Nanoparticles Synthesis in Wet-Operating Stirred Media: Investigation on the Grinding Efficiency
Abstract
:1. Introduction
2. Materials and Methods
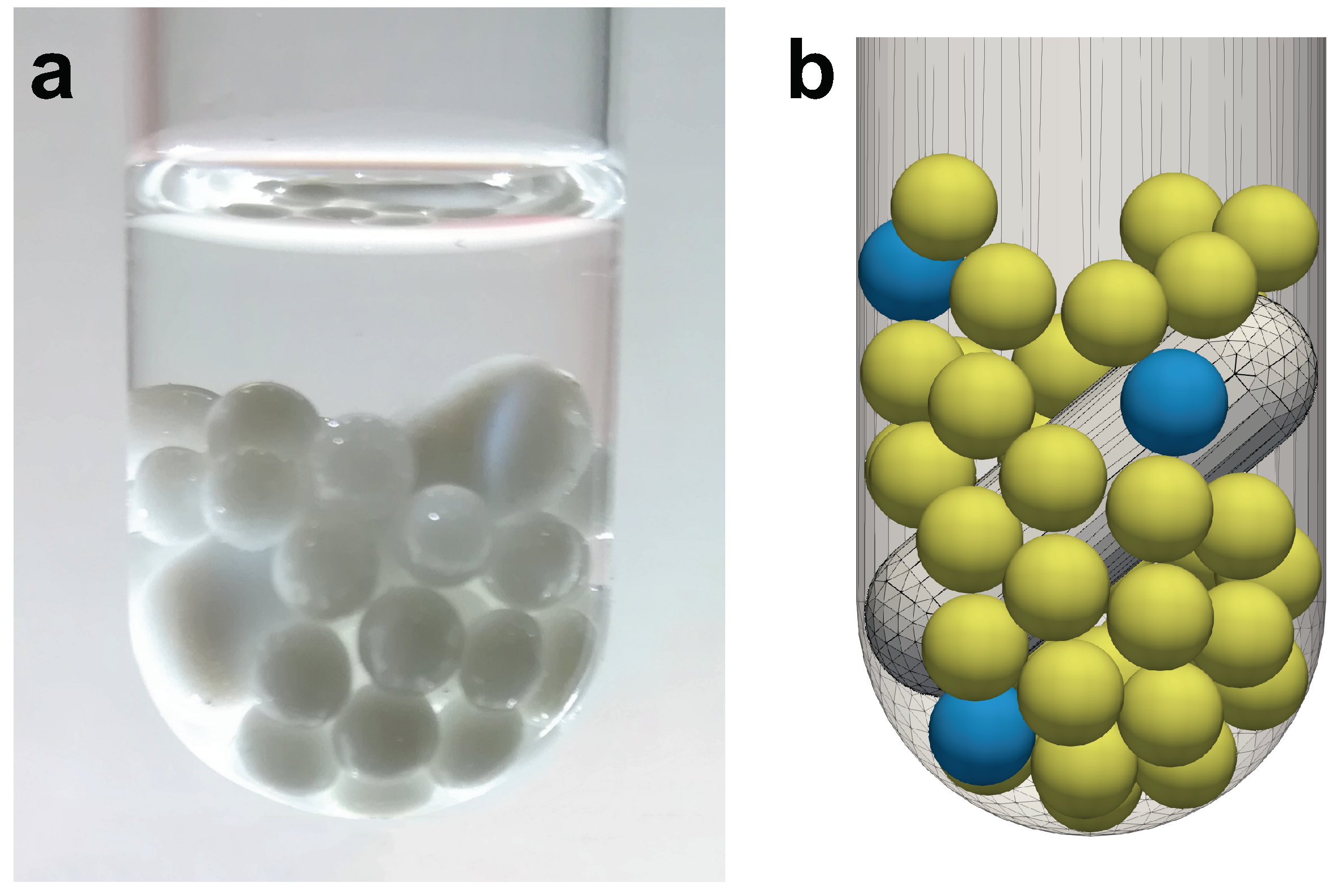
2.1. Experimental Set-Up
2.2. Numerical Set-Up
3. Results
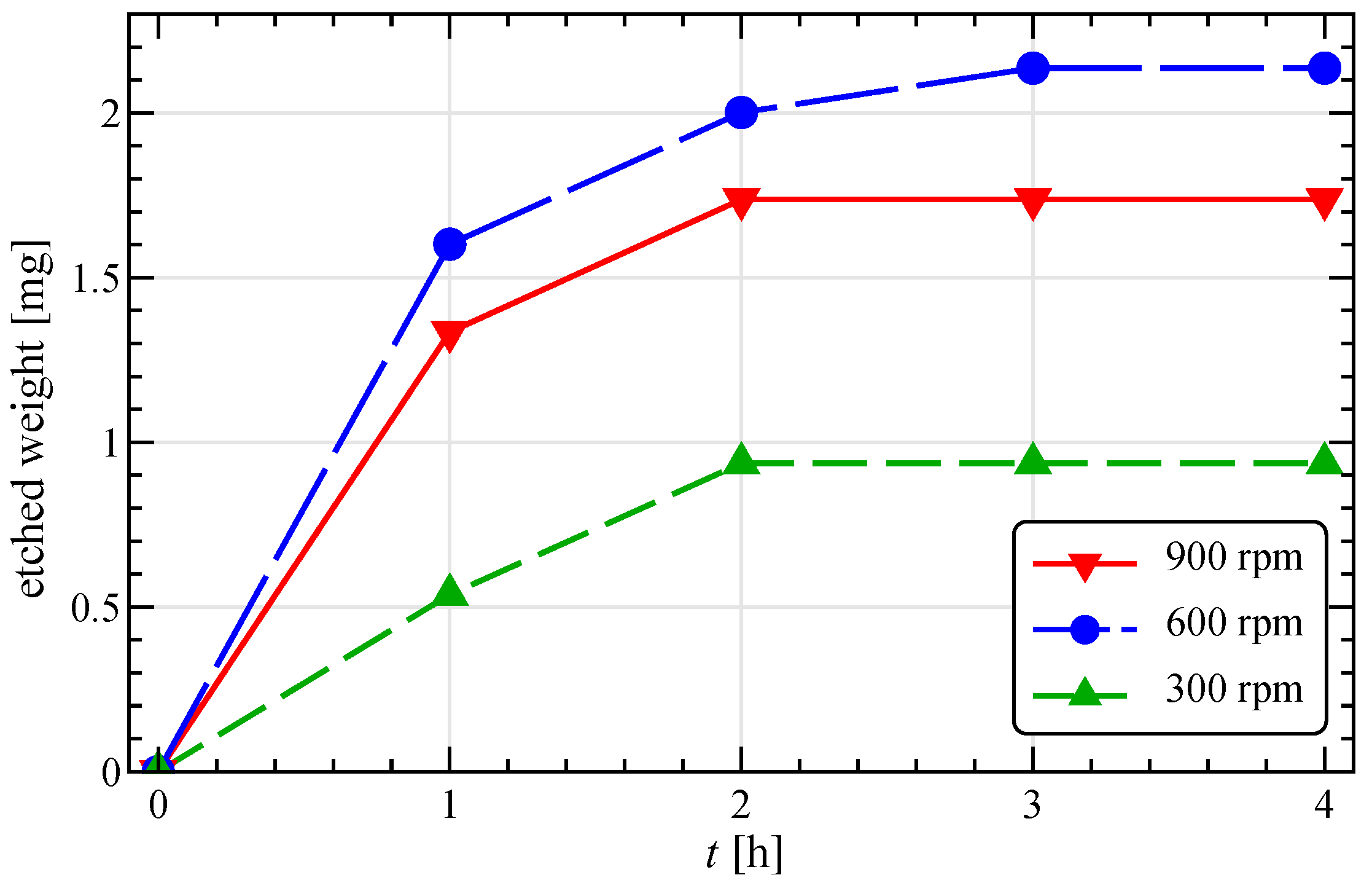
3.1. Experimental Etching Efficiency
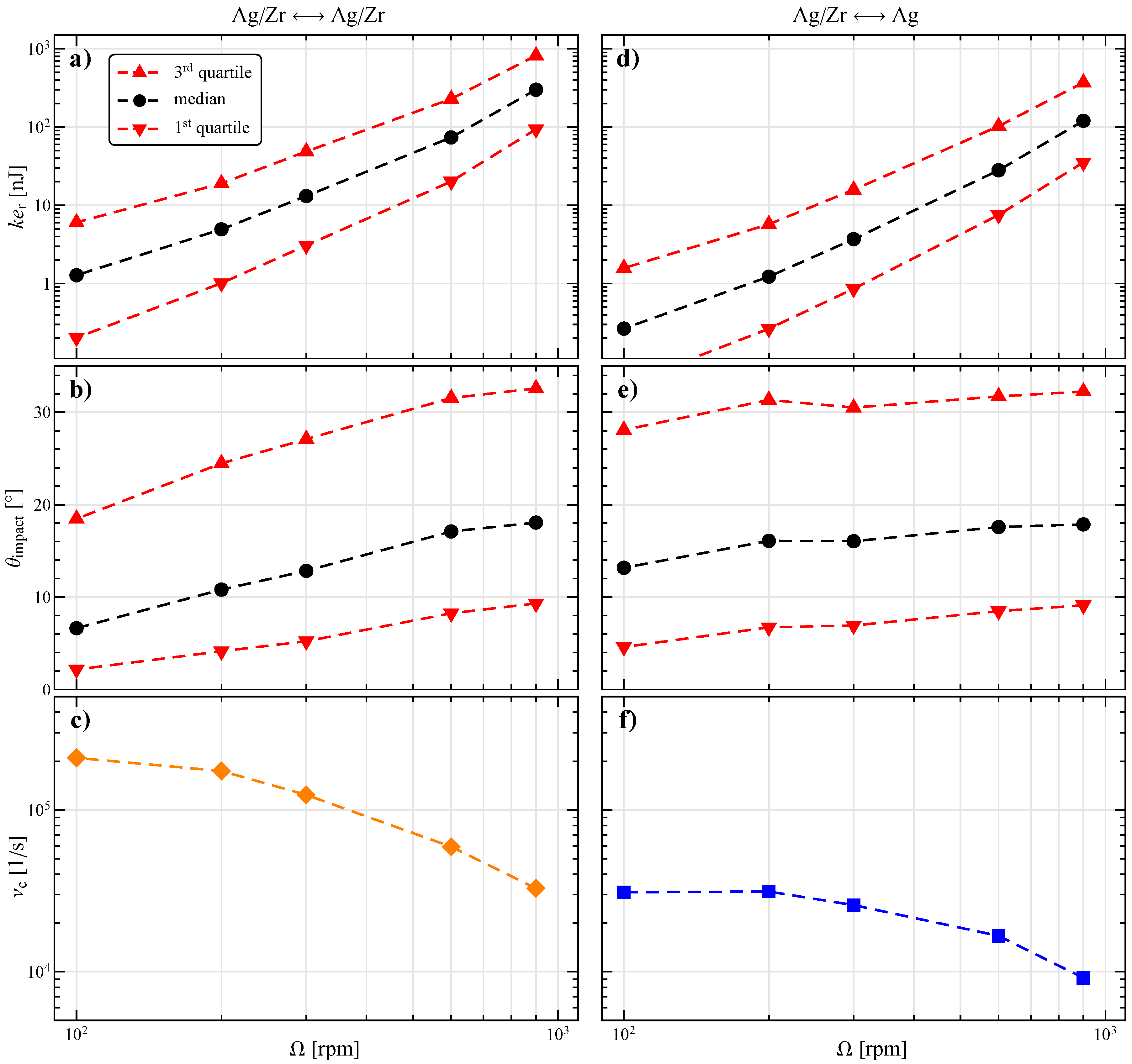
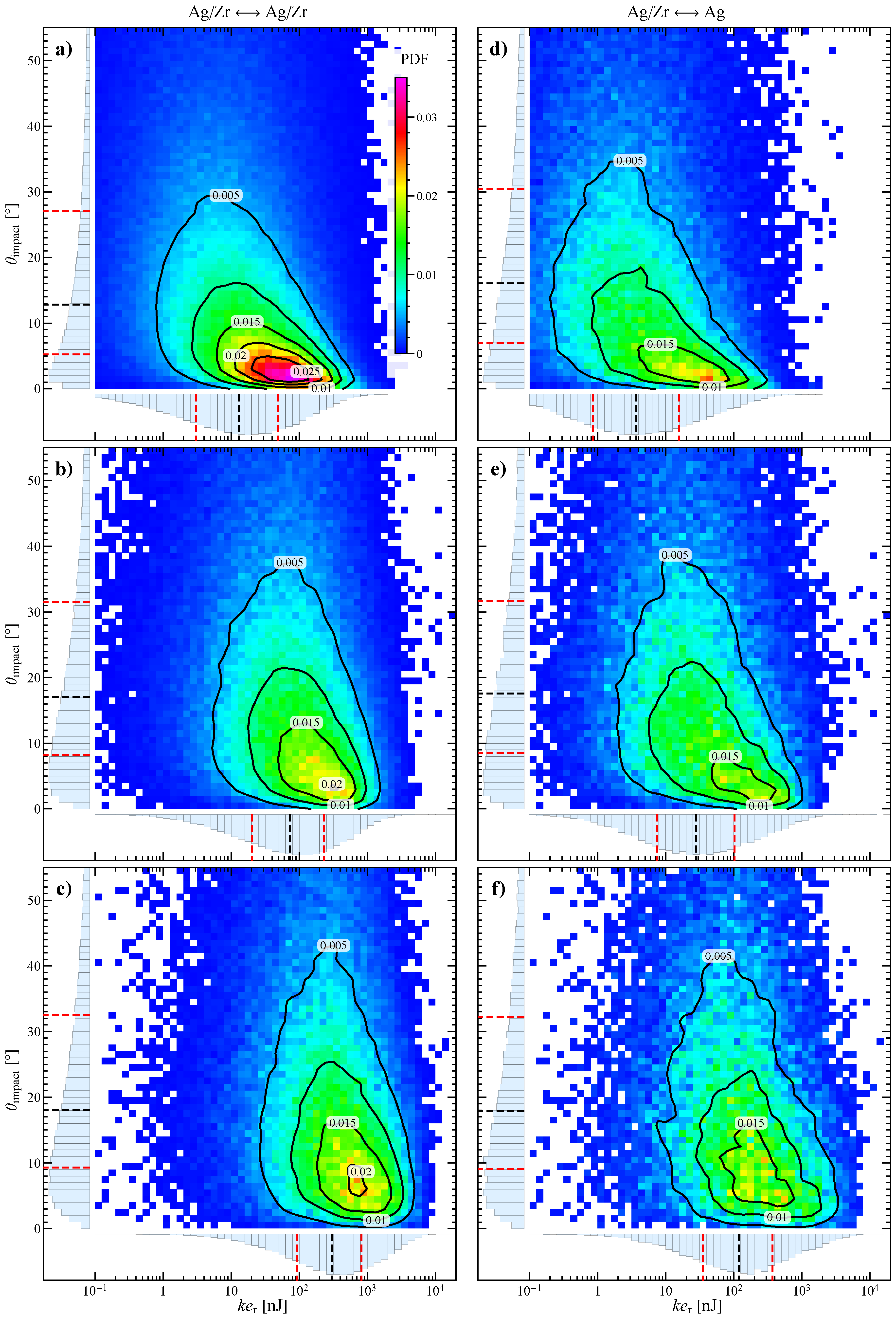
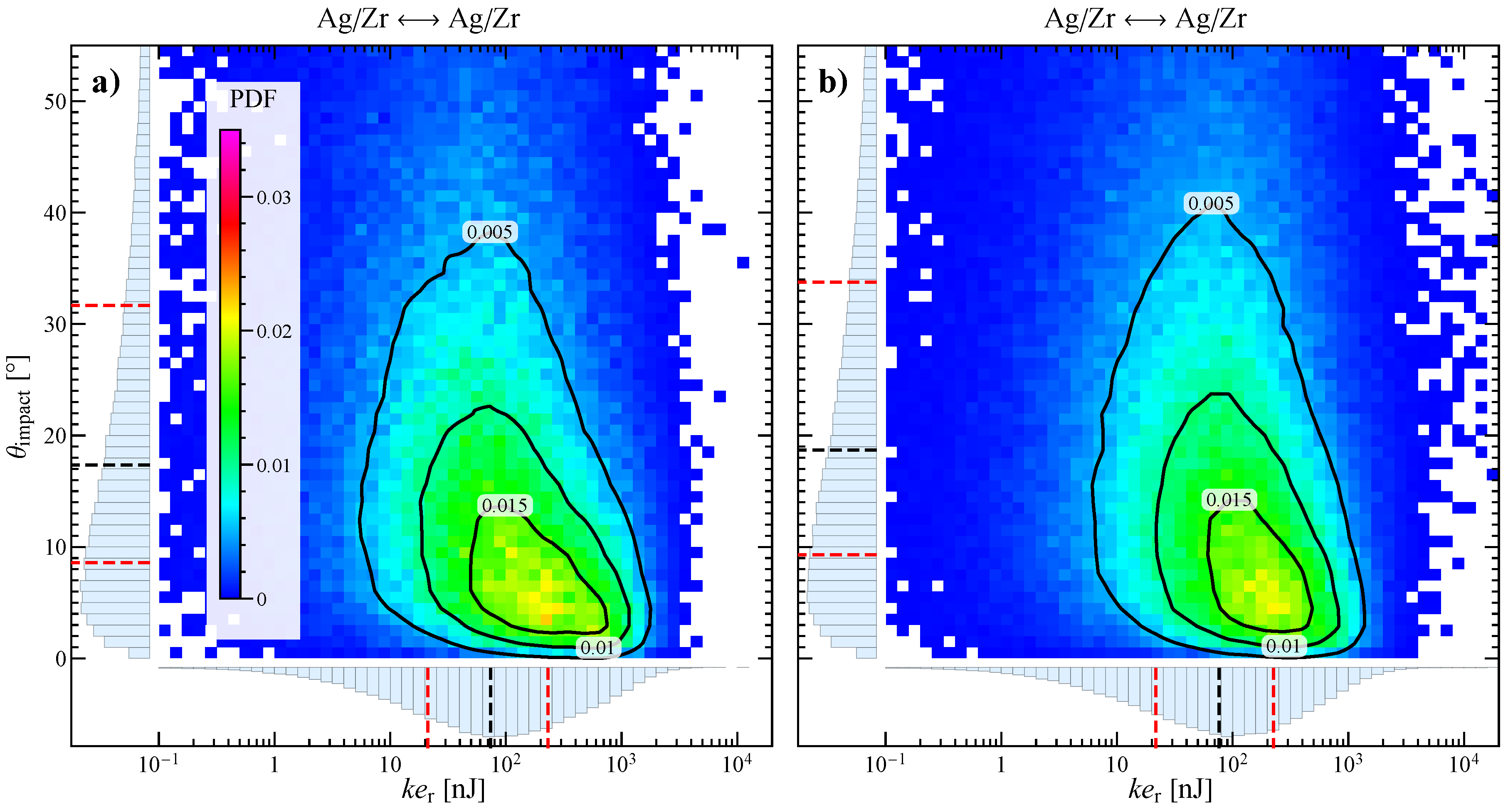
3.2. Numerical Characterization of Collisions
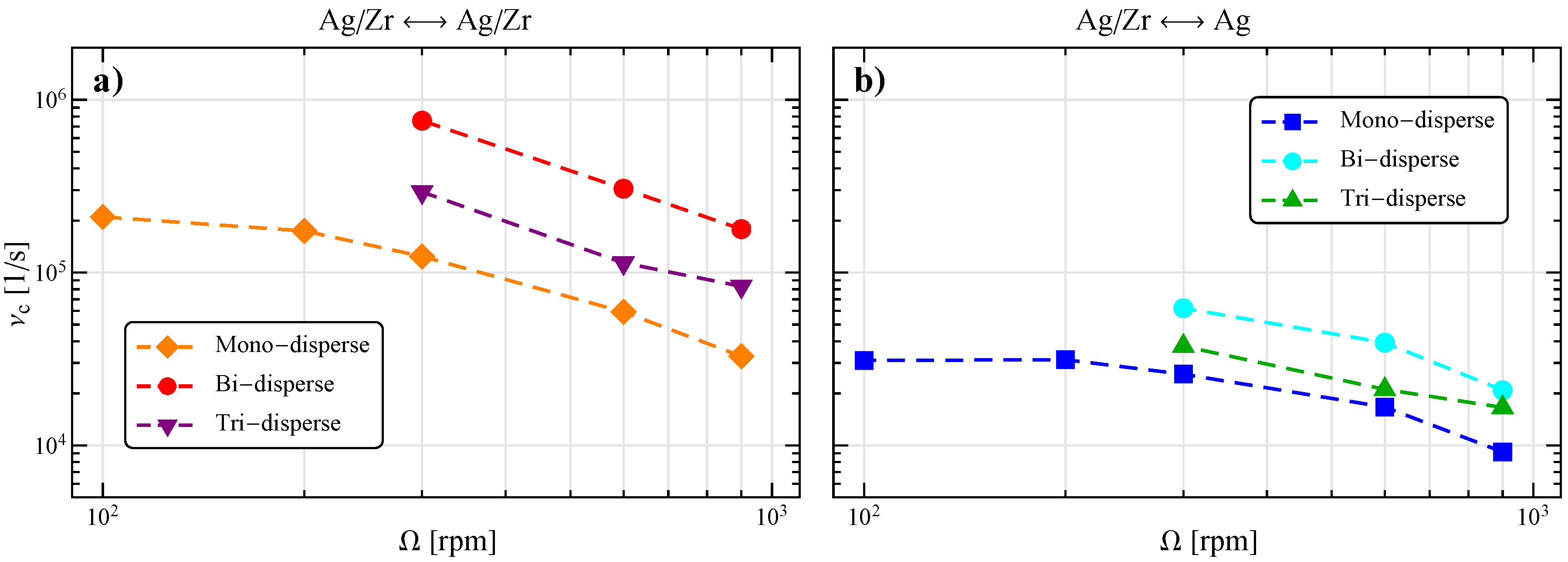
3.3. Influence of the Stirring Bar Inclination
3.4. Influence of the Bead Size
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pastorino, L.; Dellacasa, E.; Dabiri, M.H.; Fabiano, B.; Erokhina, S. Towards the fabrication of polyelectrolyte-based nanocapsules for bio-medical applications. BioNanoScience 2016, 6, 496–501. [Google Scholar] [CrossRef]
- Fabiano, B.; Pistritto, F.; Reverberi, A.; Palazzi, E. Ethylene–air mixtures under flowing conditions: A model-based approach to explosion conditions. Clean Technol. Environ. Policy 2015, 17, 1261–1270. [Google Scholar] [CrossRef]
