Lifetime Prediction Methods for Degradable Polymeric Materials—A Short Review
Abstract
:1. Introduction
2. Degradation of Polymers
2.1. Changes Caused by Degradation
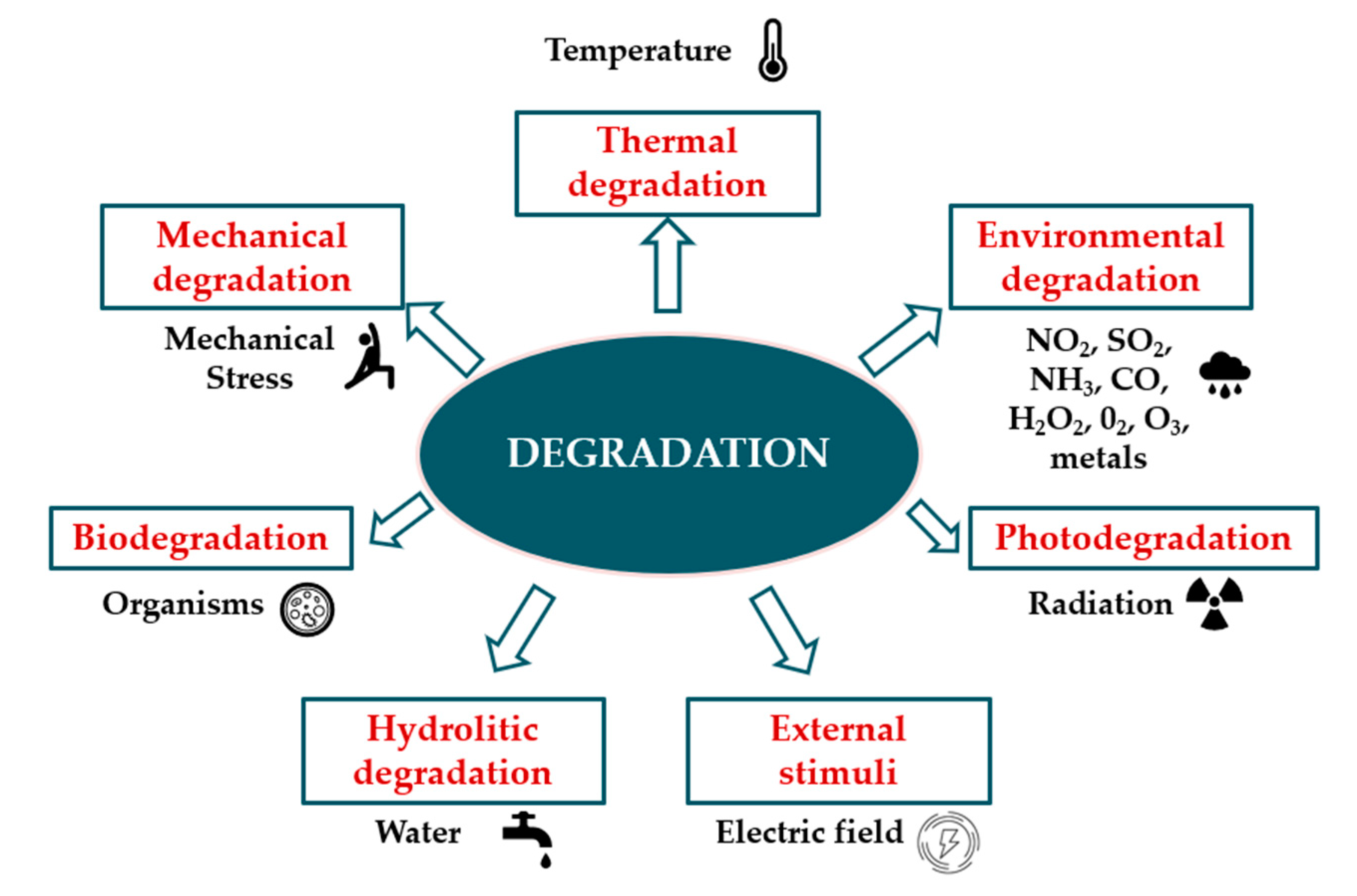
2.2. Types of Degradation
2.3. General Mechanism of Thermal Degradation
3. Lifetime of Polymers
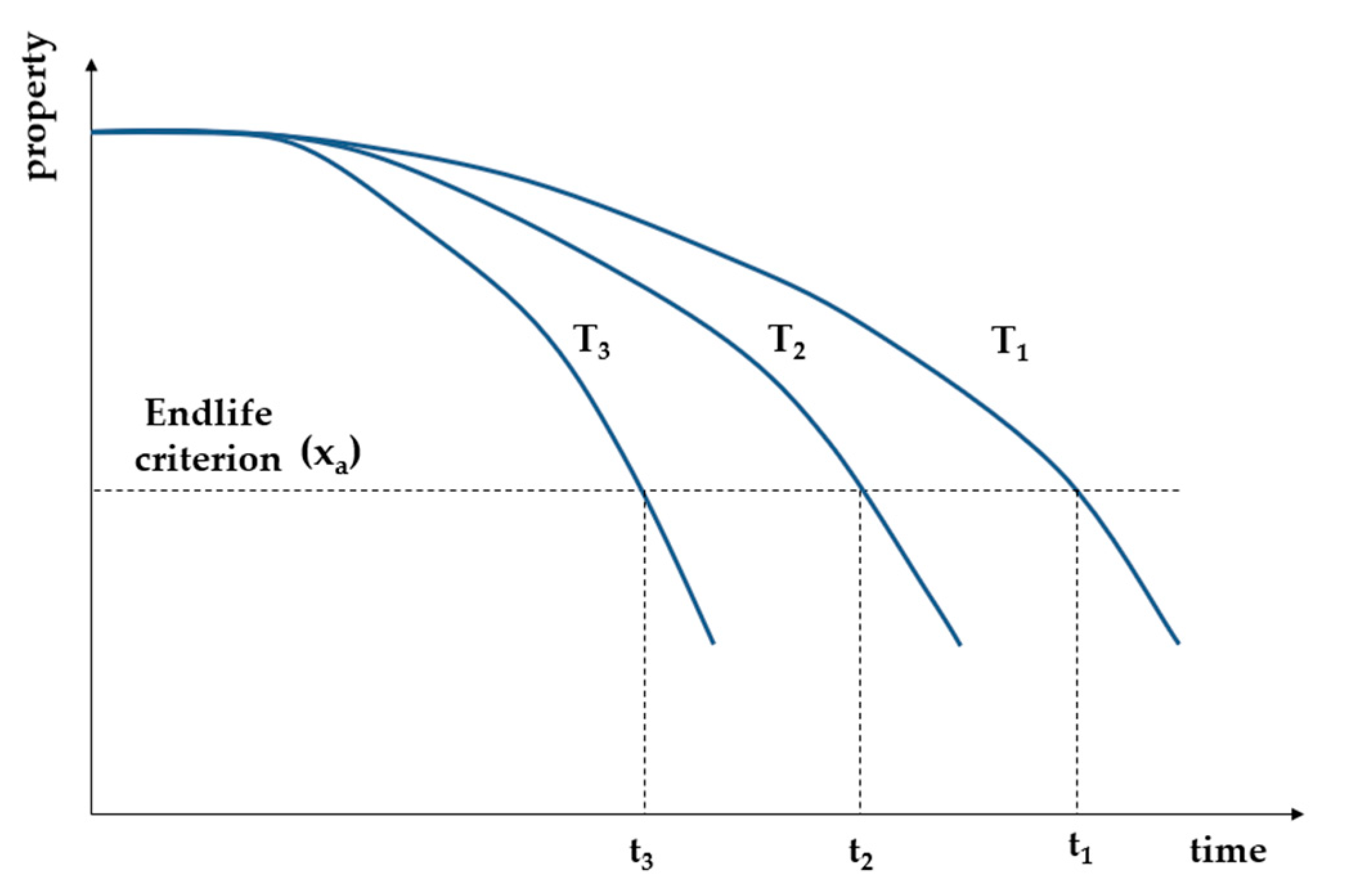
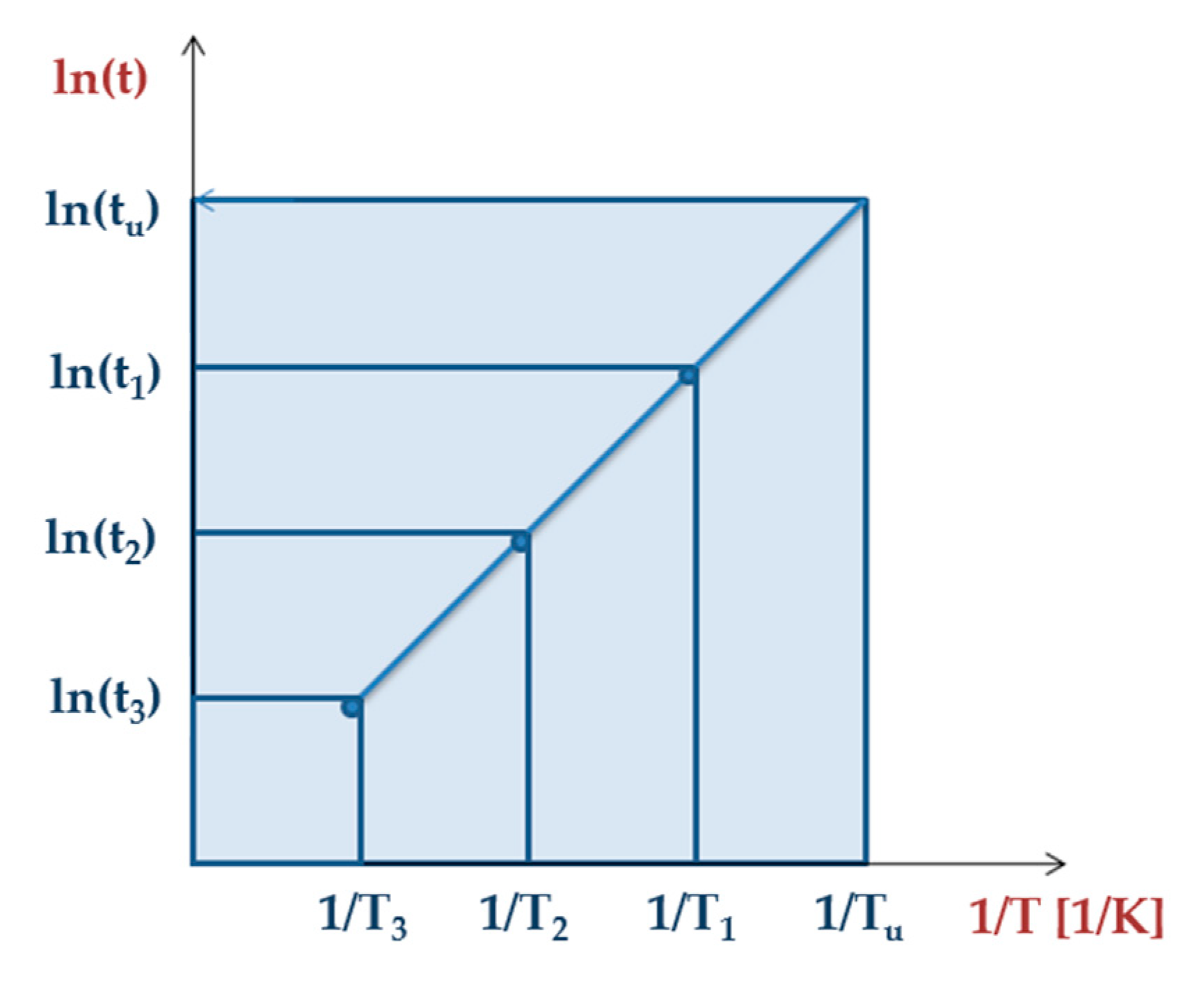
4. Accelerated Aging of Polymeric Materials
5. Methods for Predicting the Lifetime of Polymeric Materials
5.1. Analysis of Thermal Degradation Kinetics Using Thermogravimetric Analysis
5.2. Arrhenius Model
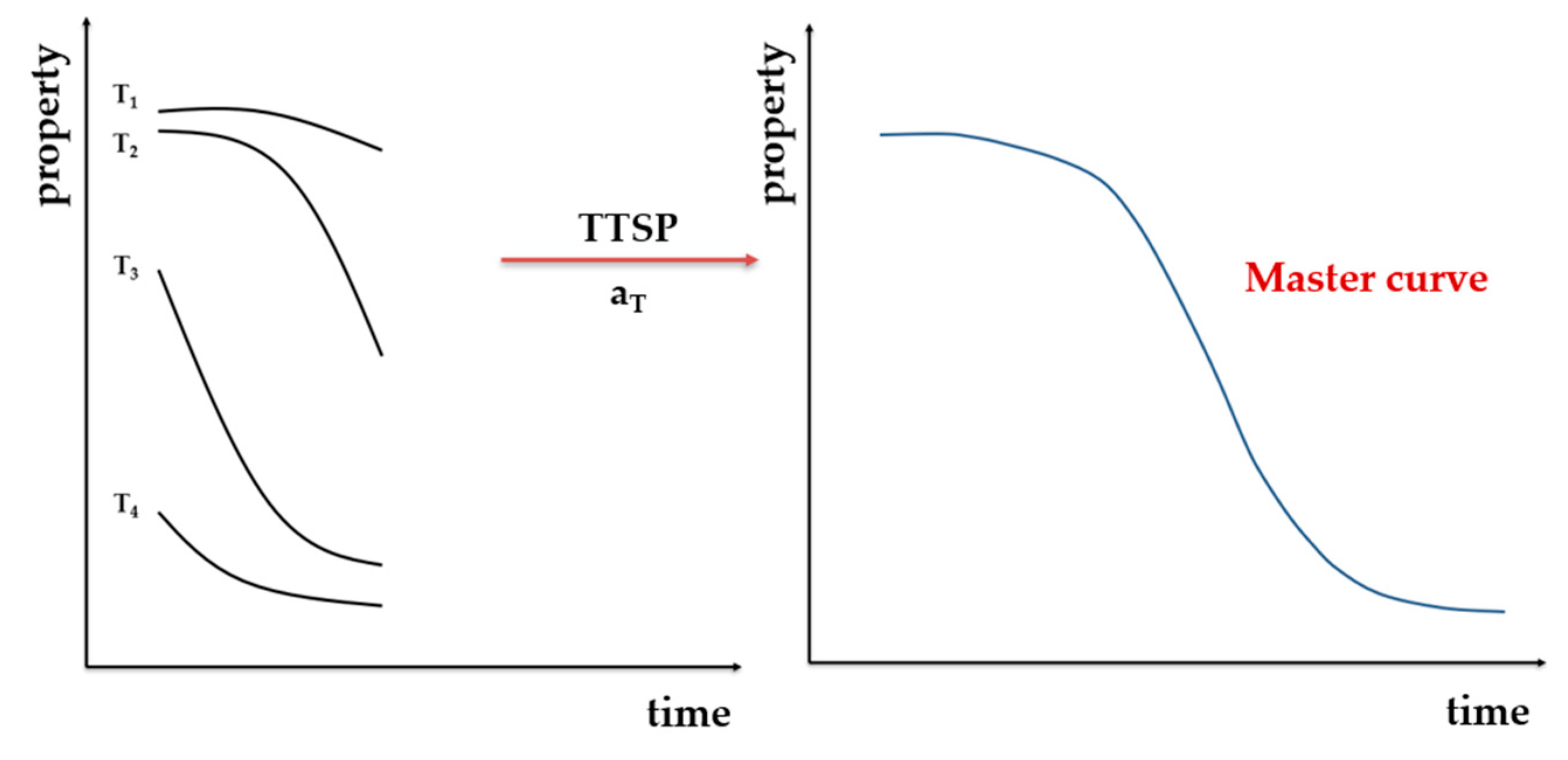
5.3. Time–Temperature Superposition
5.4. Williams–Landel–Ferry (WLF) Model
5.5. Isoconversional Methods
5.5.1. Friedman’s Method
5.5.2. Ozawa–Flynn–Wall (OFW) Method
5.5.3. Ozawa–Flynn–Wall Corrected Method by N. Sbirrazzuoli et al.
5.5.4. Kissinger–Akahira–Sunose (KAS) Method
5.5.5. Advanced Isoconversional Method by S. Vyazovkin
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jim Jem, K.; Tan, B. The Development and Challenges of Poly (lactic acid) and Poly (glycolic acid). Adv. Ind. Eng. Polym. Res. 2020, 3, 60–70. [Google Scholar] [CrossRef]
- Küçük, V.A.; Çınar, E.; Korucu, H.; Simsek, B.; Bilge Güvenç, A.; Uygunoglu, T.; Kocakerim, M. Thermal, electrical and mechanical properties of filler-doped polymer concrete. Constr. Build. Mater. 2019, 226, 188–199. [Google Scholar] [CrossRef]
- Rod, K.A.; Nguyen, M.; Elbakhshwan, M.; Gills, S.; Kutchko, B.; Varga, T.; Mckinney, A.M.; Roosendaal, T.J.; Childers, M.I.; Zhao, C.; et al. Insights into the physical and chemical properties of a cement-polymer composite developed for geothermal wellbore applications. Cem. Concr. Compos. 2019, 97, 279–287. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Jouyandeh, M.; Ganjali, M.R.; Shirkavand, B.; Mozafari, M.; Sheiko, S.S. Thermo-sensitive polymers in medicine: A review. Eur. Polym. J. 2019, 117, 402–423. [Google Scholar] [CrossRef]
- Chauhan, D.; Afreen, S.; Talreja, N.; Ashfaq, M. Multifunctional copper polymer-based nanocomposite for environmental and agricultural applications. In Multifunctional Hybrid Nanomaterials for Sustainable Agri-Food and Ecosystems; Abd-Elsalam, K.A., Ed.; Elsevier: Rotterdam, The Netherland, 2020; pp. 189–211. ISBN 9780128213544. [Google Scholar]
- Zhong, Y.; Godwin, P.; Jin, Y.; Xiao, H. Biodegradable Polymers and Green-based Antimicrobial Packaging Materials: A mini-review. Adv. Ind. Eng. Polym. Res. 2020, 3, 27–35. [Google Scholar] [CrossRef]
- Mishra, M. Household Goods: Polymers in Insulators: Polymers for High-Voltage Outdoor Use. In Encyclopedia of Polymer Applications, 3 Volume Set; CRC Press: Boca Raton, FL, USA, 2018; pp. 1549–1562. ISBN 9781351019422. [Google Scholar]
- Patil, A.; Patel, A.; Purohit, R. An overview of Polymeric Materials for Automotive Applications. Mater. Today Proc. 2017, 4, 3807–3815. [Google Scholar] [CrossRef]
