitious Composite. Materials 2017, 10, 20. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.N.; Kessler, M.R.; Sottos, N.R.; White, S.R. In situ poly(urea-formaldehyde) microencapsulation of dicyclopentadiene. J. Microencapsul. 2003, 20, 719–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, T.; Wang, X.; Li, D.; Li, D.; Xing, F.; Ren, J.; Han, N. Stress-strain behaviour and pore structure of microcapsule-based self-healing cementitious composite under triaxial tests. Constr. Build. Mater. 2020, 241, 118009. [Google Scholar] [CrossRef]
- Zhang, M.; Xing, F.; Shi, K.Y.; Du, X.X. Study on Organic Microcapsule Based Self-Healing Cementitious Composite. Adv. Mater. Res. 2011, 239–242, 764–767. [Google Scholar] [CrossRef]
- Bekas, D.G.; Tsirka, K.; Baltzis, D.; Paipetis, A.S. Self-healing materials: A review of advances in materials, evaluation, characterization and monitoring techniques. Compos. Part B 2016, 87, 92–119. [Google Scholar] [CrossRef]
- Zhang, M. A Study on Microcapsule Based Self-Healing Method and Mechanism for Cementitious Composites. Ph.D. Thesis, Central South University, Changsha, China, 2013. [Google Scholar]
- Li, V.C.; Lim, Y.M.; Chan, Y.-W. Feasibility study of a passive smart self-healing cementitious composite. Compos. Part B 1998, 29, 819–827. [Google Scholar] [CrossRef]
- Wu, W.; Al-Ostaz, A.; Cheng, A.H.-D.; Song, R.C. Concrete as a hierarchical structural composite material. Int. J. Multiscale Comput. Eng. 2010, 8, 585–595. [Google Scholar] [CrossRef]
- Lin, F.; Christian, M. Micromechanics model for the effective elastic properties of hardened cement pastes. Acta Mater. Compos. Sin. 2007, 24, 184–189. [Google Scholar]
- Haecker, C.J.; Garboczi, E.J.; Bullard, J.W.; Bohn, R.B.; Sun, Z.; Shah, S.P.; Voigt, T. Modeling the linear elastic properties of Portland cement paste. Cement Concr. Res. 2005, 35, 1948–1960. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Cao, D.; Shen, J.; Gao, Y. Computational simulation of elastomer nanocomposites: Current progress and future challenges. Rubber Chem. Technol. 2012, 85, 450–481. [Google Scholar] [CrossRef]
- Qianrui, Z.; Yong, H.; Leichao, W.; Measurement, T. Influence of electrostatic field on the adsorption of phenol on single-walled carbon nanotubes: A study by molecular dynamics simulation. Chem. Eng. J. 2019, 363, 278–284. [Google Scholar]
- Macphee, D.E.; Folli, A. Photocatalytic concretes—The interface between photocatalysis and cement chemistry. Cement Concr. Res. 2016, 85, 48–54. [Google Scholar] [CrossRef]
- Hou, D.; Zhu, Y.; Lu, Y.; Li, Z. Mechanical properties of calcium silicate hydrate (C–S–H) at nano-scale: A molecular dynamics study. Mater. Chem. Phys. 2014, 146, 503–511. [Google Scholar] [CrossRef]
