Sustainable Ecocements: Chemical and Morphological Analysis of Granite Sawdust Waste as Pozzolan Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Waste/Lime System
3.2. Waste/Mortar System
3.3. Waste/Mortar System with Substitution
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Angulo, S.C.; Carrijo, P.M.; Figueiredo, A.D.; Chaves, A.P.; John, V.M. On the classification of mixed construction and demolition waste aggregate by porosity and its impact on the mechanical performance of concrete. Mater. Struct. 2009, 43, 519–528. [Google Scholar] [CrossRef]
- Miller, S.A.; John, V.M.; Pacca, S.A.; Horvath, A. Carbon dioxide reduction potential in the global cement industry by 2050. Cem. Concr. Res. 2018, 114, 115–124. [Google Scholar] [CrossRef]
- Schneider, M.; Romer, M.; Tschudin, M.; Bolio, H. Sustainable cement production—present and future. Cem. Concr. Res. 2011, 41, 642–650. [Google Scholar] [CrossRef]
- Zhang, T.; Gao, P.; Gao, P.; Wei, J.; Yu, Q. Effectiveness of novel and traditional methods to incorporate industrial wastes in cementitious materials—An overview. Resour. Conserv. Recycl. 2013, 74, 134–143. [Google Scholar] [CrossRef]
- Yagüe, S.; Sánchez, I.; De La Villa, R.V.; García-Giménez, R.; Zapardiel, A.; Frías, M. Coal-Mining Tailings as a Pozzolanic Material in Cements Industry. Minerals 2018, 8, 46. [Google Scholar] [CrossRef] [Green Version]
- Akcil, A.; Agcasulu, I.; Swain, B. Valorization of waste LCD and recovery of critical raw material for circular economy: A review. Resour. Conserv. Recycl. 2019, 149, 622–637. [Google Scholar] [CrossRef]
- Chen, J.; Kwan, A.; Jiang, Y. Adding limestone fines as cement paste replacement to reduce water permeability and sorptivity of concrete. Constr. Build. Mater. 2014, 56, 87–93. [Google Scholar] [CrossRef]
