Preparation and Thermal Properties of Modified Cu2O/Polypropylene (PP) Composite
Abstract
:1. Introduction
2. Experimental Section
2.1. Raw Materials
2.2. Synthesized of Superfine Cu2O Sphere
2.3. Modification of Cu2O
2.4. Synthesis of Cu2O/PP Composite
2.5. Characterization Methods
3. Results and Discussion
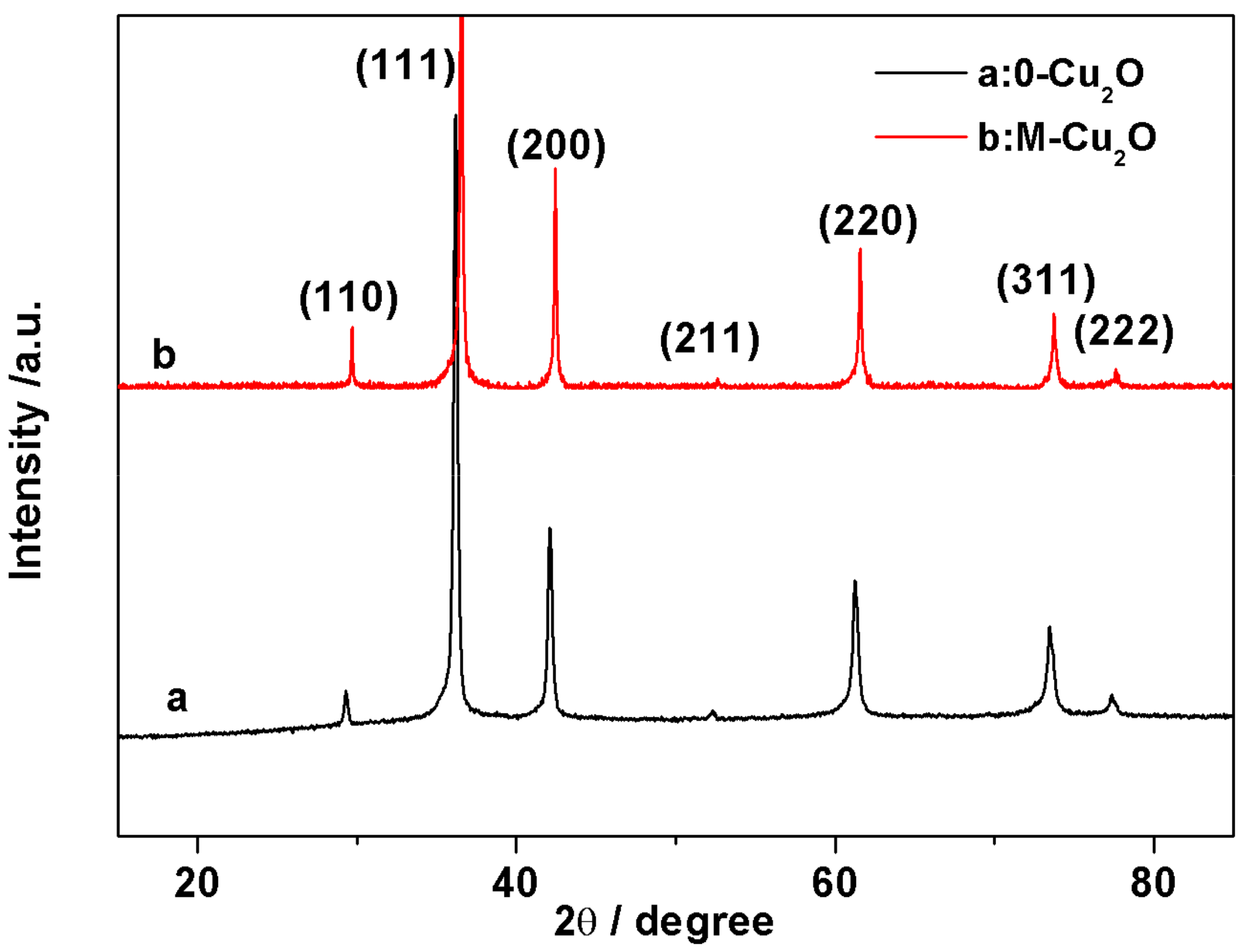
3.1. XRD and SEM of As-Synthesized Cu2O and M-Cu2O
3.2. Characterization of Surface Modification of Cuprous Oxide
3.2.1. Fourier-Transform Infrared Spectroscopy (FTIR)
3.2.2. Thermogravimetric Analysis (TGA) of Inorganic Particles Cu2O
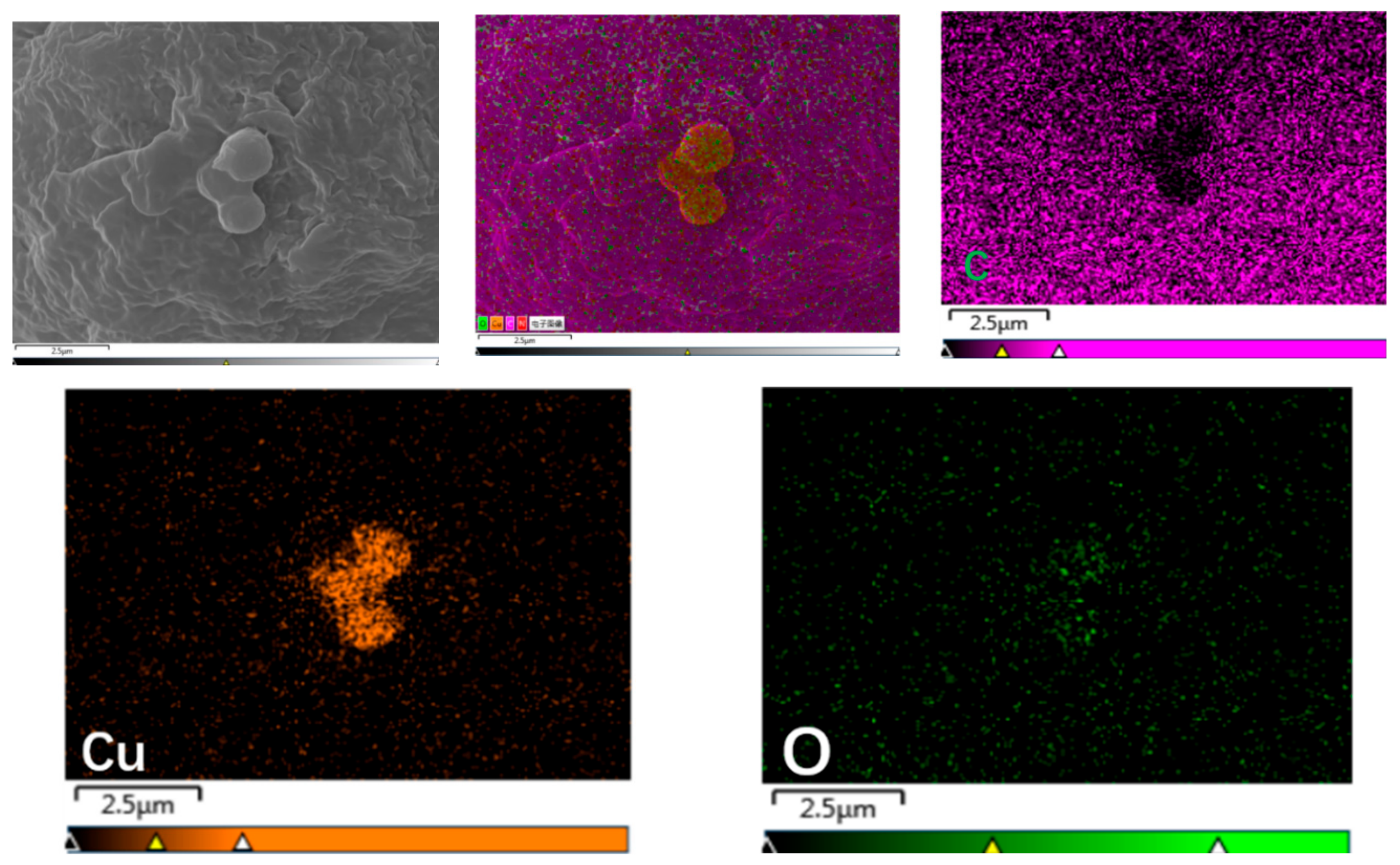
3.2.3. Dispersion of 0-Cu2O and M-Cu2O in PP
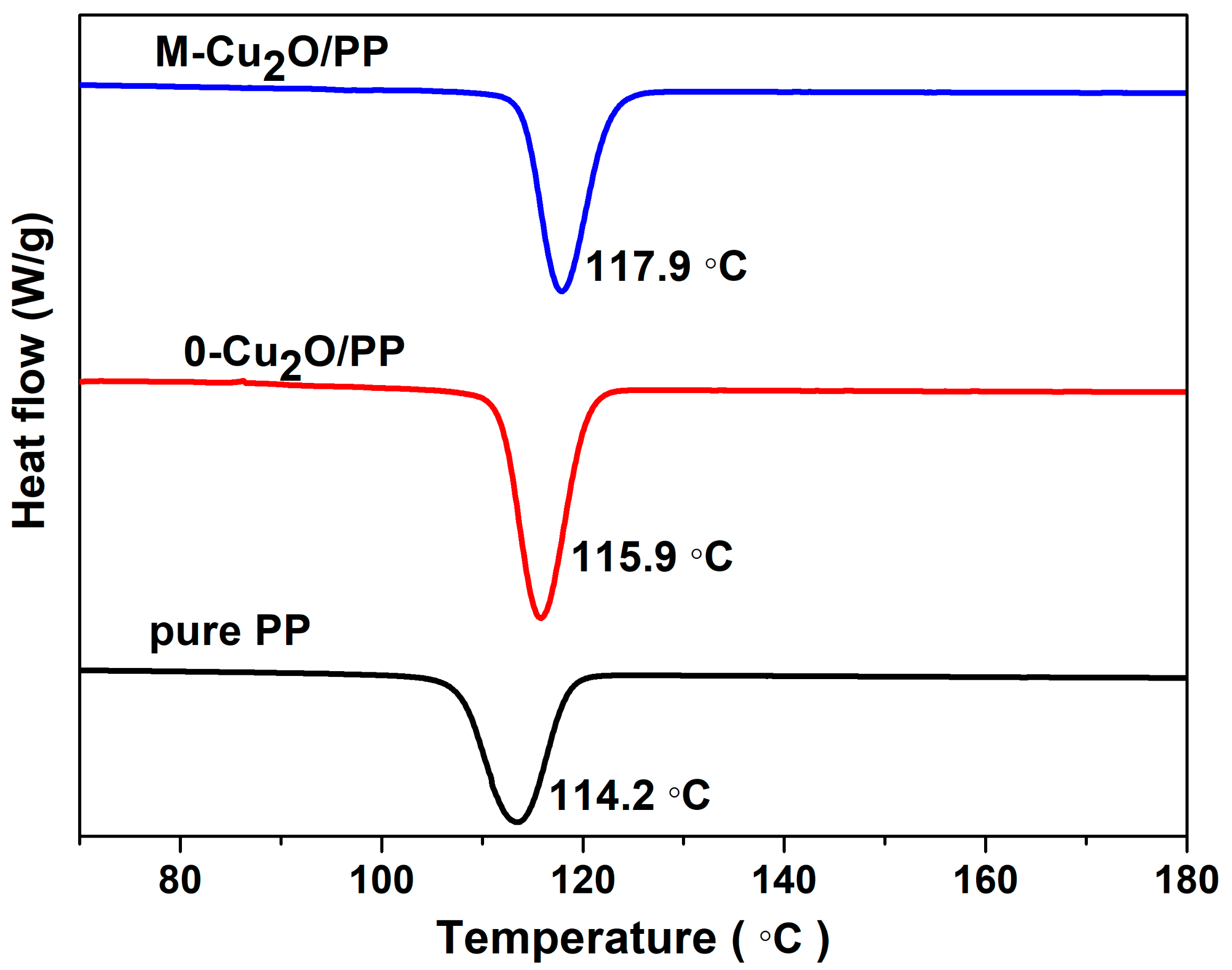
3.3. Thermal Properties of 0-Cu2O/PP and M-Cu2/PP Composites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ganb, M.; Satapathy, B.K.; Thunga, M.; Weidisch, R.; Pötschke, P.; Jehnichen, D. Structural interpretations of deformation and fracture behavior of poly-propylene/multi-walled carbon nanotube composites. Acta Mater. 2008, 56, 2247–2261. [Google Scholar]
- Seomk, M.K.; Lee, J.R.; Park, S.J. Crystallization kinetics and interfacial behaviors of polypropylene composites reinforced with multi-walled carbon nanotubes. Mater. Sci. Eng. A 2005, 404, 79–84. [Google Scholar]
- Gawish, S.M.; Avci, H.; Ramadan, A.M.; Mosleh, S.; Monticello, R.; Breidt, F.; Kotek, R. Properties of antibacterial polypropylene/nanometal composite fibers. J. Biomater. Sci. 2012, 23, 43–61. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, C.; Duraccio, D.; Cimmino, S. Food packaging based on polymer nanomaterials. Progr. Polym. Sci. 2011, 36, 1766–1782. [Google Scholar] [CrossRef]