- Qomi, M.A.; Krakowiak, K.J.; Bauchy, M.; Stewart, K.L.; Shahsavari, R.; Jagannathan, D.; Brommer, D.B.; Baronnet, A.; Buehler, M.J.; Yip, S.; et al. Combinatorial molecular optimization of cement hydrates. Nat. Commun. 2014, 5, 1–10. [Google Scholar]
- Zhou, J.; Liang, Y. Effect of Water on the Dynamic Tensile Mechanical Properties of Calcium Silicate Hydrate: Based on Molecular Dynamics Simulation. Materials 2019, 12, 2837. [Google Scholar] [CrossRef] [Green Version]
- Hou, D.; Ma, H.; Zhu, Y.; Li, Z. Calcium silicate hydrate from dry to saturated state: Structure, dynamics and mechanical properties. Acta Mater. 2014, 67, 81–94. [Google Scholar] [CrossRef]
- Hajilar, S.; Shafei, B. Nano-scale investigation of elastic properties of hydrated cement paste constituents using molecular dynamics simulations. Comput. Mater. Sci. 2015, 101, 216–226. [Google Scholar] [CrossRef]
- Wang, X.F.; Li, T.R.; Wei, P.; Li, D.W.; Han, N.X.; Xing, F.; Gan, Y.; Chen, Z. Computational study of the nanoscale mechanical properties of C–S–Hcomposites under different temperatures. Comput. Mater. Sci. 2018, 146, 42–53. [Google Scholar] [CrossRef]
- Powers, T.C.; Brownyard, T.L. Studies of the Physical Properties of Hardened Portland Cement Paste. J. Proc. 1946, 43, 1946. [Google Scholar]
- Taylor, H.F. Proposed Structure for Calcium Silicate Hydrate Gel. J. Am. Ceram. Soc. 1986, 69, 464–467. [Google Scholar] [CrossRef]
- Wai-Yim, C.; Lokendra, P.; Saro, S.; Khagendra, B. Interfacial Interaction between Suolunite Crystal and Silica Binding Peptide for Novel Bioinspired Cement. ACS Comb. Sci. 2019, 21, 794–804. [Google Scholar]
- Rouhi, S.; Atfi, A. Molecular Dynamics Simulations of Adsorption of Polymer Chains on the Surface of BmNn Graphyne-Like Monolayers. Braz. J. Phys. 2017, 47, 239–267. [Google Scholar] [CrossRef]
- Alkhateb, H.; Al-Ostaz, A.; Cheng, H.D.; Li, X. Materials Genome for Graphene-Cement Nanocomposites. J. Nanomech. Micromech. 2013, 3, 67–77. [Google Scholar] [CrossRef]
- Du, J.; Bu, Y.; Shen, Z. Interfacial properties and nanostructural characteristics of epoxy resin in cement matrix. Constr. Build. Mater. 2018, 164, 103–112. [Google Scholar] [CrossRef]
- Moon, J.; Yoon, S.; Monteiro, P.J.M. Mechanical properties of jennite: A theoretical and experimental study. Cement Concr. Res. 2015, 71, 106–114. [Google Scholar] [CrossRef] [Green Version]
- Makar, J.M.; Chan, G.W. Growth of Cement Hydration Products on Single-Walled Carbon Nanotubes. J. Am. Ceram. Soc. 2009, 92, 1303–1310. [Google Scholar] [CrossRef]