- Jasso-Gastinel, C.F.; Soltero-Martínez, J.F.A.; Mendizábal, E. Introduction: Modifiable Characteristics and Applications. In Modification of Polymer Properties; William Andrew: Norwich, NY, USA, 2017; pp. 1–21. ISBN 9780323443982. [Google Scholar]
- Kaur, R.; Marwaha, A.; Chhabra, V.A.; Kaushal, K.; Kim, K.; Tripathi, S.K. Facile synthesis of a Cu-based metal-organic framework from plastic waste and its application as a sensor for acetone. J. Clean. Prod. 2020, 263, 121492. [Google Scholar] [CrossRef]
- Laycock, B.; Nikolić, M.; Colwell, J.M.; Gauthier, E.; Halley, P.; Bottle, S.; George, G. Lifetime prediction of biodegradable polymers. Prog. Polym. Sci. 2017, 71, 144–189. [Google Scholar] [CrossRef] [Green Version]
- Barba, D.; Arias, A.; Garcia-gonzalez, D. Temperature and strain rate dependences on hardening and softening behaviours in semi-crystalline polymers: Application to PEEK. Int. J. Solids Struct. 2020, 182–183, 205–217. [Google Scholar] [CrossRef]
- Yan, C.; Huang, W.; Lin, P.; Zhang, Y.; Lv, Q. Chemical and rheological evaluation of aging properties of high content SBS polymer modified asphalt. Fuel 2019, 252, 417–426. [Google Scholar] [CrossRef]
- Awaja, F.; Zhang, S.; Tripathi, M.; Nikiforov, A.; Pugno, N. Cracks, microcracks and fracture in polymer structures: Formation, detection, autonomic repair. Prog. Mater. Sci. 2016, 83, 536–573. [Google Scholar] [CrossRef]
- Lewandowski, M.; Pawłowska, U. Part I. Degradation of elastomers and prediction of lifetime. Elastomery 2016, 20, 24–30. [Google Scholar]
- Gijsman, P.; Meijers, G.; Vitarelli, G. Comparison of the UV-degradation chemistry of polypropylene, polyethylene, polyamide 6 and polybutylene terephthalate. Polym. Degrad. Stab. 1999, 65, 433–441. [Google Scholar] [CrossRef]
- Gillen, K.T.; Celina, M. The wear-out approach for predicting the remaining lifetime of materials. Polym. Degrad. Stab. 2000, 71, 15–30. [Google Scholar] [CrossRef] [Green Version]
- Hondred, P.R. Polymer Damage Mitigation-Predictive Lifetime Models of Polymer Insulation Degradation and Biorenewable Thermosets through Cationic Polymerization for Self-Healing Applications. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2013. [Google Scholar]
- Ashter, S.A. Mechanisms of Polymer Degradation. In Introduction to Bioplastics Engineering; William Andrew: Norwich, NY, USA, 2016; pp. 31–59. ISBN 978-0-323-39396-6. [Google Scholar]
- Gogate, P.R.; Prajapat, A.L. Depolymerization using sonochemical reactors: A critical review. Ultrason. Sonochem. 2015, 27, 480–494. [Google Scholar] [CrossRef]
- Godiya, C.B.; Gabrielli, S.; Materazzi, S.; Pianesi, M.S.; Stefanini, N.; Marcantoni, E. Depolymerization of waste poly(methyl methacrylate) scraps and purification of depolymerized products. J. Environ. Manag. 2019, 231, 1012–1020. [Google Scholar] [CrossRef]