- Rungruang, P.; Grady, B.P.; Supaphol, P. Surface-modified calcium carbonate particles by admicellar polymerization to be used as filler for isotactic polypropylene. Colloids Surf. A Physicochem. Eng. Asp. 2006, 275, 114–125. [Google Scholar] [CrossRef]
- Avella, M.; Cosco, S.; Lorenzo, M.L.D.; Pace, E.D.; Errico, M.E.; Gentile, G. iPP Based Nanocomposites Filled with Calcium Carbonate Nanoparticles: Structure/Properties Relationships; Weinh. WILEY-VCH Verl: Weinheim, Germany, 2006; pp. 156–162. [Google Scholar]
- Hui, C.Y.; Shia, D. Simple formulae for the effective moduli of unidirectional aligned composites. Polym. Eng. Sci. 1998, 38, 774–782. [Google Scholar] [CrossRef]
- Santos, M.A.; Maliska, A.M.; Klein, A.N.; Junior, W.R. Debinding of injected parts using an abnormal glow discharge. Mater. Sci. Eng. A 2005, 407, 71–76. [Google Scholar] [CrossRef]
- Bhuiyan, M.A.; Pucha, R.V.; Karevan, M.; Kalaitzidou, K. Tensile modulus of carbon nanotube/polypropylene composites–A computational study based on experimental characterization. Comput. Mater. Sci. 2011, 50, 2347–2353. [Google Scholar] [CrossRef]
- Liang, J.-Z.; Du, Q.; Tsui, G.C.-P.; Tang, C.-Y. Tensile properties of graphene nano-platelets reinforced polypropylene composites. Compos. Part B Eng. 2016, 95, 166–171. [Google Scholar] [CrossRef]
- Bafana, A.P.; Yan, X.; Wei, X.; Patel, M.; Guo, Z.; Wei, S.; Wujcik, E.K. Polypropylene nanocomposites reinforced with low weight percent graphene nanoplatelets. Compos. Part B Eng. 2017, 109, 101–107. [Google Scholar] [CrossRef]
- Liang, J.Z. Effects of tension rates and filler size on tensile properties of polypropylene/graphene nano-platelets composites. Compos. Part B Eng. 2019, 167, 241–249. [Google Scholar] [CrossRef]
- Liang, J.; Wang, Y.; Huang, Y.; Ma, Y.; Liu, Z.; Cai, J.; Zhang, C.; Gao, H.; Chen, Y. Electromagnetic interference shielding of graphene/epoxy composites. Carbon 2009, 47, 922–925. [Google Scholar] [CrossRef]