- Mosaferi, M.; Dianat, I.; Khatibi, M.S.; Mansour, S.N.; Fahiminia, M.; Hashemi, A.A. Review of environmental aspects and waste management of stone cutting and fabrication industries. J. Mater. Cycles Waste Manag. 2013, 16, 721–730. [Google Scholar] [CrossRef]
- Baeza, F.; Payá, J.; Galao, Ó.; Saval, J.; Garcés, P. Blending of industrial waste from different sources as partial substitution of Portland cement in pastes and mortars. Constr. Build. Mater. 2014, 66, 645–653. [Google Scholar] [CrossRef]
- Galetakis, M.; Soultana, A. A review on the utilisation of quarry and ornamental stone industry fine by-products in the construction sector. Constr. Build. Mater. 2016, 102, 769–781. [Google Scholar] [CrossRef]
- Singh, M.; Srivastava, A.; Bhunia, D. An investigation on effect of partial replacement of cement by waste marble slurry. Constr. Build. Mater. 2017, 134, 471–488. [Google Scholar] [CrossRef]
- Singh, S.; Nagar, R.; Agrawal, V. Performance of granite cutting waste concrete under adverse exposure conditions. J. Clean. Prod. 2016, 127, 172–182. [Google Scholar] [CrossRef]
- Choudhary, R.; Gupta, R.; Nagar, R. Impact on fresh, mechanical, and microstructural properties of high strength self-compacting concrete by marble cutting slurry waste, fly ash, and silica fume. Constr. Build. Mater. 2020, 239, 117888. [Google Scholar] [CrossRef]
- Piasta, W.; Góra, J.; Turkiewicz, T. Properties and durability of coarse igneous rock aggregates and concretes. Constr. Build. Mater. 2016, 126, 119–129. [Google Scholar] [CrossRef]
- Ramos, T.; Matos, A.M.; Schmidt, B.; Rio, J.; Sousa-Coutinho, J. Granitic quarry sludge waste in mortar: Effect on strength and durability. Constr. Build. Mater. 2013, 47, 1001–1009. [Google Scholar] [CrossRef]