- Wiles, D.M.; Scott, G. Polyolefins with controlled environmental degradability. Polym. Degrad. Stab. 2006, 91, 1581–1592. [Google Scholar] [CrossRef]
- Bhuvaneswari, G.H. Degradability of Polymers. In Recycling of Polyurethane Foams; Thomas, S., Rane, A.V., Kanny, K., Abitha, V.K., Thomas, G.M., Eds.; William Andrew: Norwich, NY, USA, 2018; pp. 29–44. ISBN 9780323511339. [Google Scholar]
- Anju, S.; Prajitha, N.; Sukanya, V.S.; Mohanan, P.V. Complicity of degradable polymers in health-care applications. Mater. Today Chem. 2020, 16, 100236. [Google Scholar] [CrossRef]
- Melnikov, M.; Seropegina, E.N. Photoradical ageing of polymers. Int. J. Polym. Mater. 1996, 31, 41–93. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Morreale, M.; Botta, L.; Mistretta, M.C.; Ceraulo, M.; Scaffaro, R. Degradation of polymer blends: A brief review. Polym. Degrad. Stab. 2017, 145, 79–92. [Google Scholar] [CrossRef]
- White, J.R. Polymer ageing: Physics, chemistry or engineering? Time to reflect. C. R. Chim. 2006, 9, 1396–1408. [Google Scholar] [CrossRef]
- Chmielnicki, B. Niektóre aspekty starzenia wytworów z poliamidów wzmocnionych. Cz. 1 Podatność poliamidów na procesy starzenia. Przetwórstwo Tworzyw 2009, 15, 116–122. [Google Scholar]
- Rojek, M. Metodologia Badań Diagnostycznych Warstwowych Materiałów Kompozytowych o Osnowie Polimerowej; Dobrzański, L.A., Ed.; International OCSCO World Press: Gliwice, Poland, 2011; ISBN 83-89728-89-3. [Google Scholar]
- Sobków, D.; Czaja, K. Influence of accelerated aging conditions on the process of polyolefines degradation. Polimery 2003, 48, 627–632. [Google Scholar] [CrossRef]
- Gijsman, P. Review on the thermo-oxidative degradation of polymers during processing and in service. E-Polymers 2008, 8, 1–34. [Google Scholar] [CrossRef] [Green Version]
- Moraczewski, K.; Stepczyńska, A.; Malinowski, R.; Karasiewicz, T.; Jagodziński, B.; Rytlewski, P. The Effect of Accelerated Aging on Polylactide Containing Plant Extracts. Polymers 2019, 11, 575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masłowski, M.; Zaborski, M. Effect of thermooxidative and photooxidative aging processes on mechanical properties of magnetorheological elastomer composites. Polimery 2015, 60, 264–271. [Google Scholar] [CrossRef]
- Król-Morkisz, K.; Pielichowska, K. Thermal Decomposition of Polymer Nanocomposites With Functionalized Nanoparticles. In Polymer Composites with Functionalized Nanoparticles; Elsevier: Rotterdam, The Netherland, 2019; pp. 405–435. ISBN 978-0-12-814064-2. [Google Scholar]
- Mierzwa-Hersztek, M.; Gondek, K.; Kopeć, M. Degradation of Polyethylene and Biocomponent-Derived Polymer Materials: An Overview. J. Polym. Environ. 2019, 27, 600–611. [Google Scholar] [CrossRef] [Green Version]
- Muthukumar, A.; Veerappapillai, S. Biodegradation of Plastics—A Brief Review. Int. J. Pharm. Sci. Rev. Res. 2015, 31, 204–209. [Google Scholar]