- Kim, H.; Miura, Y.; Macosko, C.W. Graphene/polyurethane nanocomposites for improved gas barrier and electrical conductivity. Chem. Mater. 2010, 22, 3441–3450. [Google Scholar] [CrossRef]
- Inuwa, I.M.; Hassan, A.; Samsudin, S.A.; Kassim, M.H.M.; Jawaid, M. Mechanical and thermal properties of exfoliated graphite nanoplatelets reinforced polyethylene terephthalate/polypropylene composites. Polym. Compos. 2014, 35, 2029–2035. [Google Scholar] [CrossRef]
- Sultana, S.; Khan, M.Z.; Umar, K.; Muneer, M. Electrical, thermal, photocatalytic and antibacterial studies of metallic oxide nanocomposite doped polyaniline. J. Mater. Sci. Technol. 2013, 29, 795–800. [Google Scholar] [CrossRef]
- Nezbedova, E.; Krcma, F.; Majer, Z.; Hutar, P. Effect of particles size on mechanical properties of polypropylene particulate composites. Int. J. Struct. Integr. 2016, 7, 690–699. [Google Scholar] [CrossRef]
- Jiasheng, Q.; Pingsheng, H.; Kangming, N. Nonisothermal Crystallization of PP/Nano-SiO2 Composites. J. Appl. Polym. Sci. 2004, 91, 1013–1019. [Google Scholar]
- Ma, J.; Qi, Z.; Hu, Y. Synthesis and characterization of polypropylene/clay nanocomposites. J. Appl. Polym. Sci. 2001, 82, 3611–3617. [Google Scholar] [CrossRef]
- Niederberger, N.; Pinna, N. Metal Oxide Nanoparticles in Organic Solvents, Synthesis, Formation, Assembly and Application; Springer Dordrecht Heidelberg: New York, NY, USA, 2009. [Google Scholar]
- Bottero, J.Y.; Auffan, M.; Rose, J.; Mouneyrac, C.; Botta, C.; Labille, J.; Masion, A.; Thill, A.; Chaneac, C. Manufactured metal and metal-oxide nanoparticles: Properties and perturbing mechanisms of their biological activity in ecosystems. C.R. Geosci. 2011, 343, 168–176. [Google Scholar] [CrossRef]
