Corrosion and Corrosion Protection of Additively Manufactured Aluminium Alloys—A Critical Review
Abstract
:1. Introduction
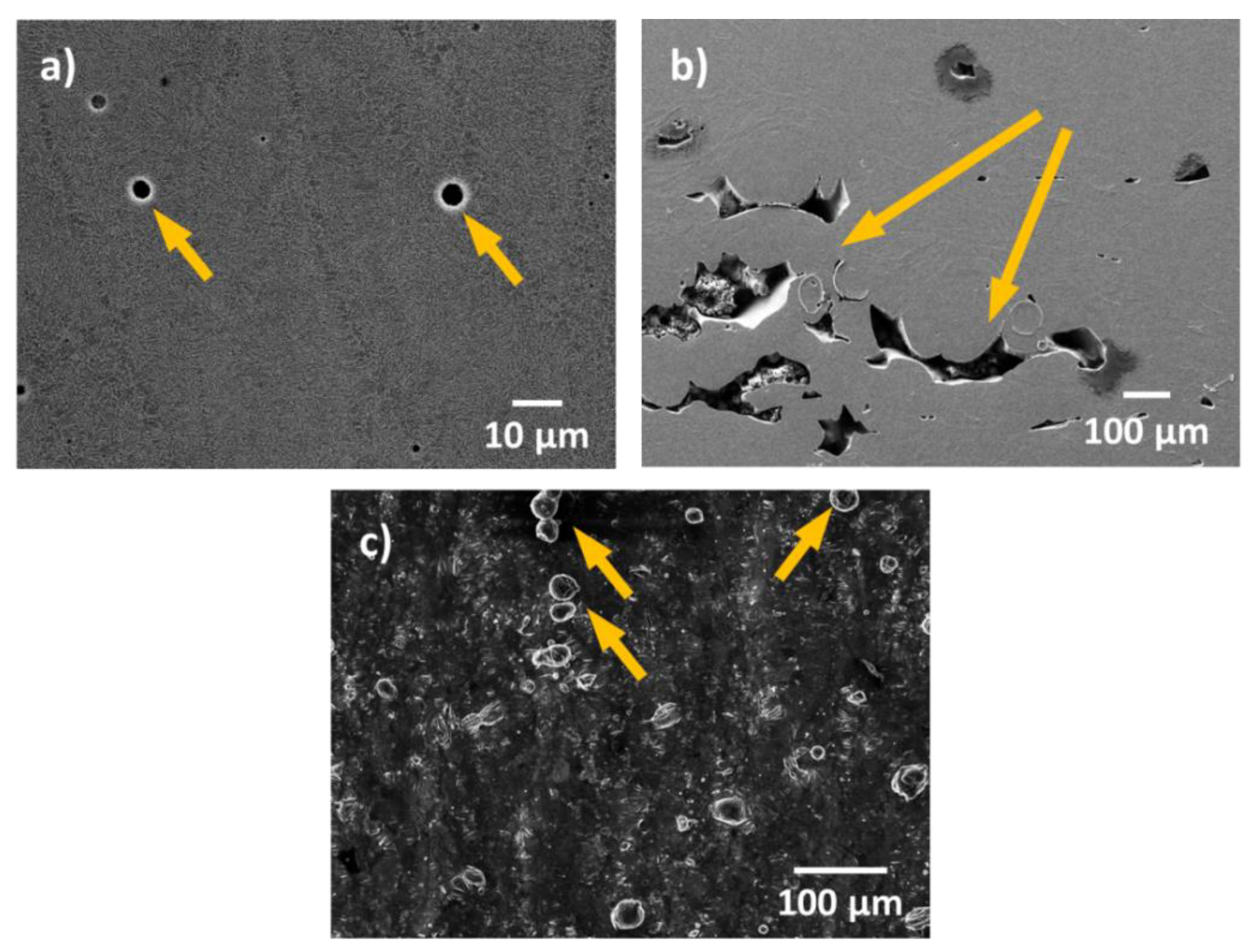
2. Influence of Defects in AM Specimens on Corrosion Resistance
3. Al-Si Alloys
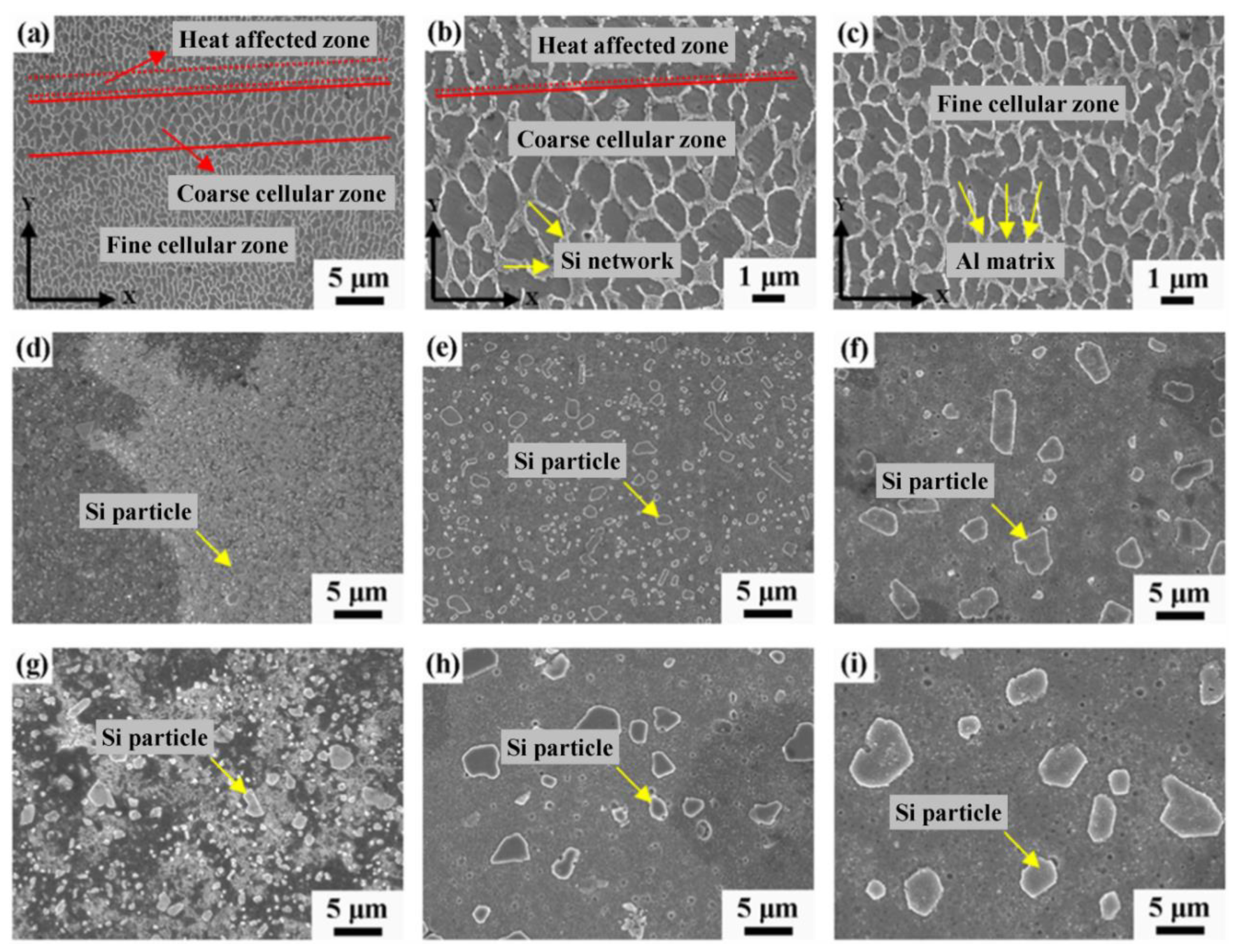
3.1. Effect of Microstructure on Corrosion Behavior
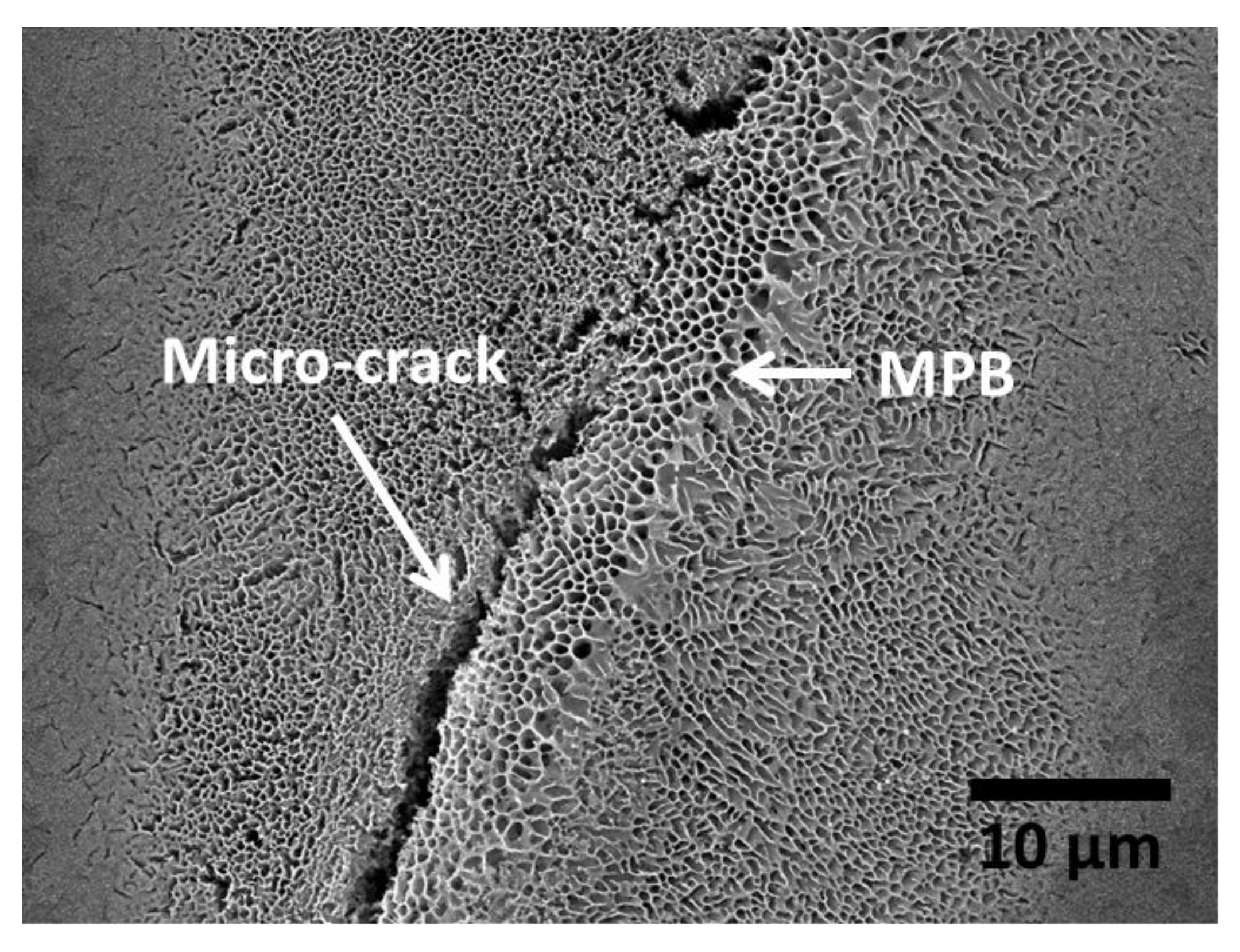
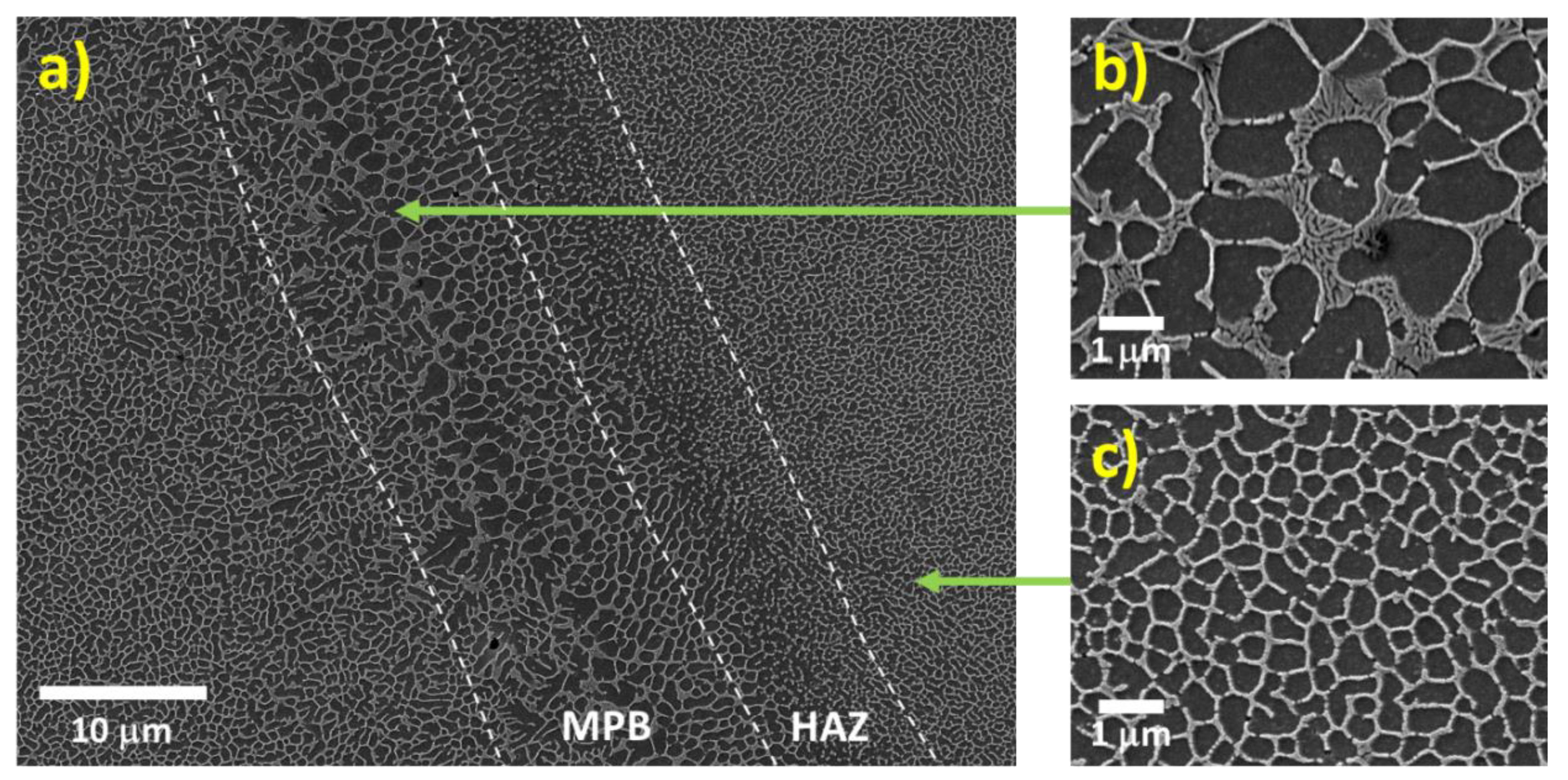
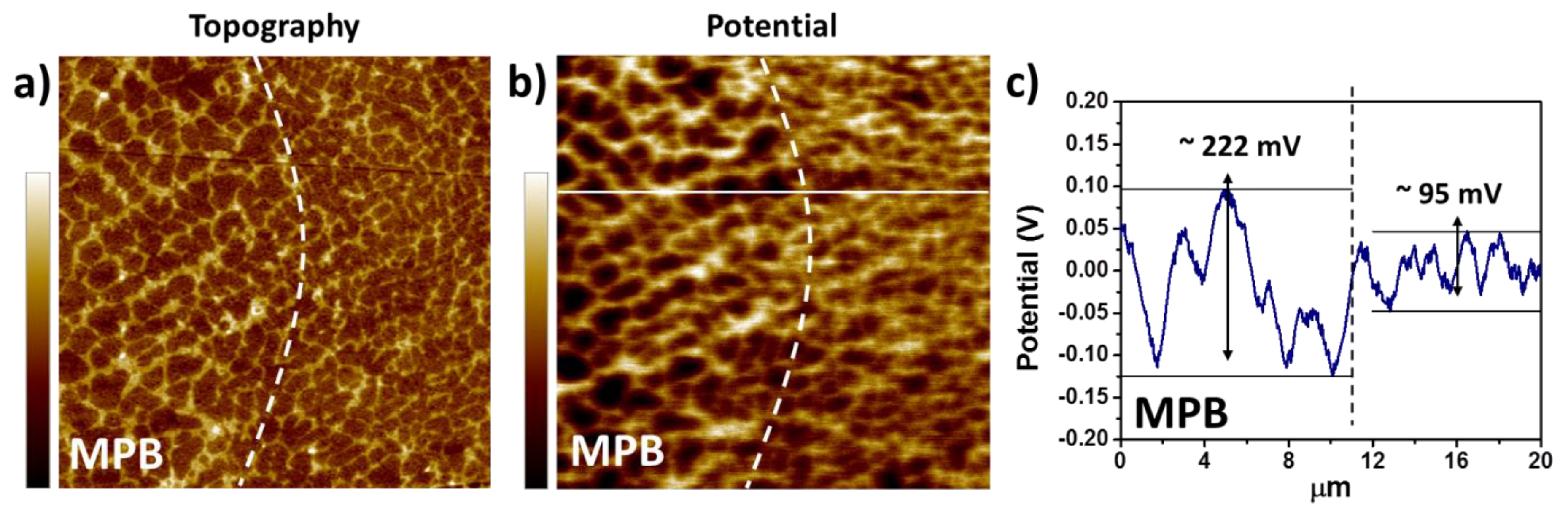
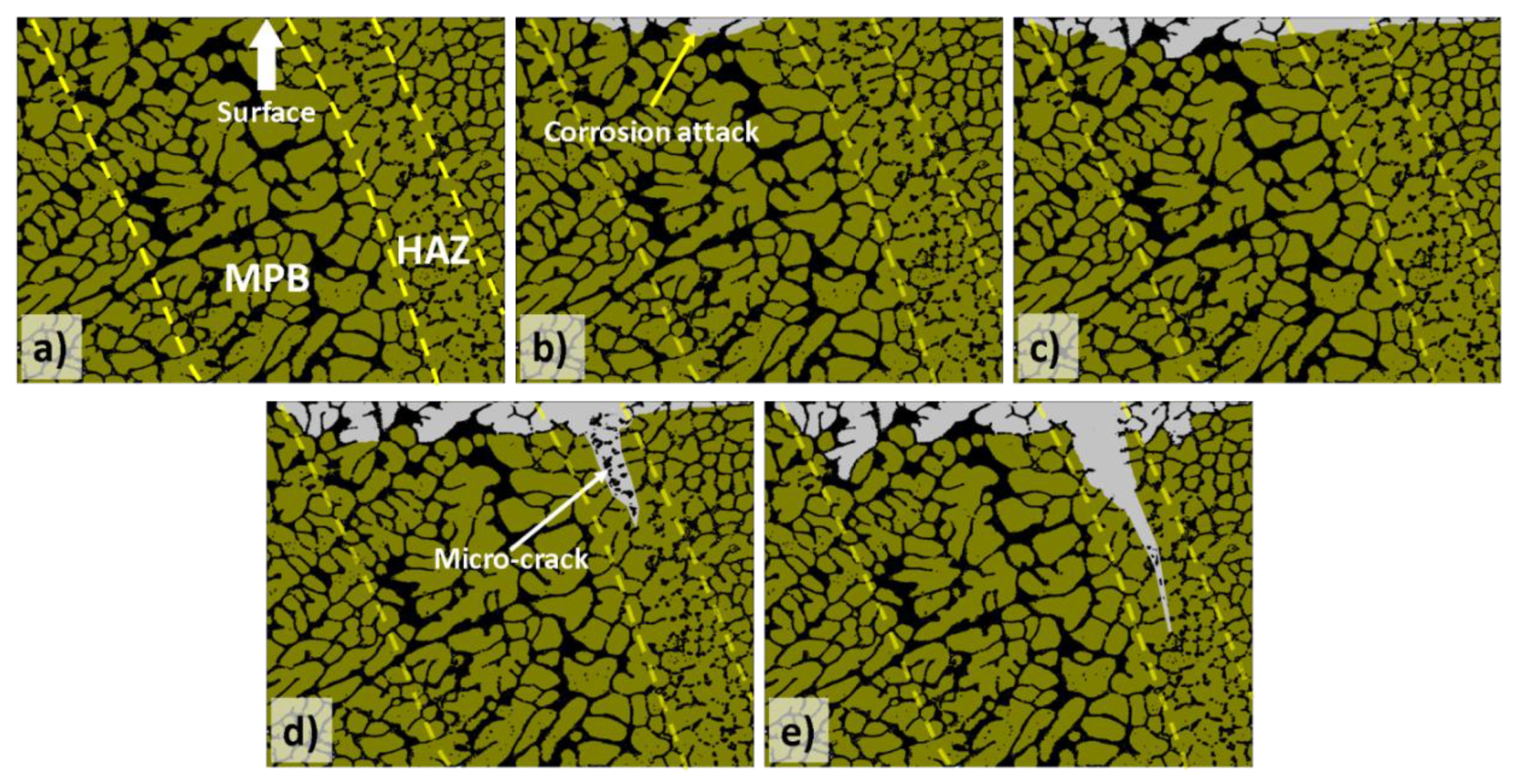