- Lyu, S.; Untereker, D. Degradability of Polymers for Implantable Biomedical Devices. Int. J. Mol. Sci. 2009, 10, 4033–4065. [Google Scholar] [CrossRef] [Green Version]
- Wojtala, A. The effect of properties of polyolefines and outdoor factors on the course of polyolefines degradation. Polimery 2001, 46, 120–124. [Google Scholar] [CrossRef]
- Laurence, W.M. Introduction to the Effect of Heat Aging on Plastics. In The Effect of Long Term Thermal Exposure on Plastics and Elastomers; William Andrew: Norwich, NY, USA, 2014; pp. 17–42. ISBN 978-0-323-22108-5. [Google Scholar]
- Reis, A.; Spickett, C.M. Chemistry of phospholipid oxidation. Biochim. Biophys. Acta 2012, 1818, 2374–2387. [Google Scholar] [CrossRef] [Green Version]
- Kröhnke, C. Polymer Additives. In Polymer Science: A Comprehensive Reference; Elsevier, B.V.: Amsterdam, The Netherlands, 2012; Volume 8, pp. 349–375. ISBN 9780444533494. [Google Scholar]
- Liu, X.; Gao, C.; Sangwan, P.; Yu, L.; Tong, Z. Accelerating the degradation of polyolefins through additives and blending. J. Appl. Polym. Sci. 2014, 131, 9001–9015. [Google Scholar] [CrossRef]
- Tolinski, M. Ultraviolet Light Protection and Stabilization. In Additives for Polyolefins; William Andrew: Norwich, NY, USA, 2015; pp. 32–43. ISBN 9780323358842. [Google Scholar]
- Lu, M.; Zhang, H.; Sun, L. Quantitative prediction of elastomer degradation and mechanical behavior based on diffusion–reaction process. J. Appl. Polym. Sci. 2020, 1–9. [Google Scholar] [CrossRef]
- Bin Samsuri, A.; Abdullahi, A.A. Degradation of Natural Rubber and Synthetic Elastomers. In Reference Module in Materials Science and Materials Engineering; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 1–32. ISBN 9780128035818. [Google Scholar]
- Tobolsky, A.V. Properties and Structure of Polymers; Wiley: New York, NY, USA, 1960. [Google Scholar]
- Rybiński, P.; Kucharska-Jastrząbek, A.; Janowska, G. Thermal Properties of Diene Elastomers. Modif. Polym. 2014, 56, 477–486. [Google Scholar] [CrossRef] [Green Version]
- Coquillat, M.; Verdu, J.; Colin, X.; Audouin, L.; Nevie, R. Thermal oxidation of polybutadiene. Part 1: Effect of temperature, oxygen pressure and sample thickness on the thermal oxidation of hydroxyl-terminated polybutadiene. Polym. Degrad. Stab. 2007, 92, 1326–1333. [Google Scholar] [CrossRef]
- Masek, A. Flavonoids as Natural Stabilizers and Color Indicators of Ageing for Polymeric Materials. Polymers 2015, 7, 1125–1144. [Google Scholar] [CrossRef]
- Thompson, R.C.; Moore, C.J.; Saal, F.S.; Swan, S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2153–2166. [Google Scholar] [CrossRef]
- ISO, the International Organization for Standardization. Rubber, Vulcanized or Thermoplastic—Estimation of Life-Time and Maximum Temperature of Use (Standard No. ISO 11346:2014); International Organization for Standardization: Geneva, Switzerland; pp. 1–10.
