Waste Rubber Pyrolysis: Product Yields and Limonene Concentration
Abstract
:1. Introduction
2. Experimental Part
2.1. Materials and Methods
2.2. Pyrolysis Process
2.3. Analytical Methods
3. Results and Discussion
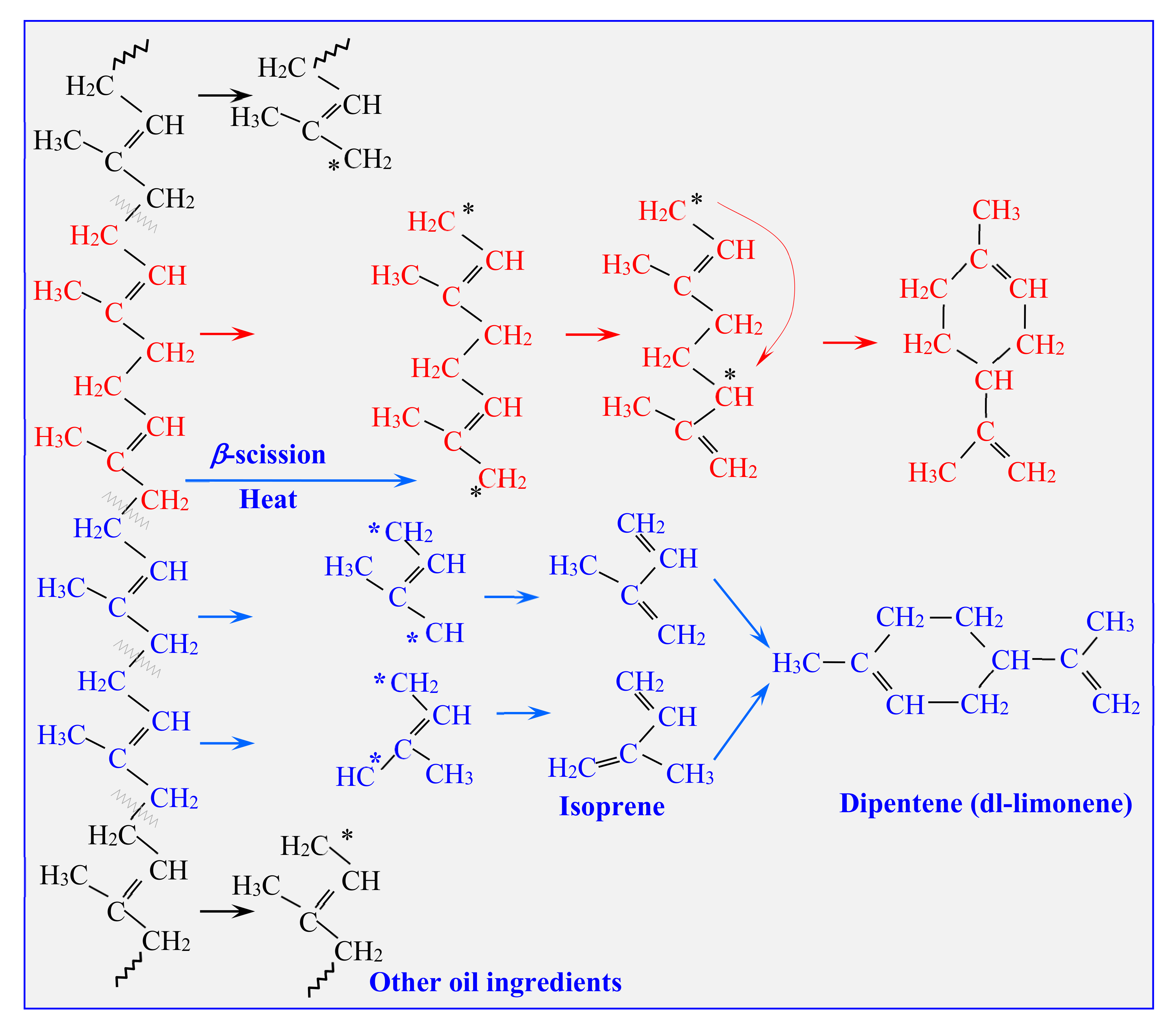
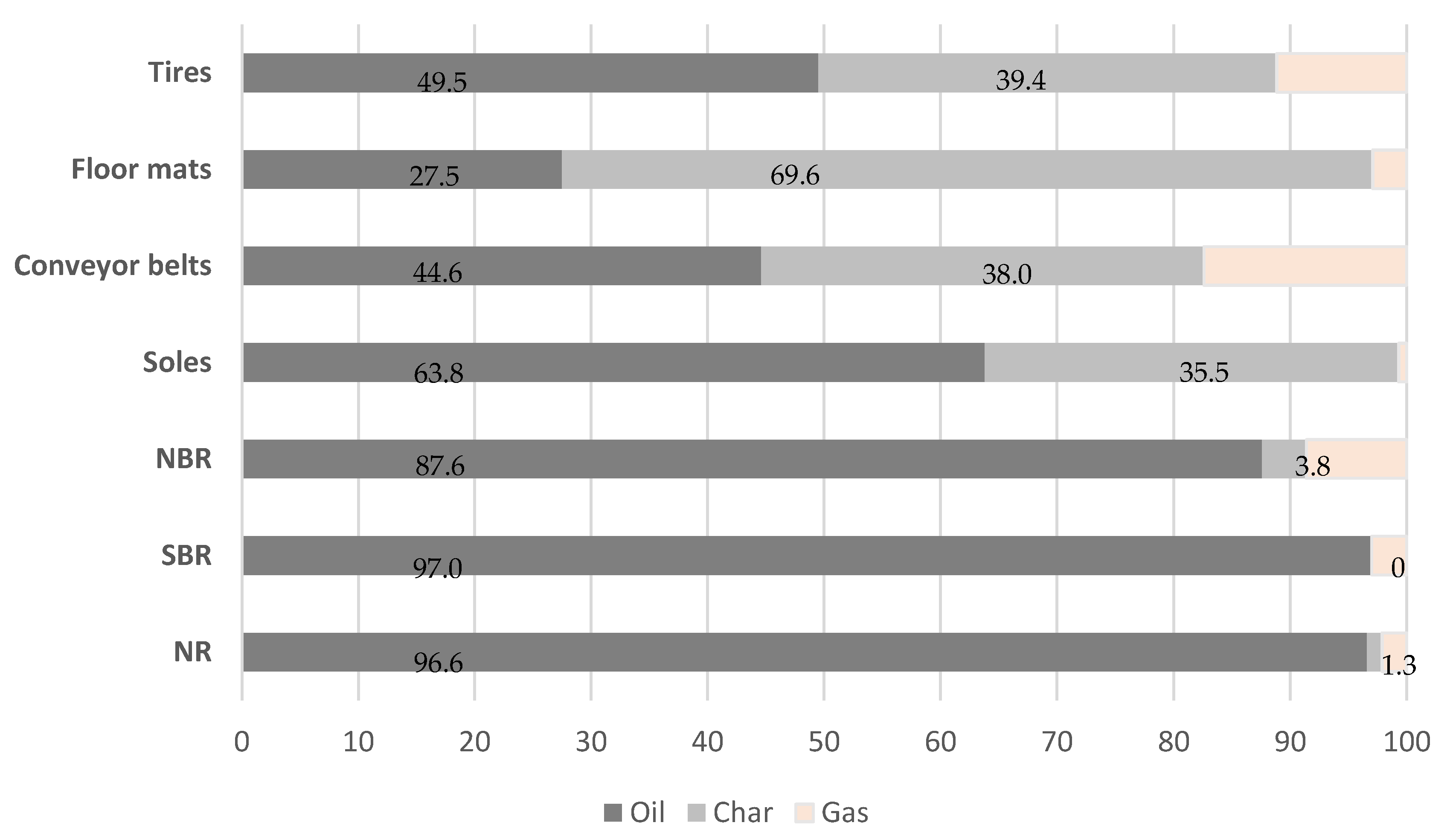
3.1. Pyrolysis Process on a Microgram Scale
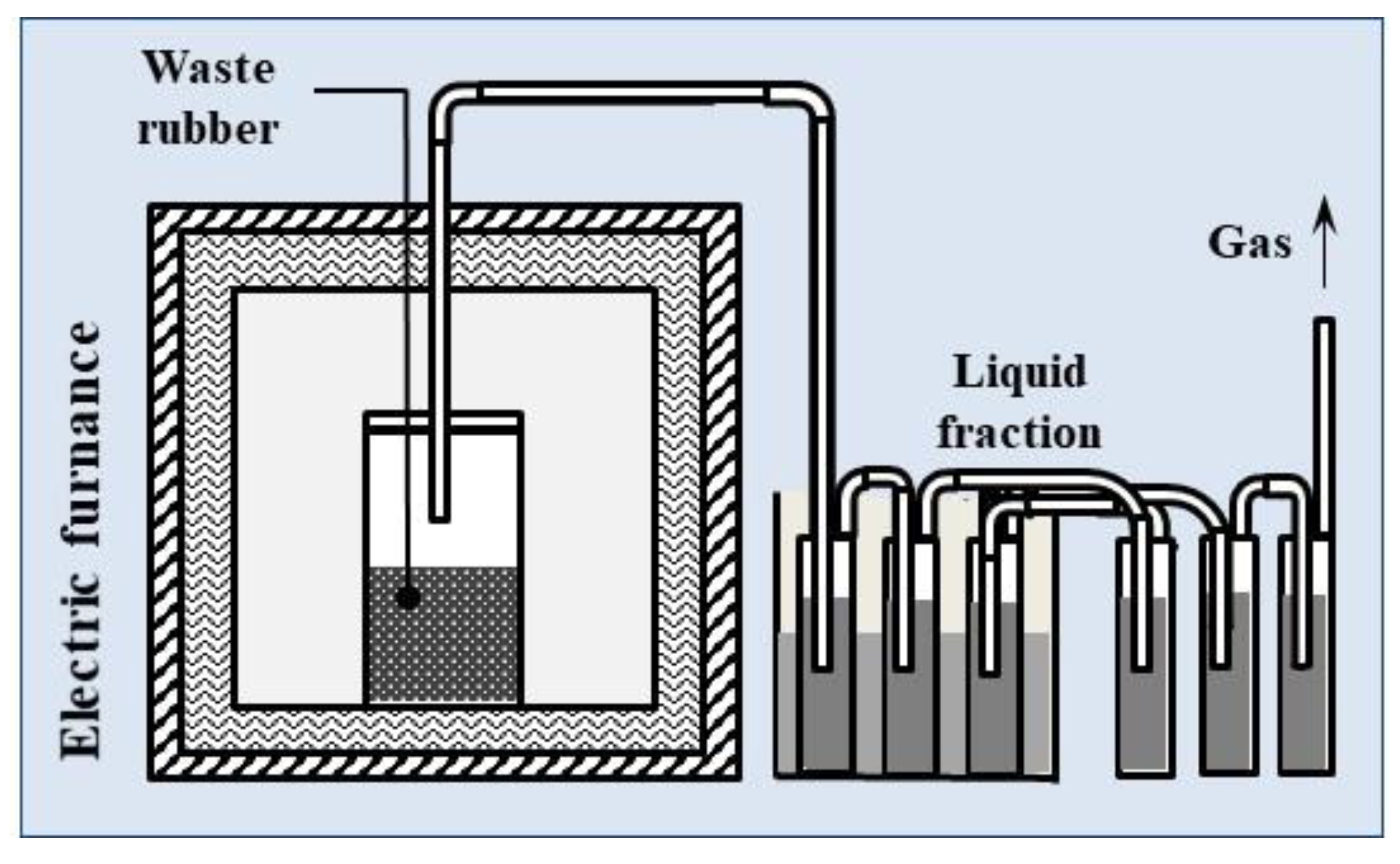
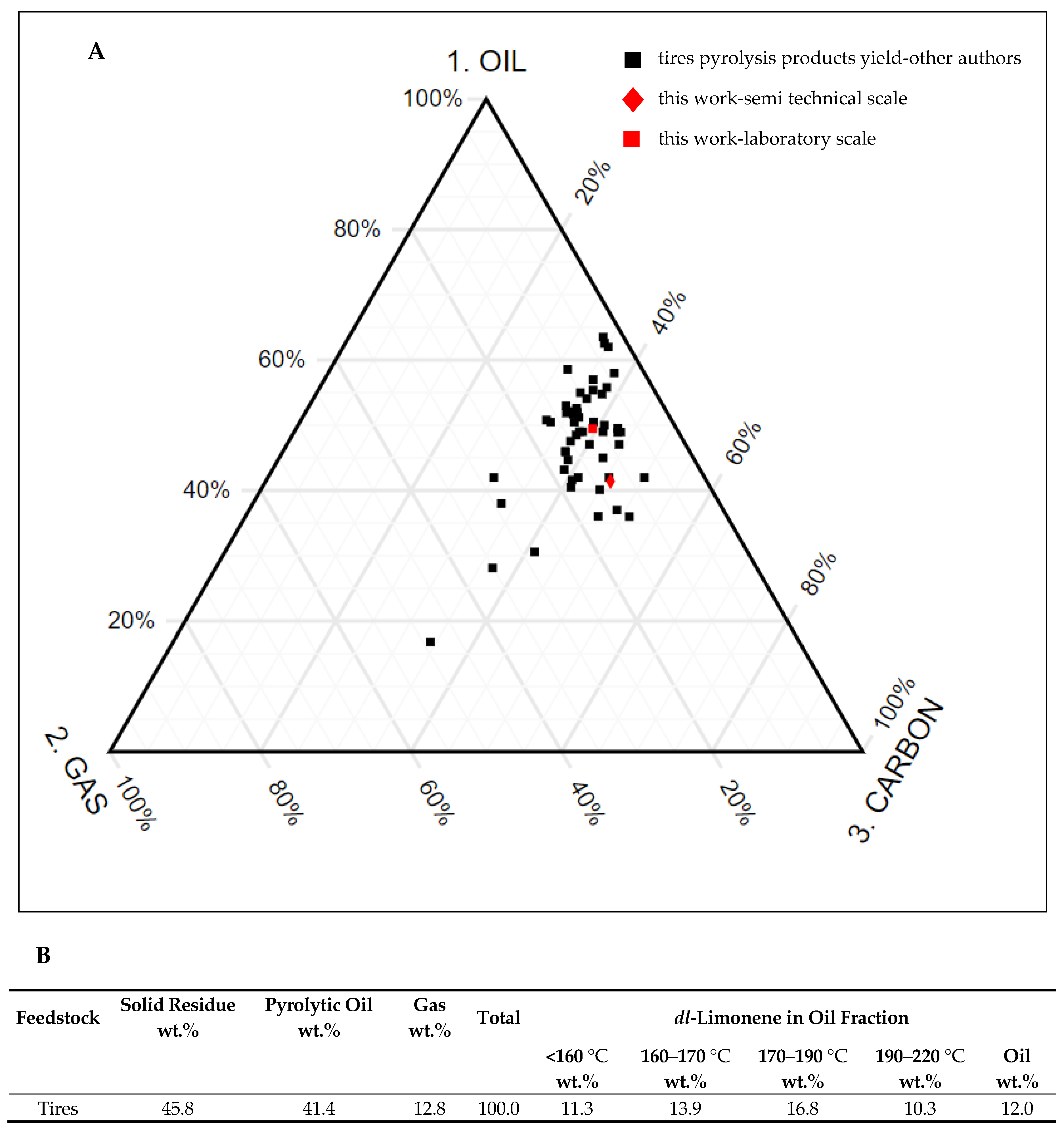
3.2. Pyrolysis Process on a Laboratory Scale
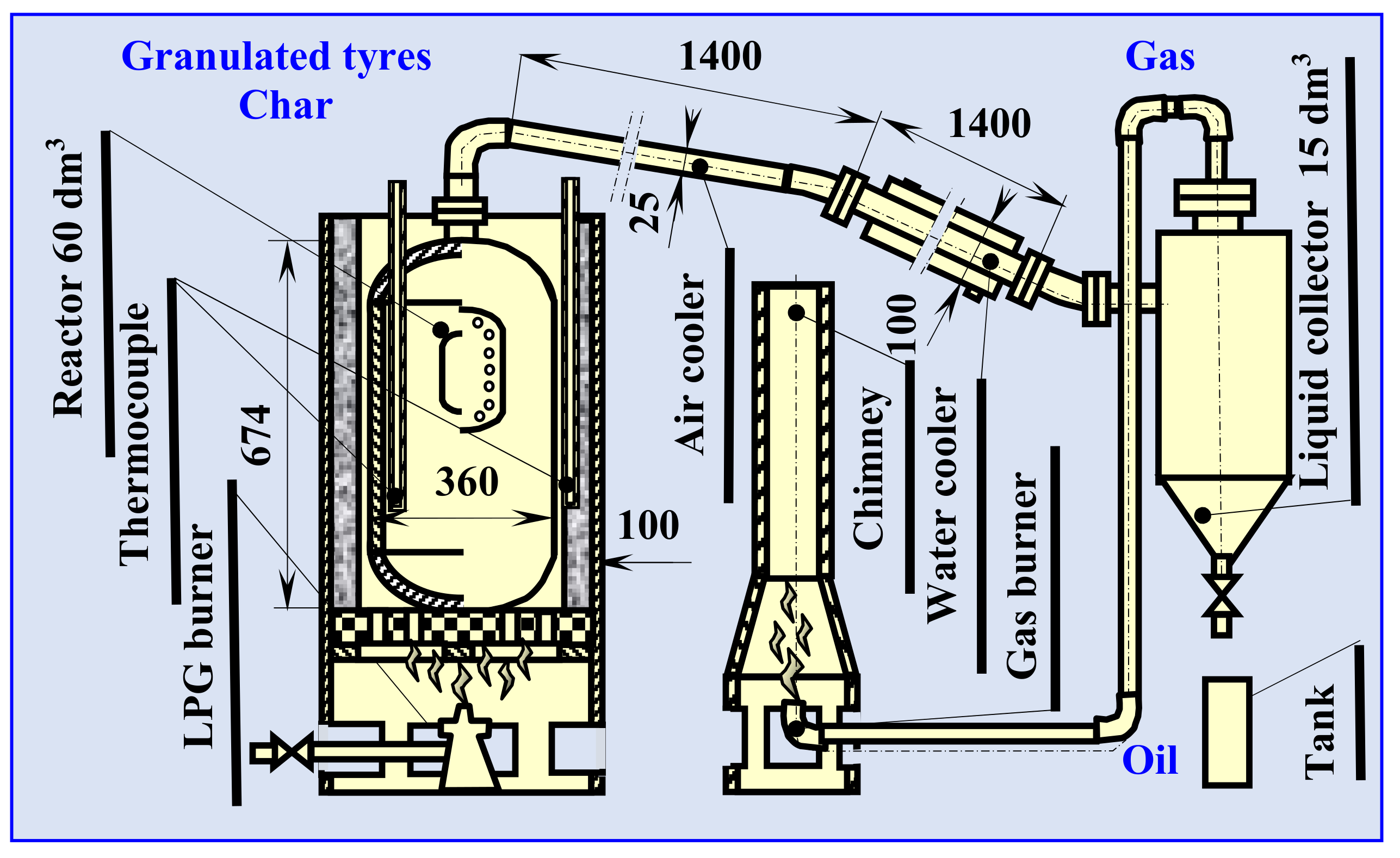
3.3. Semi-Technical Tires Pyrolysis Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Europe Tire Market by Vehicle Type (PC, LCV, M&HCV, 2W & OTR), by Online vs. Offline, by Radial vs. Bias, by Demand Category (OEM vs. Replacement), by Country, Competition Forecast & Opportunities, 2013–2023; TechSci Research: Noida, India, 2018.
- Singh, R.K.; Ruj, B.; Jana, A.; Mondal, S.; Jana, B.; Sadhukhan, A.K.; Gupta, P. Pyrolysis of three different categories of automotive tyre wastes: Product yield analysis and characterization. J. Anal. Appl. Pyrolysis 2018, 135, 379. [Google Scholar] [CrossRef]
- Rofiqul Islam, M.; Haniu, H.; Rafiqul Alam Beg, M. Liquid fuels and chemicals from pyrolysis of motorcycle tire waste: Product yields, compositions and related properties. Fuel 2008, 87, 3112–3122. [Google Scholar] [CrossRef]