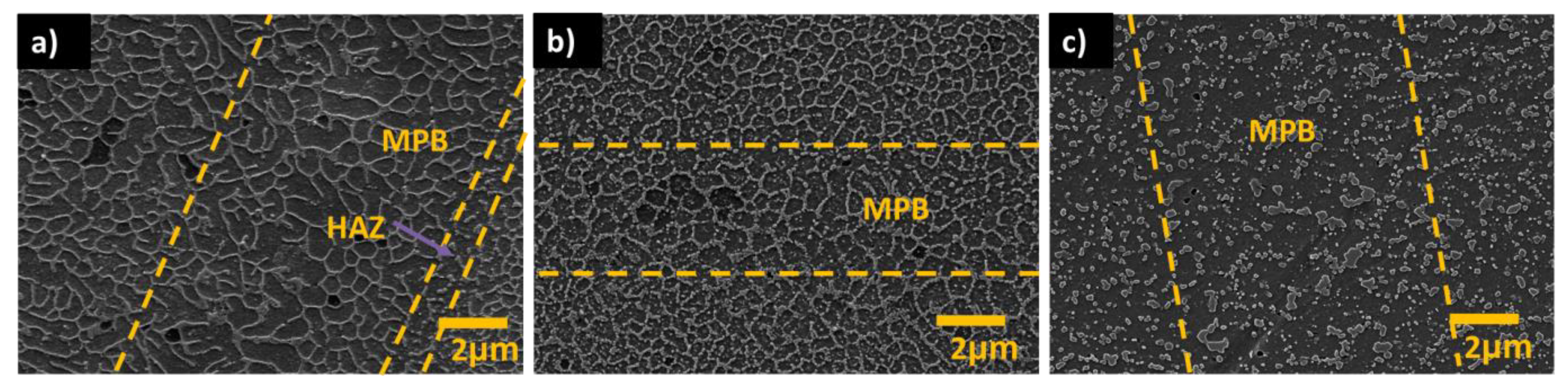
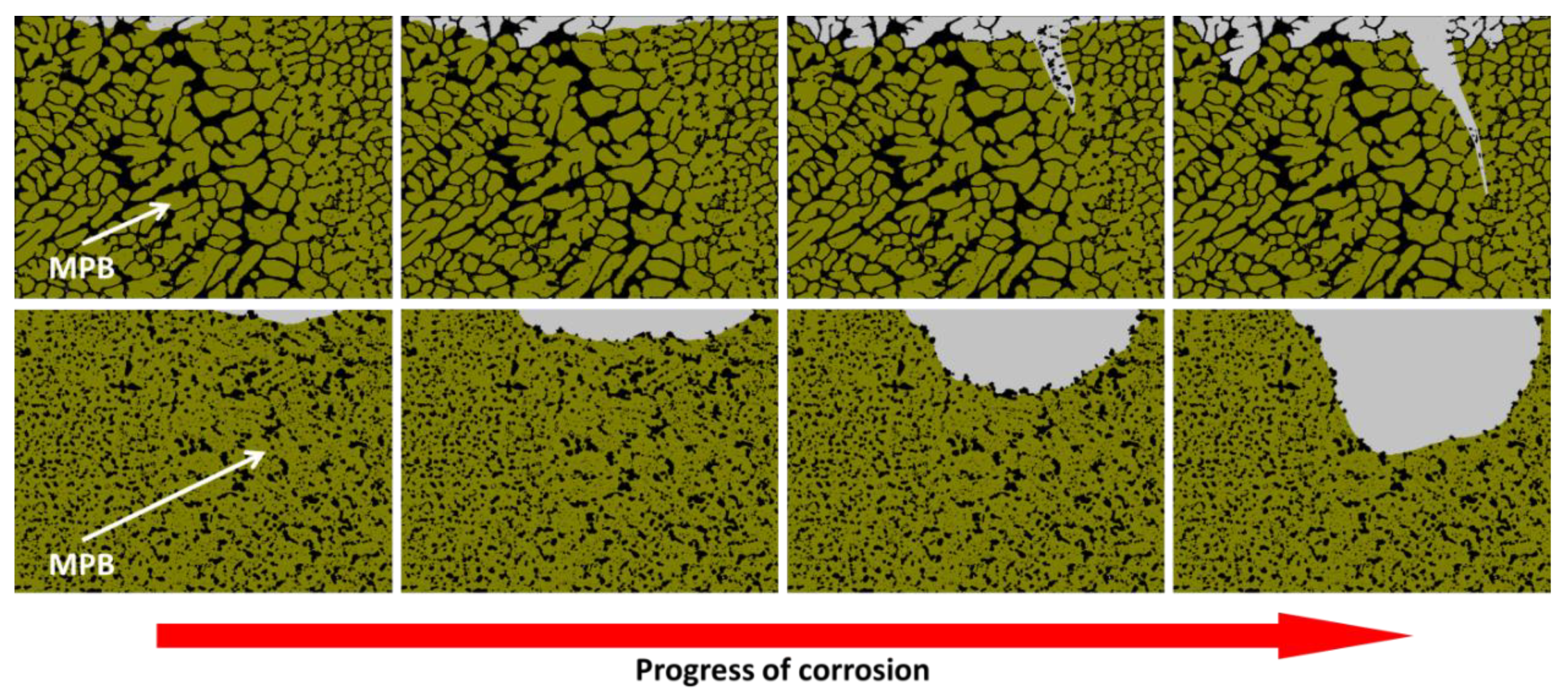
3.1.1. Influence of the Melt Pool Borders on Corrosion
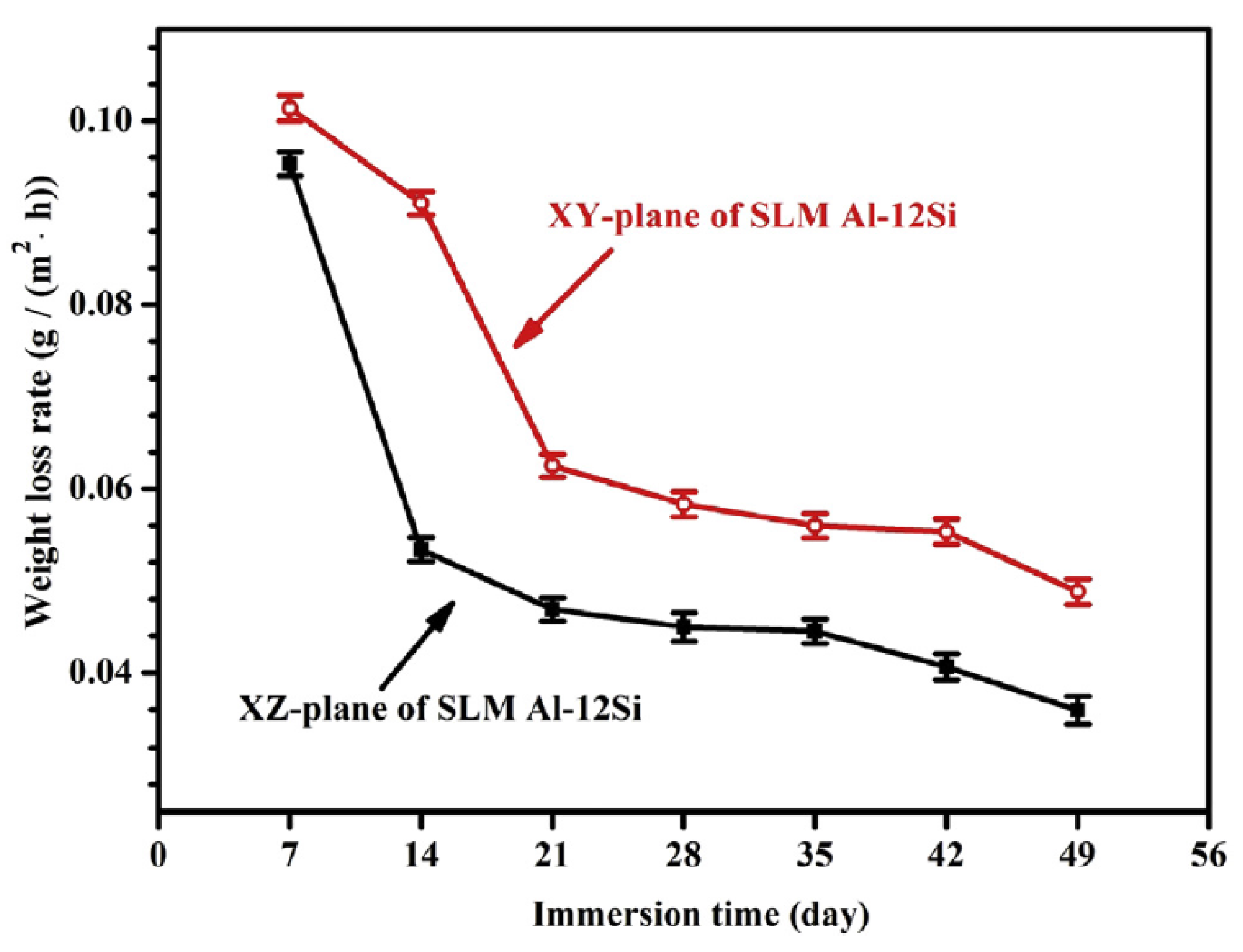
3.1.2. Anisotropy
3.1.3. Comparison with Conventional Alloys
AlSi10Mg
AlSi12
3.2. Effect of Heat Treatment on Microstructure and Corrosion
3.2.1. Effect on Microstructure
Stress Release Heat Treatments
Heating of Building Platform
Solution Treatment—T6
Artificial Aging Heat Treatments
3.2.2. Effect on Corrosion
Stress Release Heat Treatments
Heating of Building Platform
Solution Treatment—T6
Artificial Aging Heat Treatments
3.3. Effect of Si Content on Microstructure and Corrosion
3.4. Effect of Surface Roughness on Corrosion
3.5. Corrosion Protection
4. Other Al Alloys
5. Summary and Outlook
- Due to the special conditions associated with the metal additive manufacturing processes, numerous macro- and micro-structural defects can exist within the printed parts. Defects such as porosity, remaining unmolten powder, high surface roughness, and residual stresses, can greatly influence the corrosion performance of these materials. Even though the existence of some of these defects can be limited by carefully tuning the process parameters, they cannot be fully avoided due to the large number of parameters involved and the high complexity of the process. In general, more research should be conducted in order to better understand the influence of these defects and the combined impact of process parameters on the corrosion resistance of these materials.