- Reverberi, A.P.; Varbanov, P.S.; Vocciante, M.; Fabiano, B. Bismuth oxide-related photocatalysts in green nanotechnology: A critical analysis. Front. Chem. Sci. Eng. 2018, 12, 878–892. [Google Scholar] [CrossRef]
- Reverberi, A.P.; Vocciante, M.; Salerno, M.; Caratto, V.; Fabiano, B. Bi nanoparticles synthesis by a bottom-up wet chemical process. Chem. Eng. Trans. 2019, 73, 283–288. [Google Scholar] [CrossRef]
- Vocciante, M.; De Folly D’Auris, A.; Finocchi, A.; Tagliabue, M.; Bellettato, M.; Ferrucci, A.; Reverberi, A.P.; Ferro, S. Adsorption of ammonium on clinoptilolite in presence of competing cations: Investigation on groundwater remediation. J. Clean. Prod. 2018, 198, 480–487. [Google Scholar] [CrossRef]
- Toccafondi, C.; Dante, S.; Reverberi, A.P.; Salerno, M. Biomedical applications of anodic porous alumina. Curr. Nanosci. 2015, 11, 572–580. [Google Scholar] [CrossRef]
- Pascariu, V.; Avadanei, O.; Gasner, P.; Stoica, I.; Reverberi, A.P.; Mitoseriu, L. Preparation and characterization of PbTiO3–epoxy resin compositionally graded thick films. Phase Transit. 2013, 86, 715–725. [Google Scholar] [CrossRef]
- Reverberi, A.P.; Salerno, M.; Lauciello, S.; Fabiano, B. Synthesis of copper nanoparticles in ethylene glycol by chemical reduction with vanadium (+ 2) salts. Materials 2016, 9, 809. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, C.; Liu, M.; Huang, R.; Su, R.; Qi, W.; He, Z. Dechlorination of p-Chlorophenol by Fe-Pd Nanoparticles Promoted by Poly (gamma-glutamic acid)–Dopamine Composite. Chem. Eng. Trans. 2018, 70, 2095–2100. [Google Scholar] [CrossRef]
- Reverberi, A.; Vocciante, M.; Lunghi, E.; Pietrelli, L.; Fabiano, B. New trends in the synthesis of nanoparticles by green methods. Chem. Eng. Trans. 2017, 61, 667–672. [Google Scholar] [CrossRef]
- Fabiano, B.; Reverberi, A.P.; Varbanov, P.S. Safety opportunities for the synthesis of metal nanoparticles and short-cut approach to workplace risk evaluation. J. Clean. Prod. 2019, 209, 297–308. [Google Scholar] [CrossRef]