- Fernandez-Garcia, M.; Martinez-Arias, A.; Hanson, J.C.; Rodriguez, J.A. Nanostructured oxides in chemistry: Characterization and properties. Chem. Rev. 2004, 104, 4063–4104. [Google Scholar] [CrossRef]
- Faure, B.; Salazar-Alvarez, G.; Ahniyaz, A.; Villaluenga, I.; Berriozabal, G.; Miguel, Y.R.D.; Bergström, L. Dispersion and surface functionalization of oxide nanoparticles for transparent photocatalytic and UV-protecting coatings and sunscreens. Sci. Technol. Adv. Mater. 2013, 14, 023001. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.A.; Wang, H.; Zhou, D.; Lenihan, H.S.; Cherr, G.; Cardinale, B.J.; Miller, R.; Ji, Z. Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ. Sci. Technol. 2010, 44, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, S.; Hou, B.R. A Comparative Study of Neat Epoxy Coating and NanoZrO2/Epoxy Coating for Corrosion Protection on Carbon Steel. Applied Mechanics and Materials. Trans Tech Publ. 2014, 599, 3–6. [Google Scholar]
- Zhao, X.; Liu, S.; Wang, X.; Hou, B. Surface modification of ZrO2 nanoparticles with styrene coupling agent and its effect on the corrosion behaviour of epoxy coating. Chin. J. Oceanol. Limnol. 2014, 32, 1163–1171. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Bian, C.; He, J.; Xu, N.; Xue, G. Surface modification of TiO2 nanoparticles by polyaniline. Appl. Surf. Sci. 2003, 217, 16–22. [Google Scholar] [CrossRef]
- Mallakpour, S.; Madani, M. A review of current coupling agents for modification of metal oxide nanoparticles. Prog. Org. Coat. 2015, 86, 194–207. [Google Scholar] [CrossRef]
- Lu, C.; Qi, L.; Yang, J.; Wang, X.; Zhang, D.; Xie, J.; Ma, J. One-pot synthesis of octahedral Cu2O nanocages via a catalytic solution route. Adv. Mater. 2005, 17, 2562–2567. [Google Scholar] [CrossRef]