- Dȩbek, C. Modification of pyrolytic oil from waste tyres as a promising method for light fuel production. Materials 2019, 16, 880. [Google Scholar] [CrossRef] [Green Version]
- Zedler, Ł.; Kowalkowska-Zedler, D.; Vahabi, H.; Saeb, M.R.; Colom, X.; Cañavate, J.; Wang, S.; Formela, K. Preliminary investigation on auto-thermal extrusion of ground tire rubber. Materials 2019, 12, 2090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janajreh, I.; Raza, S.S. Gasification of Waste Tires; Department of Mechanical and Materials Engineering, Masdar Institute of Science and Technology: Abu Dhabi, UAE, 2002. [Google Scholar]
- Kluska, J.; Ochnio, M.; Kazimierski, P.; Kardaś, D. Comparison of downdraft and updraft gasification of biomass in a fixed bed reactor. Arch. Thermodyn. 2018, 39, 59–69. [Google Scholar]
- Lewandowski, W.M.W.M.; Januszewicz, K.; Kosakowski, W. Efficiency and proportions of waste tyre pyrolysis products depending on the reactor type—A review. J. Anal. Appl. Pyrolysis 2019, 140, 25–53. [Google Scholar] [CrossRef]
- Hita, I.; Arabiourrutia, M.; Olazar, M.; Bilbao, J.; Arandes, J.M.; Castaño Sánchez, P. Opportunities and barriers for producing high quality fuels from the pyrolysis of scrap tires. Renew. Sustain. Energy Rev. 2016, 56, 745–759. [Google Scholar] [CrossRef]
- Acosta, R.; Tavera, C.; Gauthier-Maradei, P.; Nabarlatz, D. Production of oil and char by intermediate pyrolysis of scrap tyres: Influence on yield and product characteristics. Int. J. Chem. React. Eng. 2015, 13, 189–200. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, J.; Wang, X.; Liu, P.; Li, J.; Liu, G.; Wang, K.; Li, M.; Zhong, Z.; Xu, J.; et al. Catalytic conversion of rubber wastes to produce aromatic hydrocarbons over USY zeolites: Effect of SiO2/Al2O3 mole ratio. Energy Convers. Manag. 2019, 197. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, G.; Chen, C.; Wang, Y.; Wang, W.; Li, A. Liquid oils produced from pyrolysis of plastic wastes with heat carrier in rotary kiln. Fuel Process. Technol. 2020, 206, 106455. [Google Scholar] [CrossRef]
- Costa, G.A.; Santos, R.G. Dos Fractionation of tire pyrolysis oil into a light fuel fraction by steam distillation. Fuel 2019, 241, 558–563. [Google Scholar] [CrossRef]
- Malko, M.; Wróblewska, A. Znaczenie R-(+)-limonenu, jako surowca do syntez w chemii organicznej i dla przemysłu organicznego. Chemik 2016, 70, 193–202. [Google Scholar]