- Promentilla, M.A.; Janairo, J.I.; Yu, D. A green chemistry-based decision modelling approach for optimal selection of nanomaterial’s synthesis method. Chem. Eng. Trans. 2017, 61, 241–246. [Google Scholar] [CrossRef]
- Nagar, N.; Devra, V. Green synthesis and characterization of copper nanoparticles using Azadirachta indica leaves. Mater. Chem. Phys. 2018, 213, 44–51. [Google Scholar] [CrossRef]
- Korir, D.K.; Gwalani, B.; Joseph, A.; Kamras, B.; Arvapally, R.K.; Omary, M.A.; Marpu, S.B. Facile photochemical syntheses of conjoined nanotwin gold-silver particles within a biologically-benign chitosan polymer. Nanomaterials 2019, 9, 596. [Google Scholar] [CrossRef] [Green Version]
- Reverberi, A.P.; Varbanov, P.S.; Lauciello, S.; Salerno, M.; Fabiano, B. An eco-friendly process for zerovalent bismuth nanoparticles synthesis. J. Clean. Prod. 2018, 198, 37–45. [Google Scholar] [CrossRef]
- Ogi, T.; Zulhijah, R.; Iwaki, T.; Okuyama, K. Recent progress in nanoparticle dispersion using bead mill. KONA Powder Part. J. 2017, 2017004. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.E.; Ullah, M.; Maamor, A.; Hamid, S.B.A. Surfactant Assisted Ball Milling: A Simple Top down Approach for the Synthesis of Controlled Structure Nanoparticle. Adv. Mater. Res. 2014, 832, 356–361. [Google Scholar] [CrossRef]
- Ullah, M.; Ali, M.; Hamid, S.B.A. Surfactant-assisted ball milling: A novel route to novel materials with controlled nanostructure—A review. Rev. Adv. Mater. Sci. 2014, 37, 1–14. [Google Scholar]
- Joni, I.M.; Ogi, T.; Iwaki, T.; Okuyama, K. Synthesis of a colorless suspension of TiO2 nanoparticles by nitrogen doping and the bead-mill dispersion process. Ind. Eng. Chem. Res. 2013, 52, 547–555. [Google Scholar] [CrossRef]
- Vocciante, M.; Trofa, M.; D’Avino, G.; Reverberi, A.P. Nanoparticles synthesis in wet-operating stirred media: Preliminary investigation with DEM simulations. Chem. Eng. Trans. 2019, 73, 31–36. [Google Scholar] [CrossRef]
- Trofa, M.; Vocciante, M. Discrete element simulations of nanoparticles synthesis in wet-operating stirred media: Effect of the particle material. Chem. Eng. Trans. 2020, 83. in press. [Google Scholar]