- Siegfried, M.J.; Choi, K.S. Elucidating the effect of additives on the growth and stability of Cu2O surfaces via shape transformation of pre-grown crystals. J. Am. Chem. Soc. 2006, 128, 10356–10357. [Google Scholar] [CrossRef]
- Liu, H.R.; Miao, W.F.; Yang, S.; Zhang, Z.M.; Chen, J.F. Controlled Synthesis of Different Shapes of Cu2O via γ-Irradiation. Cryst. Growth. Des. 2009, 9, 1733–1740. [Google Scholar] [CrossRef]
- Cai, J.; Liu, W.; Li, Z. One-pot self-assembly of Cu2O/RGO composite aerogel for aqueous photocatalysis. Appl. Surf. Sci. 2015, 358, 146–151. [Google Scholar] [CrossRef]
- Briskman, R.N. A study of electrodeposited cuprous oxide photovoltaic cells. Sol. Energy Mater. Sol. Cells 1992, 273, 61–368. [Google Scholar] [CrossRef]
- Ramirez-Ortiz, J.; Ogura, T.; Medina-Valtierra, J.; Lara, V. A Catalytic Application of Cu2O and CuO Films Deposited over Fiberglass. Appl. Surf. Sci. 2001, 174, 177–184. [Google Scholar] [CrossRef]
- Rubino, A.; Schiavi, P.G.; Altimari, P.; Latini, A. Ti/TiO2/Cu2O Based Electrodes as Photocatalysts in PEC Cells. Chem. Eng. Trans. 2019, 73, 73–78. [Google Scholar]
- Elsaeed, A.M.; Abd EI-Fattah, M.; Azzam, A.M.; Dardir, M.M. Synthesis of cuprous oxide epoxy nanocomposite as an environmentally antimicrobial coating. Int. J. Biol. Macromol. 2016, 89, 190–197. [Google Scholar]
- Fu, L.J.; Gao, J.; Zhang, T.; Cao, Q.; Yang, L.C.; Wu, Y.P.; Holze, R.; Wu, H.Q. Preparation of Cu2O particles with different morphologies and their application in lithium ion batteries. J. Power Sources 2007, 174, 1197–1200. [Google Scholar] [CrossRef]
- Samarasekara, P.; Yapa, N.U.S.; Kumara, N.T.R.N.; Perera, M.V.K. CO2 gas sensitivity of sputtered zinc oxide thin films. Bull. Mater. Sci. 2007, 30, 113–116. [Google Scholar] [CrossRef]