- Dobkowski, Z. Lifetime prediction for polymer materials using OIT measurements by the DSC method. Polimery 2005, 50, 213–215. [Google Scholar] [CrossRef] [Green Version]
- ISO, the International Organization for Standardization. Rubber Products—Guidelines for Storage (Standard No. ISO 2230:2002); International Organization for Standardization: Geneva, Switzerland; pp. 1–11.
- Shah, C.S.; Patni, M.J. Accelerated Aging and Life Time Prediction Analysis of Polymer Composites: A New Approach for a Realistic Prediction Using Cumulative Damage Theory. Polym. Test. 1994, 13, 295–322. [Google Scholar] [CrossRef]
- Kiliaris, P.; Papaspyrides, C.D.; Pfaendner, R. Influence of accelerated aging on clay-reinforced polyamide 6. Polym. Degrad. Stab. 2009, 94, 389–396. [Google Scholar] [CrossRef]
- Calixto, E. Accelerated Test and Reliability Growth Analysis Models. In Gas and Oil Reliability Engineering; Gulf Professional Publishing: Houston, TX, USA, 2013; pp. 63–118. ISBN 978-0-12-391914-4. [Google Scholar]
- Jachowicz, T.; Sikora, R. Methods of forecasting of the changes of polymeric products properties. Polimery 2006, 51, 177–185. [Google Scholar] [CrossRef]
- Singh, H.K. Lifetime Prediction and Durability of Elastomeric Seals for Fuel Cell Applications. Ph.D. Thesis, Virginia Polytechnic Institute, Blacksburg, VA, USA, 2009. [Google Scholar]
- Le Huy, M.; Evrard, G. Methodologies for lifetime predictions of rubber using Arrhenius and WLF models. Die Angew. Makromol. Chem. 1998, 261–262, 135–142. [Google Scholar] [CrossRef]
- Gillen, K.T.; Bernstein, R.; Celina, M. Challenges of accelerated aging techniques for elastomer lifetime predictions. Rubber Chem. Technol. 2015, 88, 1–27. [Google Scholar] [CrossRef]
- Käser, F.; Roduit, B. Lifetime prediction of rubber using the chemiluminescence approach and isoconversional kinetics. J. Therm. Anal. Calorim. 2008, 93, 231–237. [Google Scholar] [CrossRef]
- Denis, L.; Grzeskowiak, H.; Trias, D.; Delaux, D. Accelerated Life Testing. In Reliability of High-Power Mechatronic Systems 2; ISTE Press—Elsevier: London, UK, 2017; pp. 1–56. ISBN 9781785482618. [Google Scholar]
- Li, J.; Tian, Y.; Wang, D. Change-point detection of failure mechanism for electronic devices based on Arrhenius model. Appl. Math. Model. 2020, 83, 46–58. [Google Scholar] [CrossRef]
- Budrugeac, P. Theory and practice in the thermoanalytical kinetics of complex processes: Application for the isothermal and non-isothermal thermal degradation of HDPE. Thermochim. Acta 2010, 500, 30–37. [Google Scholar] [CrossRef]
- Pielichowski, J.; Pielichowski, K. Application of thermal analysis for the investigation of polymer degradation processes. J. Therm. Anal. 1995, 43, 505–508. [Google Scholar] [CrossRef]
- Kutz, M. Thermal Degradation of Polymer and Polymer Composites. In Handbook of Environmental Degradation of Materials; William Andrew: Norwich, NY, USA, 2018; pp. 185–206. ISBN 9780323524728. [Google Scholar]
- Ajitha, A.R.; Sabu, T. Applications of compatibilized polymer blends in automobile industry. In Compatibilization of Polymer Blends. Micro and Nano Scale Phase Morphologies, Interphase Characterization and Properties; Elsevier: Rotterdam, The Netherland, 2020; pp. 563–593. ISBN 9780128160060. [Google Scholar]
- Lühr, C.; Pecenka, R. Development of a model for the fast analysis of polymer mixtures based on cellulose, hemicellulose (xylan), lignin using thermogravimetric analysis and application of the model to poplar wood. Fuel 2020, 277. [Google Scholar] [CrossRef]
- Dai, L.; Wang, L.Y.; Yuan, T.Q.; He, J. Study on thermal degradation kinetics of cellulose-graft-poly(l-lactic acid) by thermogravimetric analysis. Polym. Degrad. Stab. 2014, 99, 233–239. [Google Scholar] [CrossRef]
- Seifi, H.; Gholami, T.; Seifi, S.; Ghoreishi, S.M.; Salavati-Niasari, M. A review on current trends in thermal analysis and hyphenated techniques in the investigation of physical, mechanical and chemical properties of nanomaterials. J. Anal. Appl. Pyrolysis 2020, 149, 104840. [Google Scholar] [CrossRef]
- Lau, K.S.Y. High-Performance Polyimides and High Temperature Resistant Polymers. In Handbook of Thermoset Plastics; Dodiuk, H., Goodman, S.H., Eds.; William Andrew: Norwich, NY, USA, 2014; pp. 297–424. ISBN 978-1-4557-3107-7. [Google Scholar]
- Niu, S.; Yu, H.; Zhao, S.; Zhang, X.; Li, X.; Han, K.; Lu, C.; Wang, Y. Apparent kinetic and thermodynamic calculation for thermal degradation of stearic acid and its esterification derivants through thermogravimetric analysis. Renew. Energy 2019, 133, 373–381. [Google Scholar] [CrossRef]
- Artiaga, R.; Cao, R.; Naya, S.; Garcia, A. Polymer Degradation from the Thermal Analysis Point of View. Mater. Res. Soc. 2004, 851, 499–510. [Google Scholar] [CrossRef]
- Blanco, I. Lifetime prediction of polymers: To bet, or not to bet-is this the question? Materials 2018, 11, 1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyombi, A.; Williams, M.; Wessling, R. Determination of kinetic parameters and thermodynamic properties for ash (Fraxinus) wood sawdust slow pyrolysis by thermogravimetric analysis. Energy Sources Part A Recover. Util. Environ. Eff. 2018, 40, 2660–2670. [Google Scholar] [CrossRef] [Green Version]
- Woo, C.S.; Park, H.S.; Kwang, M.C. Design and applications: Evaluation of characteristics for chevron rubber spring. In Constitutive Models for Rubber VIII; Gil-Negrete, N., Alonso, A., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 621–626. ISBN 9781138000728. [Google Scholar]
- Kaczmarek, H.; Kosmalska, D.; Malinowski, R.; Bajer, K. Advances in studies of thermal degradation of polymeric materials. Part I. Literature studies. Polimery 2019, 64, 239–314. [Google Scholar] [CrossRef] [Green Version]