- Ahmad, N.; Ahmad, N.; Maafa, I.M.; Ahmed, U.; Akhter, P.; Shehzad, N.; Amjad, U.; Hussain, M.; Javaid, M. Conversion of poly-isoprene based rubber to value-added chemicals and liquid fuel via ethanolysis: Effect of operating parameters on product quality and quantity. Energy 2020, 191, 116543. [Google Scholar] [CrossRef]
- Yan, S.; Xia, D.; Xuan, W. New insight into enhancement effect of supercritical water on scrap tire depolymerization: A study based on ReaxFF-MD simulation and DFT method. Fuel Process. Technol. 2020, 200, 106309. [Google Scholar] [CrossRef]
- Stanciulescu, M.; Ikura, M. Limonene ethers from tire pyrolysis oil. Part 2: Continuous flow experiments. J. Anal. Appl. Pyrolysis 2007, 78, 76–84. [Google Scholar] [CrossRef]
- Tavera Ruiz, C.P.; Gauthier-Maradei, P.; Capron, M.; Pirez, C.; Gardoll, O.; Katryniok, B.; Dumeignil, F. Transformation of dl Limonene into Aromatic Compounds Using Supported Heteropolyacid Catalysts. Catal. Lett. 2019, 149, 328–337. [Google Scholar] [CrossRef]
- Stanciulescu, M.; Ikura, M. Limonene ethers from tire pyrolysis oil: Part 1: Batch experiments. J. Anal. Appl. Pyrolysis 2006, 75, 217–225. [Google Scholar] [CrossRef]