- Lanas, J.; Sirera, R.; Alvarez, J.I. Compositional changes in lime-based mortars exposed to different environments. Thermochim. Acta 2005, 429, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Pacheco-Torgal, F.; Sedira, N. Influence of physical and geometrical properties of granite and limestone aggregates on the durability of a C20/25 strength class concrete. Constr. Build. Mater. 2006, 20, 1079–1088. [Google Scholar] [CrossRef]
- Zichella, L.; Bellopede, R.; Spriano, S.; Marini, P. Preliminary investigations on stone cutting sludge processing for a future recovery. J. Clean. Prod. 2018, 178, 866–876. [Google Scholar] [CrossRef]
- Ostrowski, K.; Stefaniuk, D.; Sadowski, Ł.; Krzywiński, K.; Gicala, M.; Różańska, M. Potential use of granite waste sourced from rock processing for the application as coarse aggregate in high-performance self-compacting concrete. Constr. Build. Mater. 2020, 238, 117794. [Google Scholar] [CrossRef]
- Gupta, L.K.; Vyas, A.K. Impact on mechanical properties of cement sand mortar containing waste granite powder. Constr. Build. Mater. 2018, 191, 155–164. [Google Scholar] [CrossRef]
- Mashaly, A.O.; Shalaby, B.N.; Rashwan, M. Performance of mortar and concrete incorporating granite sludge as cement replacement. Constr. Build. Mater. 2018, 169, 800–818. [Google Scholar] [CrossRef]
- Noor-E-Khuda, S.; Albermani, F.; Veidt, M. Flexural strength of weathered granites: Influence of freeze and thaw cycles. Constr. Build. Mater. 2017, 156, 891–901. [Google Scholar] [CrossRef]
- Jain, A.; Gupta, R.; Chaudhary, S. Performance of self-compacting concrete comprising granite cutting waste as fine aggregate. Constr. Build. Mater. 2019, 221, 539–552. [Google Scholar] [CrossRef]
- Abukersh, S.; Fairfield, C.A. Recycled aggregate concrete produced with red granite dust as a partial cement replacement. Constr. Build. Mater. 2011, 25, 4088–4094. [Google Scholar] [CrossRef]