- Capart, R.; Khezami, L.; Burnham, A.K. Assessment of various kinetic models for the pyrolysis of a microgranular cellulose. Thermochim. Acta 2004, 417, 79–89. [Google Scholar] [CrossRef] [Green Version]
- Materazzi, S.; Vecchio, S. Evolved Gas Analysis by Mass Spectrometry. Appl. Spectrosc. Rev. 2011, 46, 261–340. [Google Scholar] [CrossRef]
- Qin, L.; Han, J.; Zhao, B.; Wang, Y.; Chen, W.; Xing, F. Thermal degradation of medical plastic waste by in-situ FTIR, TG-MS and TG-GC/MS coupled analyses. J. Anal. Appl. Pyrolysis 2018, 136, 132–145. [Google Scholar] [CrossRef]
- Li, L.Q.; Guan, C.X.; Zhang, A.Q.; Chen, D.H.; Qing, Z.B. Thermal stabilities and thermal degradation kinetics of polyimides. Polym. Degrad. Stab. 2004, 84, 369–373. [Google Scholar] [CrossRef]
- Jin, W.P.; Sea, C.O.; Hac, P.L.; Hee, T.K.; Kyong, O.Y. A kinetic analysis of thermal degradation of polymers using a dynamic method. Polym. Degrad. Stab. 2000, 67, 535–540. [Google Scholar] [CrossRef]
- Yang, K.K.; Wang, X.L.; Wang, Y.Z.; Wu, B.; Yin, Y.D.; Yang, B. Kinetics of thermal degradation and thermal oxidative degradation of poly(p-dioxanone). Eur. Polym. J. 2003, 39, 1567–1574. [Google Scholar] [CrossRef]
- Plonka, A. Kinetics in condensed media. In Dispiersive Kinetics; Springer: Heidelberg, Germany, 2001; pp. 194–195. ISBN 978-94-015-9658-9. [Google Scholar]
- Park, B.D.; Kadla, J.F. Thermal degradation kinetics of resole phenol-formaldehyde resin/multi-walled carbon nanotube/cellulose nanocomposite. Thermochim. Acta 2012, 540, 107–115. [Google Scholar] [CrossRef]
- Van Krevelen, D.W.; Te Nijenhuis, K. Thermal Decomposition. In Properties of Polymers; Elsevier Science: Rotterdam, The Netherland, 2009; pp. 763–777. ISBN 9780080548197. [Google Scholar]
- Saha, T.K.; Purkait, P. Transformer Insulation Materials and Ageing. In Transformer Ageing: Monitoring and Estimation Techniques; Wiley-IEEE Press: New York, NY, USA, 2017; pp. 1–34. ISBN 978-1-119-23996-3. [Google Scholar]
- Celina, M.; Gillen, K.T.; Assink, R.A. Accelerated aging and lifetime prediction: Review of non-Arrhenius behaviour due to two competing processes. Polym. Degrad. Stab. 2005, 90, 395–404. [Google Scholar] [CrossRef]
- Vyazovkin, S. Activation energies and temperature dependencies of the rates of crystallization and melting of polymers. Polymers 2020, 12, 1070. [Google Scholar] [CrossRef] [PubMed]
- Moon, B.; Kim, K.; Park, K.; Park, S.; Seok, C.S. Fatigue life prediction of tire sidewall using modified Arrhenius equation. Mech. Mater. 2020, 147. [Google Scholar] [CrossRef]
- Tsuji, T.; Mochizuki, K.; Okada, K.; Hayashi, Y.; Obata, Y.; Takayama, K.; Onuki, Y. Time–temperature superposition principle for the kinetic analysis of destabilization of pharmaceutical emulsions. Int. J. Pharm. 2019, 563, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Hulme, A.; Cooper, J. Life prediction of polymers for industry. Seal. Technol. 2012, 9, 8–12. [Google Scholar] [CrossRef]
- Brown, R.P.; Greenwood, J.H. Prediction Techniques. In Practical Guide to the Assessment of the Useful Life of Plastics; Smithers Rapra Technology: Shropshire, UK, 2002; pp. 85–94. ISBN 978-1-85957-312-9. [Google Scholar]
- Moon, B.; Jun, N.; Park, S.; Seok, C.S.; Hong, U.S. A study on the modified Arrhenius equation using the oxygen permeation block model of crosslink structure. Polymers 2019, 11, 136. [Google Scholar] [CrossRef] [Green Version]
- Whitten, K.; Davis, R.; Peck, L.; Stanley, G. Chemical Kinetics. In Chemistry; Mary Finch: Boston, MA, USA, 2018; pp. 606–628. ISBN 978-0-495-39163-0. [Google Scholar]
- Xiong, Y.; Chen, G.; Guo, S.; Li, G. Lifetime prediction of NBR composite sheet in aviation kerosene by using nonlinear curve fitting of ATR-FTIR spectra. J. Ind. Eng. Chem. 2013, 19, 1611–1616. [Google Scholar] [CrossRef]
- Madej-Kiełbik, L.; Kośla, K.; Zielińska, D.; Chmal-fudali, E.; Maciejewska, M. Effect of Accelerated Ageing on the Mechanical and Structural Properties of the Material System Used in Protectors. Polymers 2019, 11, 1263. [Google Scholar] [CrossRef] [Green Version]
- Koga, Y.; Arao, Y.; Kubouchi, M. Application of small punch test to lifetime prediction of plasticized polyvinyl chloride wire. Polym. Degrad. Stab. 2019, 109013. [Google Scholar] [CrossRef]
- Wang, Y.; Lan, H.; Meng, T. Lifetime prediction of natural gas polyethylene pipes with internal pressures. Eng. Fail. Anal. 2019, 95, 154–163. [Google Scholar] [CrossRef]
- Lee, L.S. Creep and time-dependent response of composites. In Durability of Composites for Civil Structural Applications; Karbhari, V.M., Ed.; Woodhead Publishing: Cambridge, UK, 2007; pp. 150–169. ISBN 978-1-84569-035-9. [Google Scholar]
- Krauklis, A.E.; Akulichev, A.G.; Gagani, A.I.; Echtermeyer, A.T. Time-temperature-plasticization superposition principle: Predicting creep of a plasticized epoxy. Polymers 2019, 11, 1848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goertzen, W.K.; Kessler, M.R. Creep behavior of carbon fiber/epoxy matrix composites. Mater. Sci. Eng. A 2006, 421, 217–225. [Google Scholar] [CrossRef]
- Ahmed, J.; Ptaszek, P.; Basu, S. Time-Temperature Superposition Principle and its Application to Biopolymer and Food Rheology. In Advances in Food Rheology and Its Applications; Woodhead Publishing: Cambridge, UK, 2016; pp. 209–241. ISBN 978-0-08-100431-9. [Google Scholar]
- Nakada, M.; Miyano, Y.; Cai, H. Prediction of long-term viscoelastic behavior of amorphous resin based on the time-temperature superposition principle. Mech. Time Depend. Mater. 2011, 15, 309–316. [Google Scholar] [CrossRef]
- Fukushima, K.; Cai, H.; Nakada, M.; Miyano, Y. Determination of Time-Temperature Shift Factor for Long-Term Life Prediction of Polymer Composites. In Proceedings of the ICCM-17 17th International Conference on Composite Materials, Edinburgh, UK, 27–31 July 2009. [Google Scholar]