- Aside from Al-Si alloys (and specially AlSi10Mg alloy), very limited research has been published concerning the corrosion resistance of other additively manufactured Al alloy parts. More focus should be given to other types of 3D-printed Al-based alloys. Moreover, further research is needed to study the microstructure and corrosion behavior of additively manufactured parts prepared using other metal additive manufacturing methods besides selective laser melting/sintering.
- In general, most of the published works on additively manufactured Al-based alloys agree that their corrosion resistance is similar to or higher than that of the same alloys fabricated using traditional manufacturing methods.
- Due to the special conditions associated with the metal additive manufacturing processes (for instance: the use of pre-alloyed metal powder and highly localized (re)melting combined with high cooling rates), a unique microstructure formed by a fine Si network that encloses the α-Al in small cells of varying sizes across the melt pools characterizes the printed Al-Si alloys specimens. A great number of works have already shown that the corrosion behavior of these materials is greatly influenced by this special and unique microstructure. The borders of the melt pools (where larger cells are found) have been identified as vulnerable sites for corrosion to initiate and further propagate. However, in general, the existence of a rather connected Si network for the case of as-built specimens prevents/holds the corrosion from penetrating deeper into the material.
- The specific (out of equilibrium) microstructure obtained by SLM processing makes the materials susceptible to certain heat treatments (such as stress release and solution T6 heat treatments). Depending on the temperature of the heat treatment, these can disrupt the silicon network in Al-Si alloys, leading to the formation of large and separate Si precipitates. Aside from negatively impacting the mechanical properties, this also affects the corrosion resistance. The disruption of the Si network allows corrosion to penetrate deeper into the material, while larger Si precipitates disrupt the formation of a compact passive layer and form larger cathodic sites that increase the driving force for galvanic corrosion. Additionally, the presence of Mg in most Al-Si alloys makes the material susceptible to precipitation hardening through the formation of Mg2Si precipitates. However, the presence of these precipitates can greatly affect the pitting corrosion process in these materials. An alternative to stress release heat treatment is the heating of the building platform during production. In this way, alternation of the microstructure and subsequent change in corrosion behavior can be prevented. However, more research on different building platform temperatures and their effect on the microstructure and corrosion behavior is required. Moreover, further work should focus on adapting existing heat treatments to the specific microstructure of the additively manufactured materials.
- For additively manufactured Al-Si alloys, increasing the amount of Si, greatly increases the level of connectivity of the silicon network, which reduces the penetration depth of the corrosion attack. However, in these alloys, the presence of small amounts of Mg and Fe cannot be ignored during the analysis of the corrosion behavior.
- It has been shown that the choice of printing parameters can greatly affect the surface roughness of the printed parts, and thus also their corrosion resistance. The scanning speed, followed by the hatching distance, was reported to have the largest effect. Altering the manufacturing parameters to reduce the surface roughness could also affect the microstructure. In general, an improvement of the corrosion resistance has been reported for samples that underwent post-printing operations to reduce their surface roughness (such as shot peening, sandblasting, and bright dipping) compared to as-produced samples. However, further work is needed to better understand the performance of other post-printing processes (for instance electro-polishing), as well as their effect on the corrosion behavior of additively manufactured Al-based alloys.
- In general, a limited number of studies have been conducted to understand the mechanisms of corrosion protection of additively manufactured metal parts. Studies on the galvanostatic anodizing of 3D-printed Al-Si alloys demonstrated that the special microstructure of these materials greatly influences the typical voltage-time behavior as well as the characteristics of the anodic oxide layer. A significantly lower oxide growth rate was seen for additively manufactured Al-Si parts compared to cast alloy, attributed to the larger fraction of the anodic charge consumed by the oxidation of the Si phase. The porous anodic oxide film, thinner around the melt pool borders, was characterized by the formation of branched-like pores. The anodizing behavior as well as the anodic oxide layer of additively manufactured Al-Si alloys is greatly affected by defects and special features typical from the metal additive manufacturing process. The intrinsic anisotropy, melt pool borders, and internal pores are among those features affecting the anodization process. More research is still needed to better understand the impact of other defects such as unmolten powder and residual stresses on the anodizing behavior of these materials, as well as to further investigate the properties of the formed anodic oxides. Moreover, additional research is required to understand the anodizing behaviour of these materials under other anodizing conditions such as potentiostatic anodizing regime and different electrolytes.
Author Contributions
Funding
Conflicts of Interest
References
- Murr, L.E.; Gaytan, S.M.; Ramirez, D.A.; Martinez, E.; Hernandez, J.; Amato, K.N.; Shindo, P.W.; Medina, F.R.; Wicker, R.B. Metal Fabrication by Additive Manufacturing Using Laser and Electron Beam Melting Technologies. J. Mater. Sci. Technol. 2012, 28, 1–14. [Google Scholar] [CrossRef]
- Murr, L.; Martinez, E.; Amato, K.N.; Gaytan, S.M.; Hernandez, J.; Ramirez, D.A.; Shindo, P.W.; Medina, F.; Wicker, R.B. Fabrication of Metal and Alloy Components by Additive Manufacturing: Examples of 3D Materials Science. J. Mater. Res. Technol. 2012, 1, 42–54. [Google Scholar] [CrossRef] [Green Version]
- Herzog, D.; Seyda, V.; Wycisk, E.; Emmelmann, C. Additive manufacturing of metals. Acta Mater. 2016, 117, 371–392. [Google Scholar] [CrossRef]
- Sames, W.J.; List, F.A.; Pannala, S.; Dehoff, R.R.; Babu, S.S. The metallurgy and processing science of metal additive manufacturing. Int. Mater. Rev. 2016, 61, 315–360. [Google Scholar] [CrossRef]
- Gorsse, S.; Hutchinson, C.; Goune, M.; Banerjee, R. Additive manufacturing of metals: A brief review of the characteristic microstructures and properties of steels, Ti-6Al-4V and high-entropy alloys. Sci. Technol. Adv. Mater. 2017, 18, 584–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, D.; Meiners, W.; Wissenbach, K.; Poprawe, R. Laser additive manufacturing of metallic components: Materials, processes and mechanisms. Int. Mater. Rev. 2012, 57, 133–164. [Google Scholar] [CrossRef]
- ISO/ASTM52900-15. Standard Terminology for Additive Manufacturing—General Principles—Terminology; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Sun, G.; Shen, X.; Wang, Z.; Zhan, M.; Yao, S.; Zhou, R.; Ni, Z. Laser metal deposition as repair technology for 316L stainless steel: Influence of feeding powder compositions on microstructure and mechanical properties. Opt. Laser Technol. 2019, 109, 71–83. [Google Scholar] [CrossRef]
- Dilberoglu, U.M.; Gharehpapagh, B.; Yaman, U.; Dolen, M. The Role of Additive Manufacturing in the Era of Industry 4.0. Procedia Manuf. 2017, 11, 545–554. [Google Scholar] [CrossRef]
- Additive Manufacturing Report. Available online: https://additive-manufacturing-report.com/additive-manufacturing-market/ (accessed on 16 October 2020).