- Reverberi, A.P.; Vocciante, M.; Salerno, M.; Ferretti, M.; Fabiano, B. Green Synthesis of Silver Nanoparticles by Low-Energy Wet Bead Milling of Metal Spheres. Materials 2020, 13, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, J.S.; Li, S. Adhesive Particle Flow; Cambridge University Press: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Trofa, M.; D’Avino, G.; Sicignano, L.; Tomaiuolo, G.; Greco, F.; Maffettone, P.L.; Guido, S. CFD-DEM simulations of particulate fouling in microchannels. Chem. Eng. J. 2019, 358, 91–100. [Google Scholar] [CrossRef]
- Fragnière, G.; Beinert, S.; Overbeck, A.; Kampen, I.; Schilde, C.; Kwade, A. Predicting effects of operating condition variations on breakage rates in stirred media mills. Chem. Eng. Res. Des. 2018, 138, 433–443. [Google Scholar] [CrossRef]
- Smith, D.R.; Fickett, F. Low-temperature properties of silver. J. Res. Natl. Inst. Stand. Technol. 1995, 100, 119. [Google Scholar] [CrossRef]
- Gondret, P.; Lance, M.; Petit, L. Bouncing motion of spherical particles in fluids. Phys. Fluids 2002, 14, 643–652. [Google Scholar] [CrossRef]