- Barnes, P.; Bensted, J. Structure and Performance of Cements; Spoon Press: London, UK, 1983. [Google Scholar]
- García, S.Y.; González-Gaya, C. Reusing Discarded Ballast Waste in Ecological Cements. Materials 2019, 12, 3887. [Google Scholar] [CrossRef] [Green Version]
- García, S.Y.; González-Gaya, C. Durability analysis of pozzolanic cements containing recycled track ballast: Sustainability under extreme environmental conditions. Constr. Build. Mater. 2020, 242, 117999. [Google Scholar] [CrossRef]
- Asociación Española de Normalización y Certificación. UNE-EN 197-1 Cemento: Parte 1. Composición, Especificaciones y Criterios de Conformidad de los Cementos Comunes; AENOR: Madrid, Spain, 2000. [Google Scholar]
- Asociación Española de Normalización y Certificación. UNE-EN 196-1 Métodos de Ensayo de Cementos. Parte 1: Determinación de Resistencias; AENOR: Madrid, Spain, 2018. [Google Scholar]
- Young, R.A. International Workshop on the Rietveld Method (RW2000PL). Powder Diffr. 2001, 16, 48. [Google Scholar] [CrossRef]
- Asociación Española de Normalización y Certificación. UNE-EN 196-5 Métodos de Ensayo de Cementos. Parte 5: Ensayo de Puzolanicidad para los Cementos Puzolánicos; AENOR: Madrid, Spain, 2011. [Google Scholar]
- Taylor, H.F.W. Cement Chemistry; Academic Press: London, UK, 1997. [Google Scholar]
- Torres, P.; Fernandes, H.; Olhero, S.; Ferreira, J. Incorporation of wastes from granite rock cutting and polishing industries to produce roof tiles. J. Eur. Ceram. Soc. 2009, 29, 23–30. [Google Scholar] [CrossRef]
- Mármol, I.; Ballester, P.; Cerro, S.; Monrós, G.; Morales, J.; Sánchez, L.J. Use of granite sludge wastes for the production of coloured cement-based mortars. Cem. Concr. Compos. 2010, 32, 617–622. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Zheng, L.; Wen, J.; Wu, C.; Tan, Y. Compressive strength of fly ash magnesium oxychloride cement containing granite wastes. Constr. Build. Mater. 2013, 38, 1–7. [Google Scholar] [CrossRef]