- Yin, W.; Xie, Z.; Yin, Y.; Yi, J.; Liu, X.; Wu, H.; Wang, S.; Xie, Y.; Yang, Y. Aging behavior and lifetime prediction of PMMA under tensile stress and liquid scintillator conditions. Adv. Ind. Eng. Polym. Res. 2019, 2, 82–87. [Google Scholar] [CrossRef]
- Sopade, P.A.; Halley, P.; Bhandari, B.; D’Arcy, B.; Doebler, C.; Caffin, N. Application of the Williams-Landel-Ferry model to the viscosity-temperature relationship of Australian honeys. J. Food Eng. 2003, 56, 67–75. [Google Scholar] [CrossRef]
- Wise, C.W.; Cook, W.D.; Goodwin, A.A. Chemico-diffusion kinetics of model epoxy-amine resins. Polymer 1997, 38, 3251–3261. [Google Scholar] [CrossRef]
- Yildiz, M.E.; Kokini, J.L. Determination of Williams–Landel–Ferry constants for a food polymer system: Effect of water activity and moisture content. J. Rheol. 2001, 45, 903–912. [Google Scholar] [CrossRef]
- Ionita, D.; Cristea, M.; Gaina, C. Prediction of polyurethane behaviour via time-temperature superposition: Meanings and limitations. Polym. Test. 2020, 83. [Google Scholar] [CrossRef]
- Nelson, K.A.; Labuza, T.P. Water activity and food polymer science: Implications of state on Arrhenius and WLF models in predicting shelf life. J. Food Eng. 1994, 22, 271–289. [Google Scholar] [CrossRef]
- Fabre, V.; Quandalle, G.; Billon, N.; Cantournet, S. Time-Temperature-Water content equivalence on dynamic mechanical response of polyamide 6,6. Polymer 2018, 137, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.L.; Luo, W.B.; Liu, X.; Li, M.; Huang, Y.J.; Bu, J.L. Temperature and frequency dependent rheological behaviour of carbon black filled natural rubber. Plast. Rubber Compos. 2013, 42, 416–420. [Google Scholar] [CrossRef]
- Yildiz, M.E.; Sozer, N.; Kokini, J.L. Williams-Landel-Ferry (WLF) equation. In Encyclopedia of Agricultural, Food, and Biological Engineering; CRC Press: Boca Raton, FL, USA, 2003; pp. 1865–1877. ISBN 9780824709389. [Google Scholar]
- Ljubic, D.; Stamenovic, M.; Smithson, C.; Nujkic, M.; Medjo, B.; Putic, S. Time-temperature superposition principle—Application of WLF equation in polymer analysis and composites. Zast. Mater. 2014, 55, 395–400. [Google Scholar] [CrossRef] [Green Version]
- Chang, F.C.; Lam, F.; Kadla, J.F. Application of time–temperature–stress superposition on creep of wood–plastic composites. Mech. Time-Depend. Mater. 2013, 17, 427–437. [Google Scholar] [CrossRef]
- Dan-asabe, B. Thermo-mechanical characterization of banana particulate reinforced PVC composite as piping material. J. King Saud Univ.-Eng. Sci. 2016, 1–9. [Google Scholar] [CrossRef]
- Sbirrazzuoli, N. Advanced Isoconversional Kinetic Analysis for the Elucidation of Complex Reaction Mechanisms: A New Method for the Identification ofRate-Limiting Steps. Molecules 2019, 24, 1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berčič, G. The universality of Friedman’s isoconversional analysis results in a model-less prediction of thermodegradation profiles. Thermochim. Acta 2017, 650, 1–7. [Google Scholar] [CrossRef]
- Ozawa, T. A New Method of Analyzing Thermogravimetric Data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef] [Green Version]
- Flynn, J.H.; Wall, L.A. A quick, direct method for the determination of activation energy from thermogravimetric data. J. Polym. Sci. Part B Polym. Lett. 1966, 4, 323–328. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 2007, 6, 183–195. [Google Scholar] [CrossRef]
- Akahira, T.; Sunose, T. Joint Convention of Four Electrical Institutes: Method of Determining Activation Deterioration Constant of Electrical Insulating Materials. Res. Rep. Chiba Inst. Technol. 1971, 16, 22–31. [Google Scholar]
- Starink, M.J. Activation energy determination for linear heating experiments: Deviations due to neglecting the low temperature end of the temperature integral. J. Mater. Sci. 2007, 42, 483–489. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Wang, H.; Nakada, M. Iterative method to improve calculation of the pre-exponential factor for dynamic thermogravimetric analysis measurements. Polymer 2006, 47, 1590–1596. [Google Scholar] [CrossRef]
- Vyazovkin, S. Evaluation of activation energy of thermally stimulated solid-state reactions under arbitrary variation of temperature. J. Comput. Chem. 1997, 18, 393–402. [Google Scholar] [CrossRef]
- Vyazovkin, S. Modification of the integral isoconversional method to account for variation in the activation energy. J. Comput. Chem. 2001, 22, 178–183. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Chrissafis, K.; Di Lorenzo, M.L.; Koga, N.; Pijolat, M.; Roduit, B.; Sbirrazzuoli, N.; Suñol, J.J. ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim. Acta 2014, 590, 1–23. [Google Scholar] [CrossRef]
- Šimon, P. Isoconversional methods: Fundamentals, meaning and application. J. Therm. Anal. Calorim. 2004, 76, 123–132. [Google Scholar] [CrossRef]
- Criado, J.M.; Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A. Critical study of the isoconversional methods of kinetic analysis. J. Therm. Anal. Calorim. 2008, 92, 199–203. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A.; Perejón, A.; Criado, J.M. Generalized master plots as a straightforward approach for determining the kinetic model: The case of cellulose pyrolysis. Thermochim. Acta 2013, 552, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Leroy, V.; Cancellieri, D.; Leoni, E.; Rossi, J.L. Kinetic study of forest fuels by TGA: Model-free kinetic approach for the prediction of phenomena. Thermochim. Acta 2010, 497, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Das, P.; Tiwari, P. Thermal degradation kinetics of plastics and model selection. Thermochim. Acta 2017, 654, 191–202. [Google Scholar] [CrossRef]