- Allen, A.J.; Thomas, J.J.; Jennings, H.M. Composition and density of nanoscale calcium-silicate-hydrate in cement. Nat. Mater. 2007, 6, 311–316. [Google Scholar] [CrossRef]
- Richardson, I.G. Tobermorite/jennite- and tobermorite/calcium hydroxide-based models for the structure of C–S–H: Applicability to hardened pastes of tricalcium silicate, β-dicalcium silicate, Portland cement, and blends of Portland cement with blast-furnace slag, metakaol. Cement Concr. Res. 2004, 34, 1733–1777. [Google Scholar] [CrossRef]
- Tavakoli, D.; Tarighat, A. Molecular dynamics study on the mechanical properties of Portland cement clinker phases. Comput. Mater. Sci. 2016, 119, 65–73. [Google Scholar] [CrossRef]
- Wu, W.; Al-Ostaz, A.; Cheng, A.H.-D.; Song, C.R. Computation of Elastic Properties of Portland Cement Using Molecular Dynamics. J. Nanomech. Micromech. 2011, 1, 84–90. [Google Scholar] [CrossRef]
- Al-Ostaz, A.; Wu, W.; Cheng, H.D.; Song, C.R. A molecular dynamics and microporomechanics study on the mechanical properties of major constituents of hydrated cement. Compos. Part B Eng. 2010, 41, 543–549. [Google Scholar] [CrossRef]
- Wang, X.F.; Han, R.; Han, T.L.; Han, N.X.; Xing, F. Determination of elastic properties of urea-formaldehyde microcapsules through nanoindentation based on the contact model and the shell deformation theory. Mater. Chem. Phys. 2018, 215, 346–354. [Google Scholar] [CrossRef]
- Xie, J. Preparation of microcapsule for electrophoretic display. Ph.D, Thesis, Tianjin University, Tianjin, China, 2007. [Google Scholar]
- Xiaolin, P.; Francine, B.; Thibault, C.; Isabelle, P.; André, N. 27Al and 29Si solid-state NMR characterization of calcium-aluminosilicate-hydrate. Inorg. Chem. 2012, 51, 1827–1836. [Google Scholar]
- Taylor, H.F.W. Hydrated Calcium Silicates Part 1: Compound Formation at Ordinary Temperatures. J. Chem. Soc. 1950, 3, 3682–3690. [Google Scholar] [CrossRef]
- Merlino, S.; Bonaccorsi, E.; Armbruster, T. The real structures of clinotobermorite and tobermorite 9 Å: OD character, polytypes, and structural relationships. Eur. J. Mineral. 2000, 12, 411–429. [Google Scholar] [CrossRef]
- Bonaccorsi, E.; Merlino, S.; Kampf, A.R. The Crystal Structure of Tobermorite 14 Å (Plombierite), a C–S–H Phase. J. Am. Ceram. Soc. 2005, 88, 505–512. [Google Scholar] [CrossRef]
- Dongshuai, H.; Jiao, Y.; Wang, P. Molecular dynamics modeling of the structure, dynamics, energetics and mechanical properties of cement-polymer nanocomposite. Compos. Part B 2019, 162, 433–444. [Google Scholar]