- Kamaruddin, K.; Hamidah, M.S.; Norhana, A.; Hanani, A.R. Quarry dust fine powder as substitute for ordinary Portland cement in concrete mix. J. Eng. Sci. Technol. 2014, 9, 191–205. [Google Scholar]
- Medina, G.; Del Bosque, I.S.; Frías, M.; De Rojas, M.S.; Medina, C. Granite quarry waste as a future eco-efficient supplementary cementitious material (SCM): Scientific and technical considerations. J. Clean. Prod. 2017, 148, 467–476. [Google Scholar] [CrossRef]
- Souri, A.; Kazemi-Kamyab, H.; Snellings, R.; NaghiZadeh, R.; Golestani-Fard, F.; Scrivener, K.L. Pozzolanic activity of mechanochemically and thermally activated kaolins in cement. Cem. Concr. Res. 2015, 77, 47–59. [Google Scholar] [CrossRef]
- García, I.S.D.S.; De La Villa, R.V.; Frías, M.; Rodríguez, O.; Martinez-Ramirez, S.; Fernández-Carrasco, L.; De Soto, I.; Villar-Cociña, E. Mineralogical study of calcined coal waste in a pozzolan/Ca(OH)2 system. Appl. Clay Sci. 2015, 108, 45–54. [Google Scholar] [CrossRef]
- Gameiro, A.S.; Silva, A.S.; Veiga, R.; Velosa, A. Hydration products of lime–metakaolin pastes at ambient temperature with ageing. Thermochim. Acta 2012, 535, 36–41. [Google Scholar] [CrossRef]
- Frías, M.; Vigil, R.; Garcia, R.; Rodríguez, O.; Goñi, S.; Vegas, I.J. Evolution of mineralogical phases produced during the pozzolanic reaction of different metakaolinite by-products: Influence of the activation process. Appl. Clay Sci. 2012, 56, 48–52. [Google Scholar] [CrossRef]
- Bullard, J.W.; Jennings, H.M.; Livingston, R.A.; Nonat, A.; Scherer, G.W.; Schweitzer, J.S.; Scrivener, K.L.; Thomas, J.J. Mechanisms of cement hydration. Cem. Concr. Res. 2011, 41, 1208–1223. [Google Scholar] [CrossRef]