- Cui, H.W.; Jiu, J.T.; Sugahara, T.; Nagao, S.; Suganuma, K.; Uchida, H.; Schroder, K.A. Using the Friedman method to study the thermal degradation kinetics of photonically cured electrically conductive adhesives. J. Therm. Anal. Calorim. 2014, 119, 425–434. [Google Scholar] [CrossRef]
- Mittal, A.; Garg, S.; Bajpai, S. Thermal decomposition kinetics and properties of grafted barley husk reinforced PVA/starch composite fi lms for packaging applications. Carbohydr. Polym. 2020, 240, 116225. [Google Scholar] [CrossRef] [PubMed]
- Kropidłowska, A.; Rotaru, A.; Strankowski, M.; Becker, B.; Segal, E. Heteroleptic cadmium(II) complex, potential precursor for semiconducting CDS layers: TTThermal stability and non-isothermal decomposition kinetics. J. Therm. Anal. Calorim. 2008, 91, 903–909. [Google Scholar] [CrossRef]
- Venkatesh, M.; Ravi, P.; Tewari, S.P. Isoconversional kinetic analysis of decomposition of nitroimidazoles: Friedman method vs. Flynn-Wall-Ozawa method. J. Phys. Chem. A 2013, 117, 10162–10169. [Google Scholar] [CrossRef] [PubMed]
- Ali, G.; Nisar, J.; Iqbal, M.; Shah, A.; Abbas, M.; Shah, M.R.; Rashid, U.; Bhatti, I.A.; Khan, R.A.; Shah, F. Thermo-catalytic decomposition of polystyrene waste: Comparative analysis using different kinetic models. Waste Manag. Res. 2019, 38, 1–11. [Google Scholar] [CrossRef]
- Vassiliou, A.A.; Chrissafis, K.; Bikiaris, D.N. In situ prepared PET nanocomposites: Effect of organically modified montmorillonite and fumed silica nanoparticles on PET physical properties and thermal degradation kinetics. Thermochim. Acta 2010, 500, 21–29. [Google Scholar] [CrossRef]
- Benhacine, F.; Yahiaoui, F.; Hadj-hamou, A.S. Thermal Stability and Kinetic Study of Isotactic Polypropylene/Algerian Bentonite Nanocomposites Prepared via Melt Blending. J. Polym. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Sbirrazzuoli, N.; Vincent, L.; Mija, A.; Guigo, N. Integral, differential and advanced isoconversional methods. Complex mechanisms and isothermal predicted conversion-time curves. Chemom. Intell. Lab. Syst. 2009, 96, 219–226. [Google Scholar] [CrossRef]
- Lim, A.C.R.; Chin, B.L.F.; Jawad, Z.A.; Hii, K.L. Kinetic Analysis of Rice Husk Pyrolysis Using Kissinger-Akahira-Sunose (KAS) Method. Procedia Eng. 2016, 148, 1247–1251. [Google Scholar] [CrossRef] [Green Version]
- Heydari, M.; Rahman, M.; Gupta, R. Kinetic study and thermal decomposition behavior of lignite coal. Int. J. Chem. Eng. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Laborie, M.G.; Wolcott, M.P. Comparison of model-free kinetic methods for modeling the cure kinetics of commercial phenol—Formaldehyde resins. Thermochim. Acta 2005, 439, 68–73. [Google Scholar] [CrossRef]
- Gabilondo, N.; López, M.; Ramos, J.A.; Echeverría, J.M.; Mondragon, I. Curing kinetics of amine and sodium hydroxide catalyzed phenol-formaldehyde resins. J. Therm. Anal. Calorim. 2007, 90, 229–236. [Google Scholar] [CrossRef]
- Sun, H.; Liu, Y.; Wang, Y.; Tan, H. Curing Behavior of Epoxy Resins in Two-Stage Curing Process by Non-Isothermal Differential Scanning Calorimetry Kinetics Method. J. Appl. Polym. Sci. 2014, 40711, 1–8. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Sbirrazzuoli, N. Isoconversional Analysis of Calorimetric Data on Nonisothermal Crystallization of a Polymer Melt. J. Phys. Chem. B 2003, 107, 882–888. [Google Scholar] [CrossRef]
- Vyazovkin, S. A Unified Approach to Kinetic Processing of Nonisothermal Data. J. Chem. Kinet. 1996, 28, 95–101. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Sbirrazzuoli, N. Mechanism and kinetics of epoxy-amine cure studied by differential scanning calorimetry. Macromolecules 1996, 29, 1867–1873. [Google Scholar] [CrossRef]
- Dunne, R.C.; Sitaraman, S.K.; Luo, S.; Rao, Y.; Wong, C.P.; Estes, W.E.; Gonzalez, C.G.; Coburn, J.C.; Periyasamy, M. Investigation of the curing behavior of a novel epoxy photo-dielectric dry film (ViaLuxTM 81) for high density interconnect applications. J. Appl. Polym. Sci. 2000, 78, 430–437. [Google Scholar] [CrossRef]
- Li, S.Y.; Vuorimaa, E.; Lemmetyinen, H. Application of isothermal and model-free isoconversional modes in DSC measurement for the curing process of the PU system. J. Appl. Polym. Sci. 2001, 81, 1474–1480. [Google Scholar] [CrossRef]
- He, G.; Yan, N. Effect of moisture content on curing kinetics of pMDI resin and wood mixtures. Int. J. Adhes. Adhes. 2005, 25, 450–455. [Google Scholar] [CrossRef]
- Polli, H.; Pontes, L.A.M.; Araujo, A.S. Application of model-free kinetics to the study of thermal degradation of polycarbonate. J. Therm. Anal. Calorim. 2005, 79, 383–387. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Dranca, I.; Fan, X.; Advincula, R. Degradation and relaxation kinetics of polystyrene-clay nanocomposite prepared by surface initiated polymerization. J. Phys. Chem. B 2004, 108, 11672–11679. [Google Scholar] [CrossRef]

| Rubber | Abbreviation | Recommended Storage Life Without Inspection (Years) | Storage Life Extension After Visual Inspection (Years) |
|---|---|---|---|
| Natural Rubber | NR | 5 | 2 |
| Butadiene-styrene | SBR | 5 | 2 |
| Nitrile | N | 7 | 3 |
| Nitrile-butadiene | HNBR | 7 | 3 |
| Acrylic | ACM | 7 | 3 |
| Chloroprene | C | 7 | 3 |
| Ethylene-Propylene | E | 10 | 5 |
| Viton™/FKM | V | 10 | 5 |
| Kalrez™/FFKM | KLZ | 10 | 5 |
| Silicone | S | 10 | 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plota, A.; Masek, A. Lifetime Prediction Methods for Degradable Polymeric Materials—A Short Review. Materials 2020, 13, 4507. https://doi.org/10.3390/ma13204507
Plota A, Masek A. Lifetime Prediction Methods for Degradable Polymeric Materials—A Short Review. Materials. 2020; 13(20):4507. https://doi.org/10.3390/ma13204507
Chicago/Turabian StylePlota, Angelika, and Anna Masek. 2020. "Lifetime Prediction Methods for Degradable Polymeric Materials—A Short Review" Materials 13, no. 20: 4507. https://doi.org/10.3390/ma13204507