- Stronge, W.J. Impact Mechanics; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2018. [Google Scholar] [CrossRef] [Green Version]
- Kloss, C.; Goniva, C.; Hager, A.; Amberger, S.; Pirker, S. Models, algorithms and validation for opensource DEM and CFD–DEM. Prog. Comput. Fluid Dyn. Int. J. 2012, 12, 140–152. [Google Scholar] [CrossRef]
- Bitter, J. A study of erosion phenomena. Wear 1963, 6, 169–190. [Google Scholar] [CrossRef]
- Sheldon, G.L. Similarities and Differences in the Erosion Behavior of Materials. J. Basic Eng. 1970, 92, 619–626. [Google Scholar] [CrossRef]
- Hutchings, I.; Shipway, P. Tribology: Friction and Wear of Engineering Materials; Butterworth-Heinemann, an Imprint of Elsevier: Oxford, UK, 2017. [Google Scholar]


| Parameter | Equation |
|---|---|
| Equivalent radius | |
| Equivalent mass | |
| Equivalent Young modulus | |
| Equivalent shear modulus | |
| Normal overlap | |
| Tangential overlap | |
| Normal stiffness | |
| Tangential stiffness | |
| Normal damping coefficient | |
| Tangential damping coefficient |
| Zirconia, ZrO | Silver, Ag | Glass | PTFE | |
|---|---|---|---|---|
| Density [] | 6067 | 10490 | 2510 | 2200 |
| Young’s Modulus [GPa] | 210.0 | 82.0 | 70.0 | 0.50 |
| Poisson ratio | 0.31 | 0.36 | 0.24 | 0.46 |
| Coefficient of restitution | 0.92 | 0.80 | 0.99 | 0.80 |
| Coefficient of friction | 0.15 | 0.55 | 0.27 | 0.08 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trofa, M.; D’Avino, G.; Fabiano, B.; Vocciante, M. Nanoparticles Synthesis in Wet-Operating Stirred Media: Investigation on the Grinding Efficiency. Materials 2020, 13, 4281. https://doi.org/10.3390/ma13194281
Trofa M, D’Avino G, Fabiano B, Vocciante M. Nanoparticles Synthesis in Wet-Operating Stirred Media: Investigation on the Grinding Efficiency. Materials. 2020; 13(19):4281. https://doi.org/10.3390/ma13194281
Chicago/Turabian StyleTrofa, Marco, Gaetano D’Avino, Bruno Fabiano, and Marco Vocciante. 2020. "Nanoparticles Synthesis in Wet-Operating Stirred Media: Investigation on the Grinding Efficiency" Materials 13, no. 19: 4281. https://doi.org/10.3390/ma13194281