- Wu, H.-C. Re-examination of cement hydration: Sol–gel process. Adv. Cem. Res. 2014, 26, 292–301. [Google Scholar] [CrossRef]
- Gartner, E.; Maruyama, I.; Chen, J. A new model for the C-S-H phase formed during the hydration of Portland cements. Cem. Concr. Res. 2017, 97, 95–106. [Google Scholar] [CrossRef]
- Rodríguez, O. Valorización de un Residuo Industrial Procedente de la Industria Papelera como Material Puzolánico. Ph.D. Thesis, Universidad Autónoma de Madrid, Madrid, Spain, 2008. [Google Scholar]
- Richardson, I. The nature of the hydration products in hardened cement pastes. Cem. Concr. Compos. 2000, 22, 97–113. [Google Scholar] [CrossRef]
- Richardson, I. The calcium silicate hydrates. Cem. Concr. Res. 2008, 38, 137–158. [Google Scholar] [CrossRef]
- Fonseca, P.; Jennings, H. The effect of drying on early-age morphology of C–S–H as observed in environmental SEM. Cem. Concr. Res. 2010, 40, 1673–1680. [Google Scholar] [CrossRef]
- Qinhua, J.; Min, D.; Sufen, H. Investigation of deteriorated concrete railway ties. Cem. Concr. Res. 1996, 26, 999–1006. [Google Scholar] [CrossRef]
- Ferdous, W.; Manalo, A.C. Failures of mainline railway sleepers and suggested remedies–Review of current practice. Eng. Fail. Anal. 2014, 44, 17–35. [Google Scholar] [CrossRef]


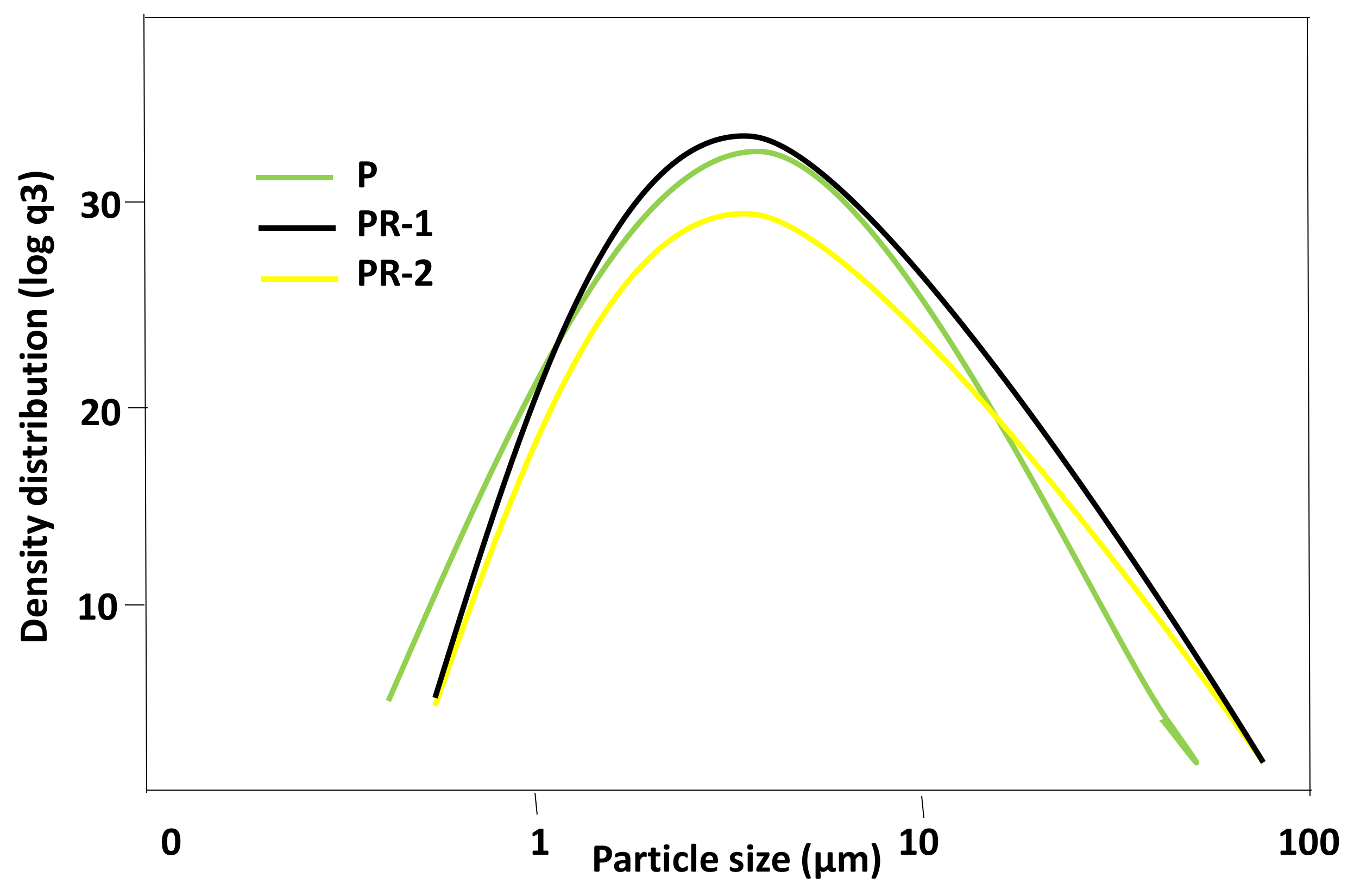
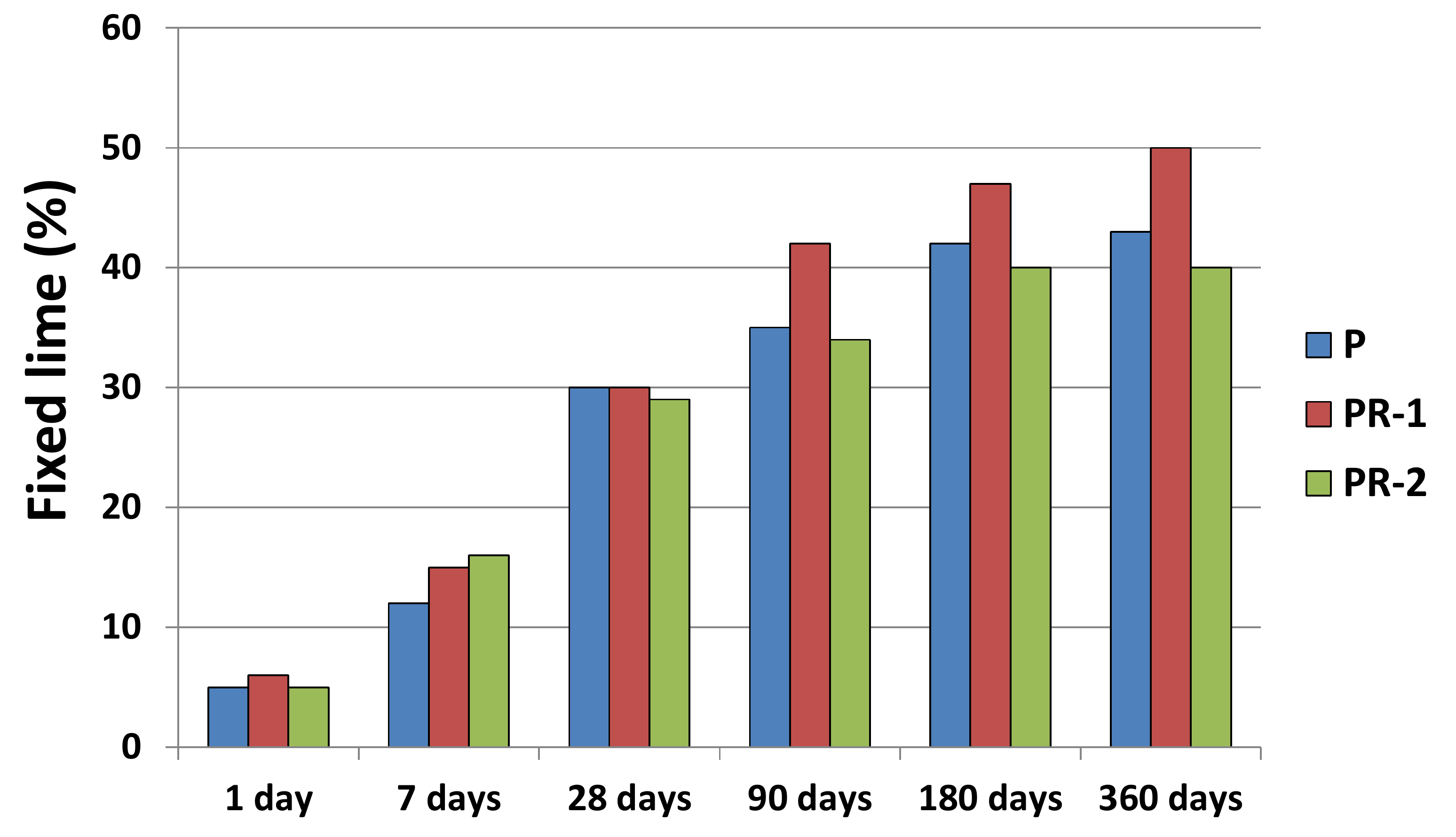
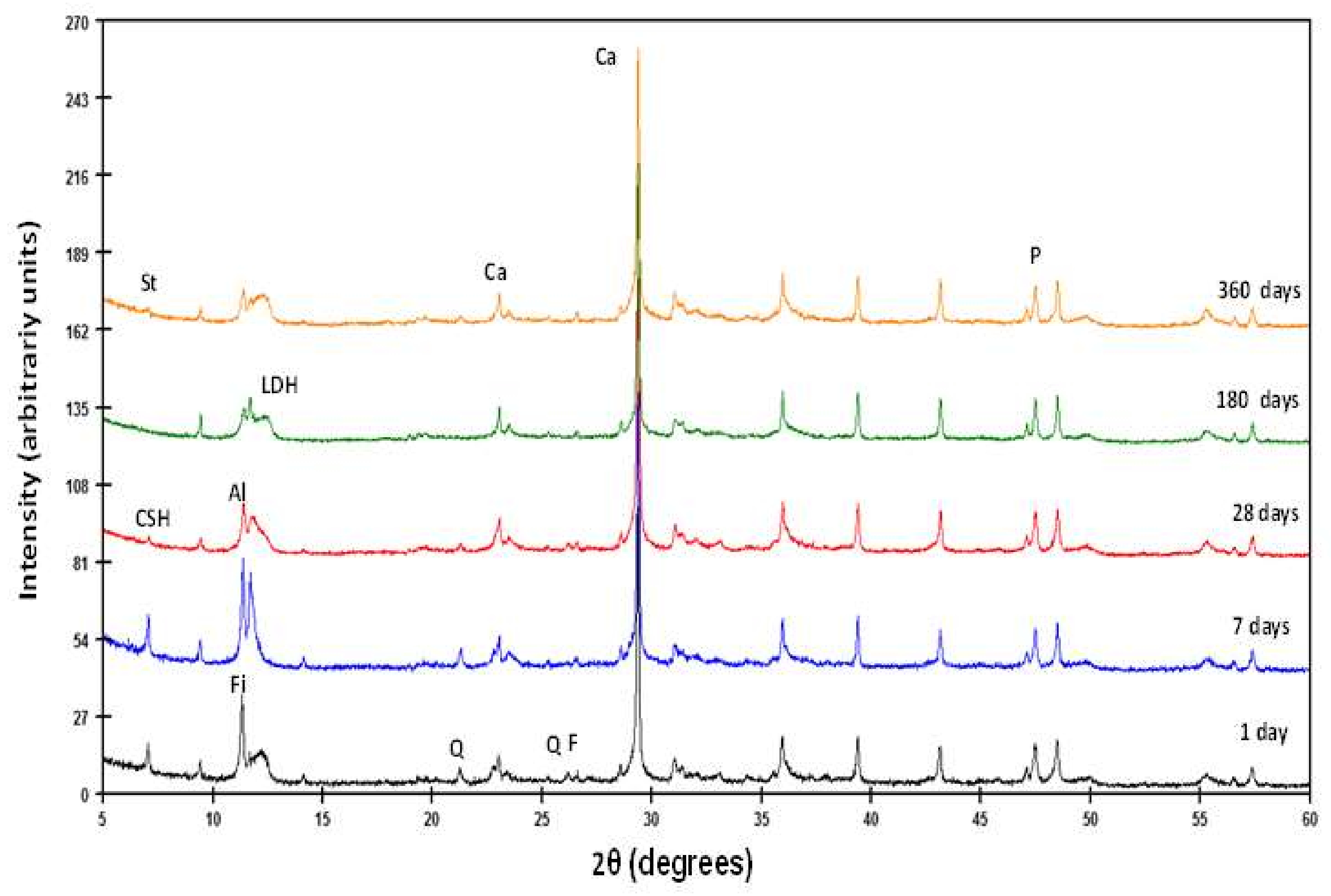


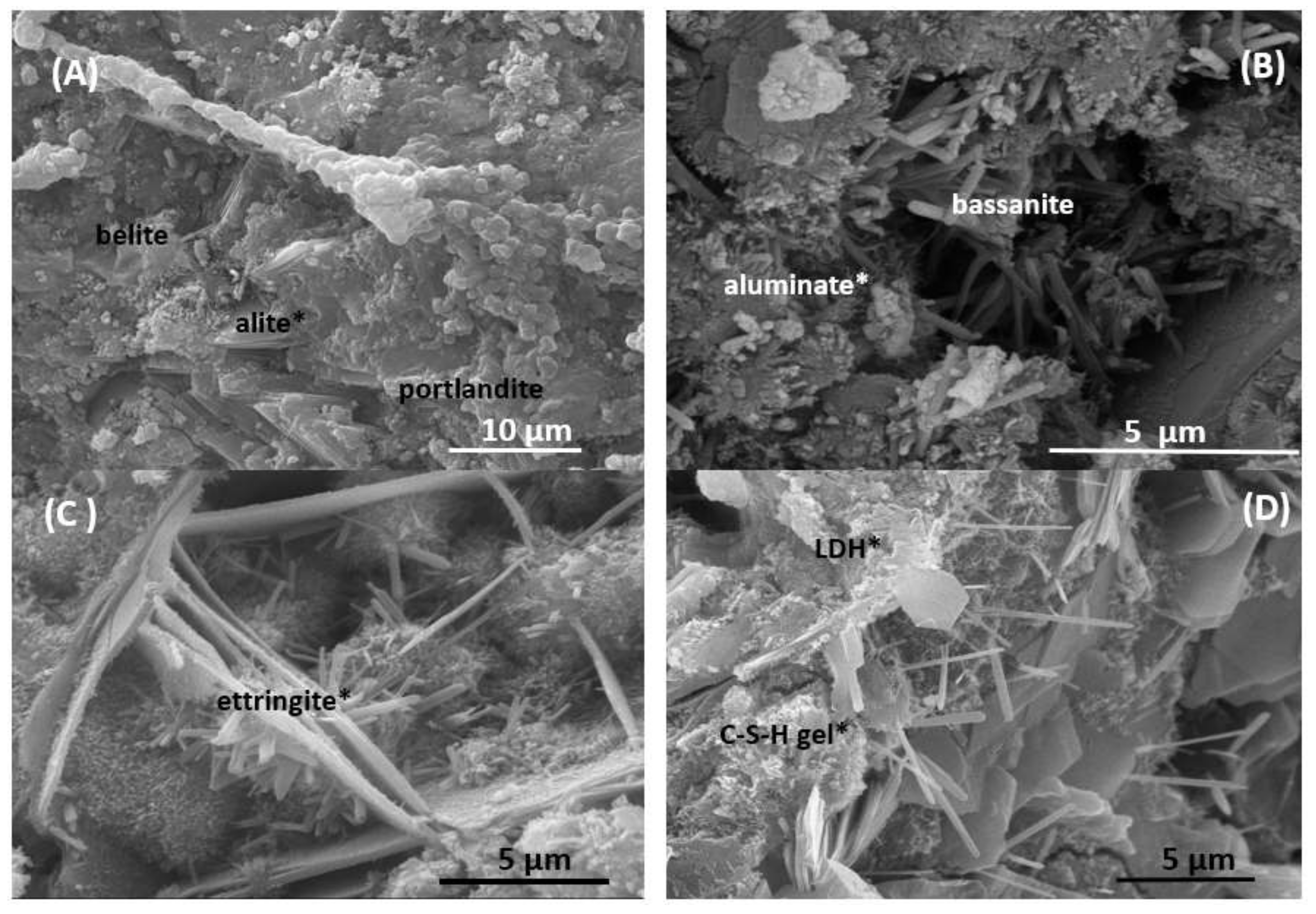


| Oxides (%) | CPO | P | PR-1 | PR-2 |
|---|---|---|---|---|
| SiO2 | 19.20 | 68.56 | 78.89 | 82.08 |
| Al2O3 | 5.62 | 18.81 | 13.03 | 13.64 |
| Fe2O3 | 3.08 | 1.95 | 2.09 | 0.86 |
| CaO | 62.44 | 1.65 | 1.47 | 0.52 |
| MgO | 1.21 | 0.39 | 0.19 | 0.06 |
| SO3 | 3.29 | n.d. | n.d. | n.d. |