- Deng, S.Z.; Verawati, T.; Hai, M.F.; Tan, H.R.; Sayle, D.C.; Olivo, M.; Mhaisalkar, S.; Wei, J.; Sow, C.H. Reduced Graphene Oxide Conjugated Cu2O Nanowire Mesocrystals for High-Performance NO2 Gas Sensor. J. Am. Chem. Soc. 2012, 134, 4905–4917. [Google Scholar] [CrossRef]
- Shi, Y.; Qian, X.; Zhou, K.; Tang, Q.; Jiang, S.; Wang, B.; Yu, B.; Hu, Y.; Yuen, R.K.K. CuO/graphene nanohybrids: Preparation and enhancement on thermal stability and smoke suppression of polypropylene. Ind. Eng. Chem. Res. 2013, 52, 13654–13660. [Google Scholar] [CrossRef]
- Palza, H.; Gutiérrez, S.; Delgado, K.; Salazar, O.; Fuenzalida, V.; Avila, J.I.; Figueroa, G.; Quijada, R. Toward tailor-made biocide materials based on poly (propylene)/copper nanoparticles. Macromol. Rapid Commun. 2010, 31, 563–567. [Google Scholar] [CrossRef]
- Delgado, K.; Quijada, R.; Palma, R.; Palza, H. Polypropylene with embedded copper metal or copper oxide nanoparticles as a novel plastic antimicrobial agent. Lett. Appl. Microbiol. 2011, 53, 50–54. [Google Scholar] [CrossRef]
- Omrani, A.; Simon, L.C.; Rostami, A.A. The effects of alumina nanoparticle on the properties of an epoxy resin system. Mater. Chem. Phys. 2009, 114, 145–150. [Google Scholar] [CrossRef]
- Huang, L.; Peng, F.; Yu, H.; Wang, H.J. Synthesis of Cu2O nanoboxes, nanocubes and nanospheres by polyol process and their adsorption characteristic. Mater. Res. Bull. 2008, 43, 3047–3053. [Google Scholar] [CrossRef]
- He, W.; Tian, X.Y.; Du, Y.; Sun, C.Y.; Zhang, X.D.; Han, X.X.; Han, S.S.; Sun, X.N.; Du, X.Y.; Yue, Y.Z. Biologically formed hollow cuprous oxide microspheres. Mater. Sci. Eng. C 2010, 30, 758–762. [Google Scholar] [CrossRef]
- Nanayakkara, C.E.; Larish, W.A.; Grassian, V.H. Titanium dioxide nanoparticle surface reactivity with atmospheric gases, CO2, SO2, and NO2: Roles of surface hydroxyl groups and adsorbed water in the formation and stability of adsorbed products. J. Phys. Chem. C 2014, 118, 23011–23021. [Google Scholar] [CrossRef]