- Pakdel, H.; Pantea, D.M.; Roy, C. Production of dl-limonene by vacuum pyrolysis of used tires. J. Anal. Appl. Pyrolysis 2001, 57, 91–107. [Google Scholar] [CrossRef]
- Danon, B.; Van Der Gryp, P.; Schwarz, C.E.; Görgens, J.F. A review of dipentene (dl-limonene) production from waste tire pyrolysis. J. Anal. Appl. Pyrolysis 2015, 112, 1–13. [Google Scholar] [CrossRef]
- Hall, W.J.; Zakaria, N.; Williams, P.T. Pyrolysis of latex gloves in the presence of Y-zeolite. Waste Manag. 2009, 29, 797–803. [Google Scholar] [CrossRef] [Green Version]
- Pakdel, H.; Roy, C.; Aubln, H.; Jean, G.; Coulombe, S. Formation of dl-limonene in used tire vacuum pyrolysis oils. Environ. Sci. Technol. 1991, 25, 1646–1649. [Google Scholar] [CrossRef]
- Amutio, M.; Lopez, G.; Artetxe, M.; Elordi, G.; Olazar, M.; Bilbao, J. Influence of temperature on biomass pyrolysis in a conical spouted bed reactor. Resour. Conserv. Recycl. 2012, 59, 23–31. [Google Scholar] [CrossRef]
- Alvarez, J.; Lopez, G.; Amutio, M.; Mkhize, N.M.; Danon, B.; van der Gryp, P.; Görgens, J.F.; Bilbao, J.; Olazar, M. Evaluation of the properties of tyre pyrolysis oils obtained in a conical spouted bed reactor. Energy 2017, 128, 463–474. [Google Scholar] [CrossRef]