| K2O | 0.89 | 4.14 | 1.10 | 1.05 |
| Na2O | 0.27 | 4.30 | 1.36 | 1.73 |
| MnO | n.d. | 0.03 | 0.03 | 0.03 |
| TiO2 | 0.24 | 0.20 | 0.25 | 0.06 |
| P2O5 | 0.11 | n.d. | n.d. | n.d. |
| LOI | 2.72 | 1.12 | 0.47 | 0.42 |
| Sample | B (%) | C (%) | Q (%) | A (%) | O (%) | Amorphous Material (%) | H (%) | RB | Χ2 |
|---|---|---|---|---|---|---|---|---|---|
| P | 23 | 3 | 31 | 29 | 6 | 4 | 4 | 19.4 | 3.7 |
| PR-1 | 20 | 3 | 30 | 14 | 10 | 10 | 3 | 17.3 | 3.5 |
| PR-2 | 18 | n.d. | 32 | 34 | 7 | 7 | 2 | 14.3 | 4.9 |
| Reaction Time (days) | Q (%) | F (%) | Fi (%) | LDH (%) | S (%) | C3AH13 (%) | P (%) | Ca (%) | MA (%) | X2 | RB |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 25 | 18 | 15 | 4 | n.d. | traces | 27 | 6 | 4 | 11.2 | 4.3 |
| 7 | 26 | 15 | 12 | 4 | n.d. | traces | 24 | 8 | 10 | 13.6 | 5.8 |
| 28 | 30 | 14 | 10 | 4 | n.d. | 4 | 16 | 10 | 12 | 12.6 | 4.2 |
| 90 | 29 | 12 | 8 | 4 | traces | 6 | 13 | 11 | 15 | 13.4 | 4.2 |
| 180 | 30 | 9 | 4 | 7 | traces | 10 | 12 | 10 | 16 | 15.8 | 3.9 |
| 360 | 30 | 8 | 4 | 10 | traces | 11 | 9 | 6 | 18 | 14.3 | 4.8 |
| Oxides (%) | CSH Gel * | LDH * | Stratlingite * | Portlandite * | Aluminate * |
|---|---|---|---|---|---|
| Al2O3 | 18.20 ± 3.28 | 26.04 ± 0.92 | 18.23 ± 0.89 | - | 16.86 ± 0.39 |
| SiO2 | 31.07 ± 0.06 | 40.39 ± 0.67 | 16.76 ± 0.79 | - | 4.12 ± 0.18 |
| CaO | 50.32 ± 0.37 | 31.15 ± 0.89 | 64.50 ± 2.41 | 100 | 76.79 ± 1.34 |
| CaO/Al2O3 | 2.79 | 1.20 | 3.54 | - | 4.04 |
| CaO/SiO2 | 1.63 | 0.77 | 3.85 | - | 18.63 |
| SiO2/Al2O3 | 1.61 | 1.55 | 0.92 | - | 0.21 |
| Reaction Time (Day) | MA (%) | Ca (%) | A (%) | B (%) | Ba (%) | FF (%) | A3 (%) | E (%) | CSH (%) | A4 (%) | P (%) | LDH (%) | X2 | RB |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | T | 38 | 28 | T | 8 | n.d. | n.d. | n.d. | 18 | n.d. | n.d. | 17.2 | 4.6 |