- Shengwen, T.; Hubao, A.; Jingtao, C.; Wenzhi, Y.; Peng, Y.; Chen, E.; Hongyang, D.; He, Z. The interactions between water molecules and C–S–Hsurfaces in loads-induced nanopores: A molecular dynamics study. Appl. Surf. Sci. 2019, 496, 143744. [Google Scholar]
- Heyes, D.M. Electrostatic potentials and fields in infinite point charge lattices. J. Chem. Phys. 1981, 74, 1924–1929. [Google Scholar] [CrossRef]
- De Leeuw, S.W.; Perram, J.W.; Smith, E.R. Simulation of Electrostatic Systems in Periodic Boundary Conditions. I. Lattice Sums and Dielectric Constants. Proc. R. Soc. A Math. Phys. Eng. Sci. 1980, 373, 27–56. [Google Scholar]
- Kanellopoulos, A.; Giannaros, P.; Al-Tabbaa, A. The effect of varying volume fraction of microcapsules on fresh, mechanical and self-healing properties of mortars. Constr. Build. Mater. 2016, 122, 577–593. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, G.; Wang, Z.; Guan, Z. Experimental-computational approach to investigate compressive strength of magnesium phosphate cement with nanoindentation and finite element analysis. Constr. Build. Mater. 2018, 190, 414–426. [Google Scholar] [CrossRef]
- Hou, D.; Zhao, T.; Jin, Z.; Ma, H.; Li, Z. Molecular Simulation of Calcium Silicate Composites: Structure, Dynamics, and Mechanical Properties. J. Am. Ceram. Soc. 2015, 98, 758–769. [Google Scholar] [CrossRef]
- Liu, K.; Cheng, X.; Ma, Y.; Gao, X.; Zhang, C.; Li, Z.; Zhuang, J. Analysis of interfacial nanostructure and interaction mechanisms between cellulose fibres and calcium silicate hydrates using experimental and molecular dynamics simulation data. Appl. Surf. Sci. 2020, 506, 144914. [Google Scholar] [CrossRef]
- Wan, H.; Yuan, L.; Zhang, Y. Insight Into the Leaching of Sodium Alumino-Silicate Hydrate (N-A-S-H) Gel: A Molecular Dynamics Study. Front. Mater. 2020, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Tong, K.; Song, X.; Sun, S.; Xu, Y.; Yu, J. Molecular dynamics study of linear and comb-like polyelectrolytes in aqueous solution: Effect of Ca2+ ions. Mol. Phys. 2014, 112, 2176–2183. [Google Scholar] [CrossRef]
- Chung, Y.T.; Huang, C.I. Ion condensation behavior and dynamics of water molecules surrounding the sodium poly(methacrylic acid) chain in water: A molecular dynamics study. J. Chem. Phys. 2012, 136, 124903. [Google Scholar] [CrossRef]
- Eftekhari, M.; Mohammadi, S. Molecular dynamics simulation of the nonlinear behavior of the CNT-reinforced calcium silicate hydrate (C–S–H) composite. Compos. Part A 2016, 82, 78–87. [Google Scholar] [CrossRef]
- Mohiuddin, A.; Biswajit, K.B.; Zafrul, H.K.; Rafiqul, A.T. Evaluation of the fracture resistance of asphalt concrete mixes including the effect of anisotropy. Int. J. Pavement Res. Technol. 2020, 13, 121–128. [Google Scholar]