- Zwart, R.W.R.; Vreugdenhil, B.J. Tar Formation in Pyrolysis and Gasification; Energy Research Centre of the Netherlands: Petten, The Netherlands, 2009; p. 37. [Google Scholar]
- Dierkes, W. Raw Materials and Compunds in Rubber; VERT/University of Twente: Enschede, The Netherlands, 2007; pp. 1–119. [Google Scholar]
- Conesa, J.A.; Martín-Gullón, I.; Font, R.; Jauhiainen, J. Complete study of the pyrolysis and gasification of scrap tires in a pilot plant reactor. Environ. Sci. Technol. 2004, 38, 3189–3194. [Google Scholar] [CrossRef] [PubMed]
- Arabiourrutia, M.; Lopez, G.; Elordi, G.; Olazar, M.; Aguado, R.; Bilbao, J. Product distribution obtained in the pyrolysis of tyres in a conical spouted bed reactor. Chem. Eng. Sci. 2007, 62, 5271–5275. [Google Scholar] [CrossRef]
| TYPE | Natural Rubber—NR | Styrene-Butadiene Rubber—SBR | Acrylonitrile Butadiene Rubber—NBR | Conveyor Belts | Floor Mats | Waste Tires | Soles |
| RAW MATERIAL |  |  |  |  |  |  |  |
| CHAR | without char | without char | without char |  |  |  |  |
| Proximate Analysis (wt.%) | Elemental Analysis (wt.%) | ||||||
|---|---|---|---|---|---|---|---|
| Sample | Fixed Carbon | Ash | Volatile | C | H | N | S |