| 1 | 17 | T | 14 | 30 | T | 8 | T | T | T | T | 23 | n.d. | 15.2 | 5.2 |
| 7 | 13 | T | 8 | 31 | T | 4 | T | 6 | T | T | 34 | T | 16.3 | 4.8 |
| 28 | 7 | 4 | 4 | 28 | n.d. | T | T | T | T | T | 37 | 13 | 17.5 | 3.9 |
| 90 | 9 | 4 | T | 25 | n.d. | 3 | T | n.d. | T | T | 38 | 18 | 13.1 | 5.2 |
| 180 | 10 | 4 | T | 18 | n.d. | 2 | T | n.d. | T | T | 36 | 24 | 13.2 | 5.9 |
| 360 | 9 | 4 | T | 14 | n.d. | 2 | T | n.d. | T | T | 46 | 33 | 14.8 | 3.9 |
| Oxides (%) | C-S-H Gel * | LDH * | Alite * | Aluminate * | Ettringite * |
|---|---|---|---|---|---|
| Al2O3 | 8.70 ± 0.40 | 13.09 ± 0.80 | 4.30 ± 0.40 | 21.00 ± 1.00 | 39.00 ± 1.00 |
| SiO2 | 20.90 ± 0.80 | 26.92 ± 0.61 | 30.00 ± 1.00 | 2.03 ± 0.09 | 24.00 ± 2.00 |
| CaO | 59.00 ± 2.03 | 54.00 ± 1.04 | 61.21 ± 0.92 | 60.12 ± 2.06 | 33.17 ± 2.08 |
| MgO | 1.60 ± 0.40 | n.d. | 1.60 ± 0.10 | n.d. | n.d. |
| SO3 | n.d. | n.d. | n.d. | 3.91 ± 0.45 | 2.22 ± 0.32 |
| Fe2O3 | 9.52 ± 0.21 | 2.11 ± 0.45 | 2.65 ± 0.62 | 0.92 ± 0.11 | 1.61 ± 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yagüe, S.; González Gaya, C.; Rosales Prieto, V.; Sánchez Lite, A. Sustainable Ecocements: Chemical and Morphological Analysis of Granite Sawdust Waste as Pozzolan Material. Materials 2020, 13, 4941. https://doi.org/10.3390/ma13214941
Yagüe S, González Gaya C, Rosales Prieto V, Sánchez Lite A. Sustainable Ecocements: Chemical and Morphological Analysis of Granite Sawdust Waste as Pozzolan Material. Materials. 2020; 13(21):4941. https://doi.org/10.3390/ma13214941
Chicago/Turabian StyleYagüe, Santiago, Cristina González Gaya, Victor Rosales Prieto, and Alberto Sánchez Lite. 2020. "Sustainable Ecocements: Chemical and Morphological Analysis of Granite Sawdust Waste as Pozzolan Material" Materials 13, no. 21: 4941. https://doi.org/10.3390/ma13214941