- Kozo, O. Energy consumption characteristics of concrete using granulated blast-furnace slag sand related to nucleation and propagation of microcracks. Constr. Build. Mater. 2019, 218, 404–412. [Google Scholar]
- Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. New detailed insights on the role of a novel praseodymium nanofilm on the polymer/steel interfacial adhesion bonds in dry and wet conditions: An integrated molecular dynamics simulation and experimental study. J. Taiwan Inst. Chem. Eng. 2018, 85, 221–236. [Google Scholar] [CrossRef]
- Cranford, S.W.; Ortiz, C.; Buehler, M.J. Mechanomutable properties of a PAA/PAH polyelectrolyte complex: Rate dependence and ionization effects on tunable adhesion strength. Soft Matter 2015, 6, 4175. [Google Scholar] [CrossRef]
- Shalchy, F.; Rahbar, N. Nanostructural Characteristics and Interfacial Properties of Polymer Fibers in Cement Matrix. Acs Appl. Mater. Interfaces 2010, 7, 17278–17286. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Li, C.Y.; Wang, Q.; Duan, W.H. Reinforcing mechanism of graphene at atomic level: Friction, crack surface adhesion and 2D geometry. Carbon 2017, 114, 557–565. [Google Scholar] [CrossRef]
- Wan, H.; Zhang, Y. Interfacial bonding between graphene oxide and calcium silicate hydrate gel of ultra-high performance concrete. Mater. Struct. 2020, 53, 1–12. [Google Scholar] [CrossRef]



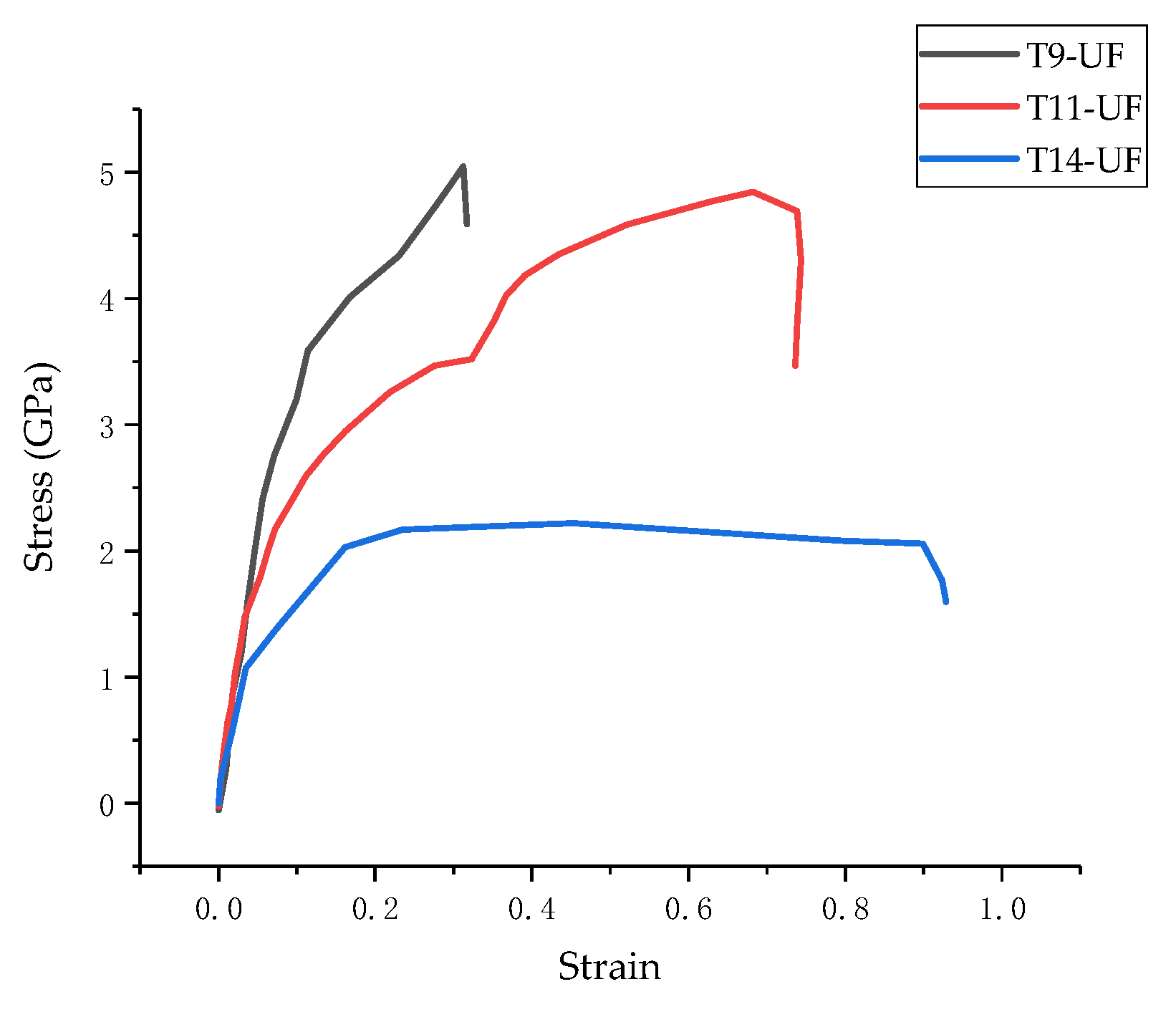
| Model | Bulk Modulus (GPa) | Shear Modulus (GPa) | Young’s Modulus (GPa) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| T9-UF | 59.163 | 23.9466 | 11.4652 | 17.6246 | 3.3996 |
| T11-UF | 28.5138 | 5.7435 | 9.7172 | 24.4057 | 11.6104 |
| T14-UF | 26.4379 | 8.6146 | 24.1895 | 36.9385 | 20.187 |
| Model | Eb (kcal/mol) |
|---|---|
| T9-UF | 1394 |
| T11-UF | 2179 |
| T14-UF | 3334 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Xie, W.; Li, T.; Ren, J.; Zhu, J.; Han, N.; Xing, F. Molecular Dynamics Study on Mechanical Properties of Interface between Urea-Formaldehyde Resin and Calcium-Silicate-Hydrates. Materials 2020, 13, 4054. https://doi.org/10.3390/ma13184054
Wang X, Xie W, Li T, Ren J, Zhu J, Han N, Xing F. Molecular Dynamics Study on Mechanical Properties of Interface between Urea-Formaldehyde Resin and Calcium-Silicate-Hydrates. Materials. 2020; 13(18):4054. https://doi.org/10.3390/ma13184054
Chicago/Turabian StyleWang, Xianfeng, Wei Xie, Taoran Li, Jun Ren, Jihua Zhu, Ningxu Han, and Feng Xing. 2020. "Molecular Dynamics Study on Mechanical Properties of Interface between Urea-Formaldehyde Resin and Calcium-Silicate-Hydrates" Materials 13, no. 18: 4054. https://doi.org/10.3390/ma13184054