- DebRoy, T.; Wei, H.L.; Zuback, J.S.; Mukherjee, T.; Elmer, J.W.; Milewski, J.O.; Beese, A.M.; Wilson-Heid, A.; De, A.; Zhang, W. Additive manufacturing of metallic components—Process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Additive Manufacturing for the Aerospace Industry; Elsevier: Amsterdam, The Netherlands, 2019.
- Miller, W.; Zhuang, L.; Bottema, J.; Wittebrood, A.; De Smet, P.; Haszler, A.; Vieregge, A. Recent development in aluminium alloys for the automotive industry. Mater. Sci. Eng. A 2000, 280, 37–49. [Google Scholar] [CrossRef]
- Available Materials for Metal Additive Manufacturing: Characteristics & Applications, Farinia Group. Available online: https://www.farinia.com/additive-manufacturing/3dmaterials/characteristics-and-applications-ofavailable-metals-for-additive-manufacturing (accessed on 7 September 2020).
- Aboulkhair, N.T.; Simonelli, M.; Parry, L.; Ashcroft, I.; Tuck, C.; Hague, R. 3D printing of Aluminium alloys: Additive Manufacturing of Aluminium alloys using selective laser melting. Prog. Mater. Sci. 2019, 106, 100578. [Google Scholar] [CrossRef]
- Vojtech, D.; Serak, J.; Ekrt, O. Improving the casting properties of high-strength aluminium alloys. Mater. Technol. 2004, 38, 99–102. [Google Scholar]
- Zhang, J.; Song, B.; Wei, Q.; Bourell, D.; Shi, Y. A review of selective laser melting of aluminum alloys: Processing, microstructure, property and developing trends. J. Mater. Sci. Technol. 2019, 35, 270–284. [Google Scholar] [CrossRef]
- ASM Handbook; ASM International: Materials Park, OH, USA, 1991; Volume 4.
- Sander, G.; Tan, J.; Balan, P.; Gharbi, O.; Feenstra, D.; Singer, L.; Thomas, S.; Kelly, R.; Scully, J.; Birbilis, N. Corrosion of Additively Manufactured Alloys: A Review. Corrosion 2018, 74, 1318–1350. [Google Scholar] [CrossRef] [Green Version]
- Kong, D.; Dong, C.; Ni, X.; Li, X. Corrosion of metallic materials fabricated by selective laser melting. NPJ Mater. Degrad. 2019, 3, 24. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, C.; Jia, D.; Wellmann, D.; Liu, W. Corrosion Behaviors of Selective Laser Melted Aluminum Alloys: A Review. Metals 2020, 10, 102. [Google Scholar] [CrossRef] [Green Version]
- Ornek, C. Additive manufacturing–a general corrosion perspective. Corr. Eng. Sci. Technol. 2018, 53, 531–535. [Google Scholar] [CrossRef]
- Kempen, K.; Thijs, L.; Van Humbeeck, J.; Kruth, J.-P. Processing AlSi10Mg by selective laser melting: Parameter optimisation and material characterisation. Mater. Sci. Technol. 2014, 31, 917–923. [Google Scholar] [CrossRef]
- Prashanth, K.G.; Scudino, S.; Maity, T.; Das, J.; Eckert, J. Is the energy density a reliable parameter for materials synthesis by selective laser melting? Mater. Res. Lett. 2017, 5, 386–390. [Google Scholar] [CrossRef] [Green Version]
- Leung, C.L.A.; Marussi, S.; Towrie, M.; Atwood, R.C.; Withers, P.J.; Lee, P.D. The effect of powder oxidation on defect formation in laser additive manufacturing. Acta Mater. 2019, 166, 294–305. [Google Scholar] [CrossRef] [Green Version]
- Coeck, S.; Bisht, M.; Plas, J.; Verbist, F. Prediction of lack of fusion porosity in selective laser malting based on melt pool monitoring data. Addit. Manuf. 2019, 25, 347–356. [Google Scholar]
- Sander, G.; Thomas, S.; Cruz, V.; Jurg, M.; Birbilis, N.; Gao, X.; Brameld, M.; Hutchinson, C.R. On the Corrosion and Metastable Pitting Characteristics of 316L Stainless Steel Produced by Selective Laser Melting. J. Electrochem. Soc. 2017, 164, C250–C257. [Google Scholar] [CrossRef]
- Schaller, R.; Taylor, J.; Rodelas, J.; Schindelholz, E. Corrosion Properties of Powder Bed Fusion Additively Manufactured 17-4 PH Stainless Steel. Corrosion 2017, 73, 796–807. [Google Scholar] [CrossRef]
- Kumbhar, N.N.; Mulay, A.V. Post Processing Methods used to Improve Surface Finish of Products which are Manufactured by Additive Manufacturing Technologies: A Review. J. Inst. Eng. Ser. C 2016, 99, 481–487. [Google Scholar] [CrossRef]
- Scherillo, F. Chemical surface finishing of AlSi10Mg components made by additive manufacturing. Manuf. Lett. 2019, 19, 5–9. [Google Scholar] [CrossRef]
- Li, C.; Liu, Z.; Fang, X.; Guo, Y. Residual Stress in Metal Additive Manufacturing. Procedia CIRP 2018, 71, 348–353. [Google Scholar] [CrossRef]
- Revilla, R.I.; Liang, J.; Godet, S.; De Graeve, I. Local Corrosion Behavior of Additive Manufactured AlSiMg Alloy Assessed by SEM and SKPFM. J. Electrochem. Soc. 2016, 164, C27–C35. [Google Scholar] [CrossRef]
- Revilla, R.I.; De Graeve, I. Influence of Si content on the microstructure and corrosion behaviour of additive manufactured Al-Si alloys. J. Electrochem. Soc. 2018, 165, C926–C932. [Google Scholar] [CrossRef]
- Brandl, E.; Heckenberger, U.; Holzinger, V.; Buchbinder, D. Additive manufactured AlSi10Mg samples using Selective Laser Melting (SLM): Microstructure, high cycle fatigue, and fracture behavior. Mater. Des. 2012, 34, 159–169. [Google Scholar] [CrossRef]
- Manfredi, D.; Calignano, F.; Krishnan, M.; Canali, R.; Ambrosio, E.P.; Atzeni, E. From Powders to Dense Metal Parts: Characterization of a Commercial AlSiMg Alloy Processed through Direct Metal Laser Sintering. Materials 2013, 6, 856–869. [Google Scholar] [CrossRef] [Green Version]
- Thijs, L.; Kempen, K.; Kruth, J.-P.; Van Humbeeck, J. Fine-structured aluminium products with controllable texture by selective laser melting of pre-alloyed AlSi10Mg powder. Acta Mater. 2013, 61, 1809–1819. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.; Hao, L.; Hussein, A.; Young, P.; Huang, J.; Zhu, W. Microstructure and mechanical properties of aluminium alloy cellular lattice structures manufactured by direct metal laser sintering. Mater. Sci. Eng. A 2015, 628, 238–246. [Google Scholar] [CrossRef]