| Tires | 48.3 | 8.7 | 43.0 | 81.0 | 6.6 | 0.6 | 1.9 |
| Char tires | 65.1 | 0.4 | 0.2 | 0.9 | |||
| Conveyor belts | 28.1 | 7.8 | 64.1 | 78.5 | 6.9 | 0.6 | 0.7 |
| Char con. belts | 53.1 | 1.6 | 0.4 | 0.0 | |||
| Floor mats | 11.4 | 45.6 | 43 | 31.6 | 3.5 | 0.0 | 0.2 |
| Char f. mats | 21.4 | 0.2 | 0.0 | 0.0 | |||
| Soles | 2.2 | 28.6 | 68.9 | 55.7 | 6.9 | 0.6 | 0.7 |
| Char soles | 32.3 | 0.6 | 0.3 | 0.5 | |||
| NR | - | 0.0 | 100.0 | 85.8 | 11.4 | 1.0 | 0.0 |
| NBR | - | 0.0 | 100.0 | 80.6 | 9.9 | 7.6 | 0.0 |
| SBR | - | 0.0 | 100.0 | 85.8 | 10.9 | 0.3 | 0.0 |
| (A) | |||||||
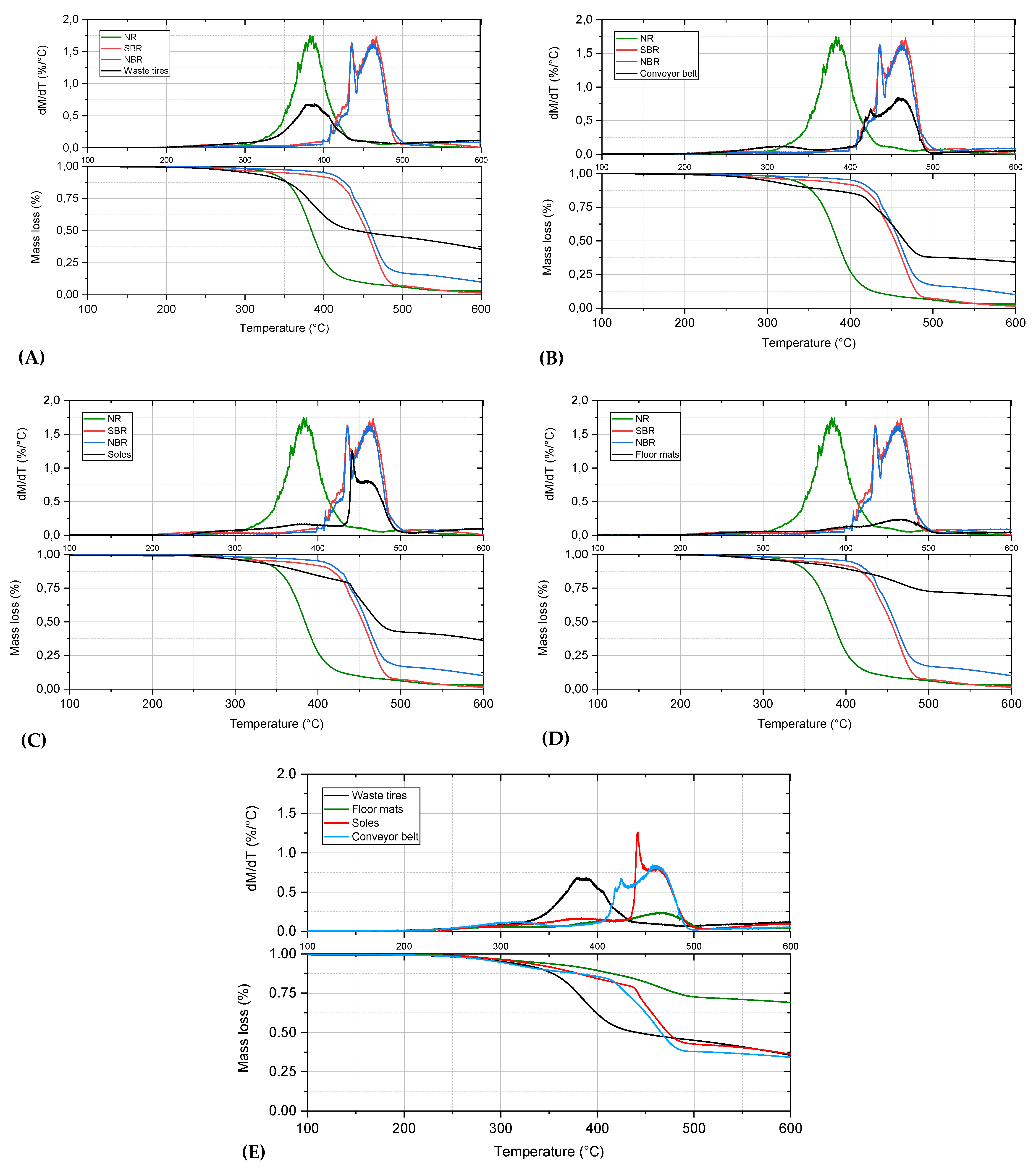
| Raw Material | Decomposition Temperature (C) | DTG Maxima | Char at 750 (wt.%) | ||||
| T2% | T5% | T10% | T50% | Tmax (°C) | (dm/dT)max (%/°C) | ||
| Tires | 254.8 | 302.6 | 343.4 | 441.1 | 381.6 | 0.7 | 20.5 |
| Conveyor belt | 256.8 | 296.9 | 343.5 | 465.7 | 459.2 | 0.8 | 26.5 |
| Soles | 274.6 | 317.1 | 363.5 | 472.7 | 441.5 | 1.3 | 23.9 |
| Floor mats | 272.6 | 328.2 | 395.5 | 763.5 | 463.4 | 0.2 | 54 |
| NBR | 315.8 | 401.9 | 421.6 | 458.8 | 461.2 | 1.6 | 1.9 |
| SBR | 267.3 | 350.4 | 411.6 | 453.9 | 461.2 | 1.7 | 1.6 |
| NR | 302.9 | 330.7 | 348.2 | 384.4 | 382.4 | 1.8 | 3 |
| (B) | |||||||