- Tangchantra, N.; Kruenate, J.; Aumnate, C.; Sooksomsong, T. The effect of surface modification of TiO2 on mechanical properties of polyethylene composite film. Advanced Materials Research. Trans Tech Publ. 2010, 93, 300–303. [Google Scholar]
- Varga, J. β-modification of isotactic polypropylene: Preparation, structure, processing, properties, and application. J. Macromol. Sci. Part B 2002, 41, 1121–1171. [Google Scholar] [CrossRef]
- Hybiak, D.; Garbarczyk, J. Silver nanoparticles in isotactic polypropylene (iPP). Part I. Silver nanoparticles as metallic nucleating agents for β-iPP polymorph. Polimery 2014, 59, 585–591. [Google Scholar] [CrossRef]
- Garbarczyk, J.; Paukszta, D.; Borysiak, S. Polymorphism of isotactic polypropylene in presence of additives, in blends and in composites. J. Macromol. Sci. Part B 2002, 41, 1267–1278. [Google Scholar] [CrossRef]
- Sterzynski, T.; Lambla, M.; Georgi, F.; Thomas, M. Studies of the Trans-Quinacridone Nucleation of Poly-(ethylene-b-propylene) Dedicated to the memory of Prof. Morand Lambla. Int. Polym. Process. 1997, 12, 64–71. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Xu, L.; Jiang, Y. Preparation and Thermal Properties of Modified Cu2O/Polypropylene (PP) Composite. Materials 2020, 13, 309. https://doi.org/10.3390/ma13020309
Wu Y, Xu L, Jiang Y. Preparation and Thermal Properties of Modified Cu2O/Polypropylene (PP) Composite. Materials. 2020; 13(2):309. https://doi.org/10.3390/ma13020309
Chicago/Turabian StyleWu, Yurong, Longshan Xu, and Yanying Jiang. 2020. "Preparation and Thermal Properties of Modified Cu2O/Polypropylene (PP) Composite" Materials 13, no. 2: 309. https://doi.org/10.3390/ma13020309