- Li, W.; Li, S.; Liu, J.; Zhang, A.; Zhou, Y.; Wei, Q.; Yan, C.; Shi, Y. Effect of heat treatment on AlSi10Mg alloy fabricated by selective laser melting: Microstructure evolution, mechanical properties and fracture mechanism. Mater. Sci. Eng. A 2016, 663, 116–125. [Google Scholar] [CrossRef]
- Prashanth, K.; Scudino, S.; Klauss, H.; Surreddi, K.; Löber, L.; Wang, Z.; Chaubey, A.; Kühn, U.; Eckert, J. Microstructure and mechanical properties of Al–12Si produced by selective laser melting: Effect of heat treatment. Mater. Sci. Eng. A 2014, 590, 153–160. [Google Scholar] [CrossRef]
- Kimura, T.; Nakamoto, T.; Mizuno, M.; Araki, H. Effect of silicon content on densification, mechanical and thermal properties of Al-xSi binary alloys fabricated using selective laser melting. Mater. Sci. Eng. A 2017, 682, 593–602. [Google Scholar] [CrossRef] [Green Version]
- Revilla, R.I.; Verkens, D.; Couturiaux, G.; Malet, L.; Thijs, L.; Godet, S.; De Graeve, I. Galvanostatic Anodizing of Additive Manufactured Al-Si10-Mg Alloy. J. Electrochem. Soc. 2017, 164, C1027–C1034. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Gu, X.; Dai, N.; Qin, P.; Zhang, L.-C. Distinction of corrosion resistance of selective laser melted Al-12Si alloy on different planes. J. Alloys Compd. 2018, 747, 648–658. [Google Scholar] [CrossRef]
- Revilla, R.I.; Terryn, H.; De Graeve, I. Role of Si in the Anodizing Behavior of Al-Si Alloys: Additive Manufactured and Cast Al-Si10-Mg. J. Electrochem. Soc. 2018, 165, C532–C541. [Google Scholar] [CrossRef]
- Fathi, P.; Mohammadi, M.; Duan, X.; Nasiri, A. A comparative study on corrosion and microstructure of direct metal laser sintered AlSi10Mg_200C and die cast A360.1 aluminum. J. Mater. Process. Technol. 2018, 259, 1–14. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Pastore, T.; Testa, C.; Manfredi, D.; Lorusso, M.; Calignano, F.; Pavese, M.; Andreatta, F. Corrosion behavior of AlSi10Mg alloy produced by laser powder bed fusion under chloride exposure. Corros. Sci. 2019, 152, 101–108. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Pastore, T. Corrosion behavior of aluminum-silicon alloys obtained by Direct Metal Laser Sintering. In Proceedings of the EUROCORR 2017—The Annual Congress of the European Federation of Corrosion, 20th International Corrosion Congress and Process Safety Congress 2017, Prague, Czech Republic, 3–7 September 2017. [Google Scholar]
- Cabrini, M.; Lorenzi, S.; Pastore, T.; Testa, C.; Manfredi, D.; Cattano, G.; Calignano, F. Corrosion resistance in chloride solution of the AlSi10Mg alloy obtained by means of LPBF. Surf. Interface Anal. 2018, 51, 1159–1164. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Pastore, T.; Pellegrini, S.; Pavese, M.; Fino, P.; Ambrosio, E.P.; Calignano, F.; Manfredi, D. Corrosion resistance of direct metal laser sintering AlSiMg alloy. Surf. Interface Anal. 2016, 48, 818–826. [Google Scholar] [CrossRef]
- Rafieazad, M.; Chatterjee, A.; Nasiri, A.M. Effects of Recycled Powder on Solidification Defects, Microstructure, and Corrosion Properties of DMLS Fabricated AlSi10Mg. JOM 2019, 71, 3241–3252. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Pastore, T.; Pellegrini, S.; Manfredi, D.G.; Fino, P.; Biamino, S.; Badini, C.F. Evaluation of corrosion resistance of Al–10Si–Mg alloy obtained by means of Direct Metal Laser Sintering. J. Mater. Process. Technol. 2016, 231, 326–335. [Google Scholar] [CrossRef]
- Kubacki, G.; Brownhill, J.P.; Kelly, R.G. Comparison of Atmospheric Corrosion of Additively Manufactured and Cast Al-10Si-Mg over a Range of Heat Treatments. Corrosion 2019, 75, 1527–1540. [Google Scholar] [CrossRef]
- Cabrini, M.; Calignano, F.; Fino, P.; Lorenzi, S.; Lorusso, M.; Manfredi, D.; Testa, C.; Pastore, T. Corrosion Behavior of Heat-Treated AlSi10Mg Manufactured by Laser Powder Bed Fusion. Materials 2018, 11, 1051. [Google Scholar] [CrossRef] [Green Version]
- Cabrini, M.; Lorenzi, S.; Pastore, T.; Pellegrini, S.; Ambrosio, E.P.; Calignano, F.; Manfredi, D.G.; Pavese, M.; Fino, P. Effect of heat treatment on corrosion resistance of DMLS AlSi10Mg alloy. Electrochim. Acta 2016, 206, 346–355. [Google Scholar] [CrossRef]
- Rubben, T.; Revilla, R.I.; De Graeve, I. Influence of heat treatments on the corrosion mechanism of additive manufactured AlSi10Mg. Corros. Sci. 2019, 147, 406–415. [Google Scholar] [CrossRef]
- Rafieazad, M.; Mohammadi, M.; Nasiri, A. On microstructure and early stage corrosion performance of heat treated direct metal laser sintered AlSi10Mg. Addit. Manuf. 2019, 28, 107–119. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Testa, C.; Pastore, T.; Manfredi, D.; Lorusso, M.; Calignano, F.; Fino, P. Statistical approach for electrochemical evaluation of the effect of heat treatments on the corrosion resistance of AlSi10Mg alloy by laser powder bed fusion. Electrochim. Acta 2019, 305, 459–466. [Google Scholar] [CrossRef]
- Fathi, P.; Rafieazad, M.; Duan, X.; Mohammadi, M.; Nasiri, A. On microstructure and corrosion behaviour of AlSi10Mg alloy with low surface roughness fabricated by direct metal laser sintering. Corros. Sci. 2019, 157, 126–145. [Google Scholar] [CrossRef]
- Gu, X.-H.; Zhang, J.-X.; Fan, X.-L.; Zhang, L.-C. Corrosion Behavior of Selective Laser Melted AlSi10Mg Alloy in NaCl Solution and Its Dependence on Heat Treatment. Acta Met. Sin. 2019, 33, 327–337. [Google Scholar] [CrossRef] [Green Version]
- Girelli, L.; Tocci, M.; Conte, M.; Giovanardi, R.; Veronesi, P.; Gelfi, M.; Pola, A. Effect of the T6 heat treatment on corrosion behavior of additive manufactured and gravity cast AlSi10Mg alloy. Mater. Corros. 2019, 70, 1808–1816. [Google Scholar] [CrossRef]
- Fathi, P.; Mohammadi, M.; Duan, X.; Nasiri, A. Effects of Surface Finishing Procedures on Corrosion Behavior of DMLS-AlSi10Mg_200C Alloy Versus Die-Cast A360.1 Aluminum. JOM 2019, 71, 1748–1759. [Google Scholar] [CrossRef]