| Raw Material | Calculate Based on DTG (wt.%) | ||||||
| NR | NBR/SBR | Inorganic | |||||
| Tires | 26.8 | 25.3 | 20.5 | ||||
| Conveyor belt | 12.7 | 33.4 | 26.5 | ||||
| Soles | 12.9 | 28.3 | 23.9 | ||||
| Floor mats | 8.4 | 11.9 | 54.0 | ||||
| Raw Material | Limonene wt.% |
|---|---|
| Tires | 4.3 |
| Floor mats | 1.6 |
| Conveyor belts | 1.8 |
| Soles | 1.2 |
| NBR | 0.0 |
| SBR | 0.0 |
| NR | 18.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Januszewicz, K.; Kazimierski, P.; Suchocki, T.; Kardaś, D.; Lewandowski, W.; Klugmann-Radziemska, E.; Łuczak, J. Waste Rubber Pyrolysis: Product Yields and Limonene Concentration. Materials 2020, 13, 4435. https://doi.org/10.3390/ma13194435
Januszewicz K, Kazimierski P, Suchocki T, Kardaś D, Lewandowski W, Klugmann-Radziemska E, Łuczak J. Waste Rubber Pyrolysis: Product Yields and Limonene Concentration. Materials. 2020; 13(19):4435. https://doi.org/10.3390/ma13194435
Chicago/Turabian StyleJanuszewicz, Katarzyna, Paweł Kazimierski, Tomasz Suchocki, Dariusz Kardaś, Witold Lewandowski, Ewa Klugmann-Radziemska, and Justyna Łuczak. 2020. "Waste Rubber Pyrolysis: Product Yields and Limonene Concentration" Materials 13, no. 19: 4435. https://doi.org/10.3390/ma13194435