- Leon, A.; Shirizly, A.; Aghion, E. Corrosion Behavior of AlSi10Mg Alloy Produced by Additive Manufacturing (AM) vs. Its Counterpart Gravity Cast Alloy. Metals 2016, 6, 148. [Google Scholar] [CrossRef]
- Prashanth, K.; Debalina, B.; Wang, Z.; Gostin, P.; Gebert, A.; Calin, M.; Kühn, U.; Kamaraj, M.; Scudino, S.; Eckert, J. Tribological and corrosion properties of Al–12Si produced by selective laser melting. J. Mater. Res. 2014, 29, 2044–2054. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Zhang, J.; Gu, X.; Qin, P.; Dai, N.; Li, X.; Kruth, J.-P.; Zhang, L.-C. Improved corrosion behavior of ultrafine-grained eutectic Al-12Si alloy produced by selective laser melting. Mater. Des. 2018, 146, 239–248. [Google Scholar] [CrossRef]
- Yang, P.; Deibler, L.A.; Bradley, D.R.; Stefan, D.K.; Carroll, J.D. Microstructure evolution and thermal properties of an additively manufactured, solution treatable AlSi10Mg part. J. Mater. Res. 2018, 33, 4040–4052. [Google Scholar] [CrossRef] [Green Version]
- Aboulkhair, N.T.; Tuck, C.; Aschroft, I.A.; Maskery, I.; Everitt, N.M. On the Precipitation Hardening of Selective Laser Melted AlSi10Mg. Met. Mater. Trans. A 2015, 46, 3337–3341. [Google Scholar] [CrossRef]
- Yang, P.; Rodriguez, M.A.; Deibler, L.A.; Jared, B.H.; Griego, J.; Kilgo, A.; Allen, A.; Stefan, D.K. Effect of thermal annealing on microstructure evolution and mechanical behavior of an additive manufactured AlSi10Mg part. J. Mater. Res. 2018, 33, 1701–1712. [Google Scholar] [CrossRef] [Green Version]
- Rubben, T.; Revilla, R.I.; De Graeve, I. Effect of Heat Treatments on the Anodizing Behavior of Additive Manufactured AlSi10Mg. J. Electrochem. Soc. 2019, 166, C42–C48. [Google Scholar] [CrossRef]
- Gu, X.; Zhang, J.; Fan, X.; Dai, N.; Xiao, Y.; Zhang, L.-C. Abnormal corrosion behavior of selective laser melted AlSi10Mg alloy induced by heat treatment at 300 °C. J. Alloys Compd. 2019, 803, 314–324. [Google Scholar] [CrossRef]
- Fiocchi, J.; Tuissi, A.; Bassani, P.; Biffi, C.A. Low temperature annealing dedicated to AlSi10Mg selective laser melting products. J. Alloys Compd. 2017, 695, 3402–3409. [Google Scholar] [CrossRef]
- Leon, A.; Aghion, E. Effect of surface roughness on corrosion fatigue performance of AlSi10Mg alloy produced by Selective Laser Melting (SLM). Mater. Charact. 2017, 131, 188–194. [Google Scholar] [CrossRef]
- Gupta, R.K.; Sukiman, N.L.; Fleming, K.M.; Gibson, M.A.; Birbilis, N. Electrochemical Behavior and Localized Corrosion Associated with Mg2Si Particles in Al and Mg Alloys. ECS Electrochem. Lett. 2012, 1, C1–C3. [Google Scholar] [CrossRef]
- Zeng, F.-L.; Wei, Z.-L.; Li, J.; Li, C.-X.; Tan, X.; Zhang, Z.; Zheng, Z.-Q. Corrosion mechanism associated with Mg2Si and Si particles in Al–Mg–Si alloys. Trans. Nonferr. Met. Soc. China 2011, 21, 2559–2567. [Google Scholar] [CrossRef]
- Ahlatci, H. Production and corrosion behaviours of the Al–12Si–XMg alloys containing in situ Mg2Si particles. J. Alloys Compd. 2010, 503, 122–126. [Google Scholar] [CrossRef]
- Calignano, F.; Manfredi, D.; Ambrosio, E.P.; Iuliano, L.; Fino, P. Influence of process parameters on surface roughness of aluminum parts produced by DMLS. Int. J. Adv. Manuf. Technol. 2012, 67, 2743–2751. [Google Scholar] [CrossRef] [Green Version]
- Revilla, R.I.; Rojas, Y.; De Graeve, I. On the Impact of Si Content and Porosity Artifacts on the Anodizing Behavior of Additve Manufactured Al-Si Alloys. J. Electrochem. Soc. 2019, 166, C530–C537. [Google Scholar] [CrossRef]
- Gharbi, O.; Jiang, D.; Feenstra, D.; Kairy, S.; Wu, Y.; Hutchinson, C.; Birbilis, N. On the corrosion of additively manufactured aluminium alloy AA2024 prepared by selective laser melting. Corros. Sci. 2018, 143, 93–106. [Google Scholar] [CrossRef]
- Gharbi, O.; Kairy, S.K.; De Lima, P.R.; Jiang, D.; Nicklaus, J.; Birbilis, N. Microstructure and corrosion evolution of additively manufactured aluminium alloy AA7075 as a function of ageing. NPJ Mater. Degrad. 2019, 3, 1–11. [Google Scholar] [CrossRef]
- Khalil, A.; Loginova, I.; Pozdnyakov, A.V.; Mosleh, A.; Solonin, A.N. Evaluation of the Microstructure and Mechanical Properties of a New Modified Cast and Laser-Melted AA7075 Alloy. Materials 2019, 12, 3430. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Reference | Electrolyte | Ecorr | Icorr | Epit | Corrosion Rate |
|---|---|---|---|---|---|
| [44] | Aerated 3.5 wt.% NaCl | AM > Cast | Cast > AM | Cast > AM | |
| [51] | Diluted Harrison’s solution | Cast > AM | Cast ~ AM | AM > Cast | |
| [59] | Aerated 3.5 wt.% NaCl | AM > Cast | Cast > AM | Cast > AM | |
| [60] | Aerated 3.5 wt.% NaCl | AM > Cast | Cast > AM | Cast ~ AM | |
| [32] | Aerated 0.1 M NaCl | Cast ~ AM | Cast ~ AM | ||
| [61] | Aerated 3.5 wt. %NaCl | Cast ~ AM | Cast ~ AM | Cast ~ AM |
| Material | Al | Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti |
|---|---|---|---|---|---|---|---|---|---|
| AA2024 | Balance | <0.5 | <0.5 | 3.8–4.9 | 0.3–0.9 | 1.2–1.8 | <0.1 | <0.25 | <0.15 |
| AA7075 | Balance | <0.4 | <0.5 | 1.2–2 | <0.3 | 2.1–2.9 | 0.18–0.28 | 5.1–6.1 | <0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Revilla, R.I.; Verkens, D.; Rubben, T.; De Graeve, I. Corrosion and Corrosion Protection of Additively Manufactured Aluminium Alloys—A Critical Review. Materials 2020, 13, 4804. https://doi.org/10.3390/ma13214804
Revilla RI, Verkens D, Rubben T, De Graeve I. Corrosion and Corrosion Protection of Additively Manufactured Aluminium Alloys—A Critical Review. Materials. 2020; 13(21):4804. https://doi.org/10.3390/ma13214804
Chicago/Turabian StyleRevilla, Reynier I., Donovan Verkens, Tim Rubben, and Iris De Graeve. 2020. "Corrosion and Corrosion Protection of Additively Manufactured Aluminium Alloys—A Critical Review" Materials 13, no. 21: 4804. https://doi.org/10.3390/ma13214804





