Green Synthesis of Co-Zn Spinel Ferrite Nanoparticles: Magnetic and Intrinsic Antimicrobial Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanoparticles Synthesis
2.2. Structural and Magnetic Properties
2.3. Antimicrobial Activity
3. Results and Discussion
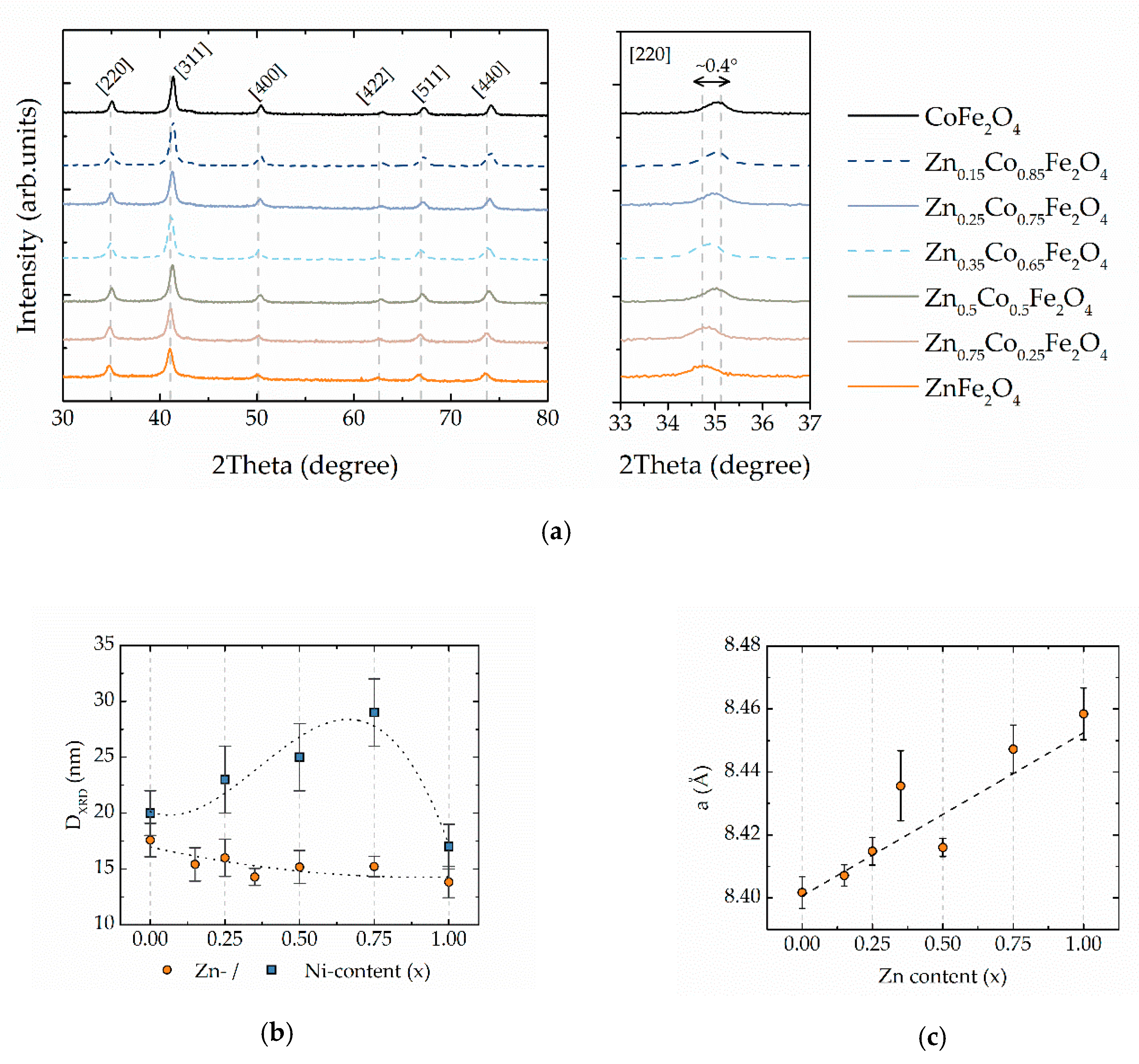
3.1. Structural and Morphological Properties
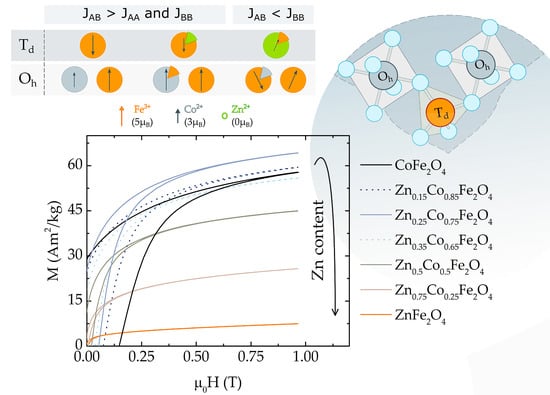
3.2. Chemical Control. of Magnetic Properties
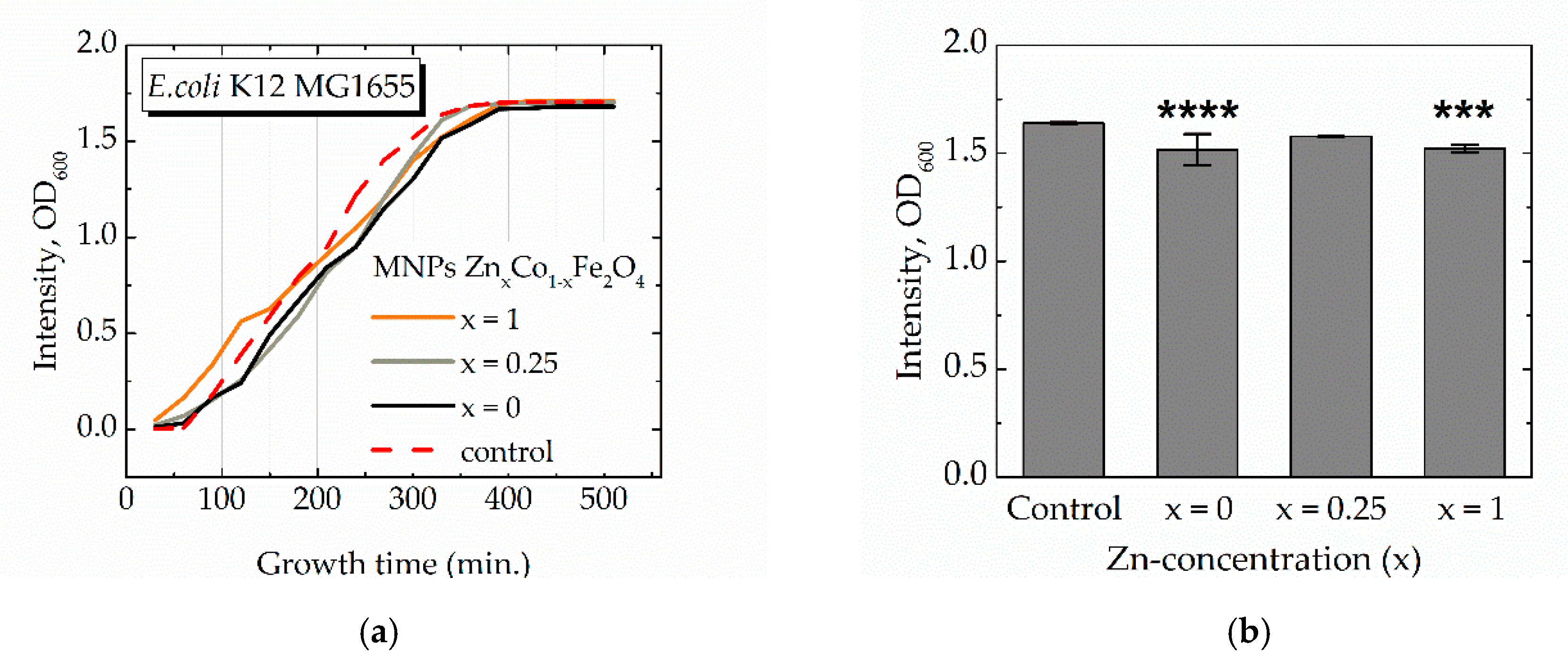
3.3. Biology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Da Silva, F.G.; Depeyrot, J.; Campos, A.F.C.; Aquino, R.; Fiorani, D.; Peddis, D. Structural and Magnetic Properties of Spinel Ferrite Nanoparticles. J. Nanosci. Nanotechnol. 2019, 19, 4888–4902. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Huh, Y.-M.; Jun, Y.; Seo, J.; Jang, J.; Song, H.-T.; Kim, S.; Cho, E.-J.; Yoon, H.-G.; Suh, J.-S.; et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat. Med. 2007, 13, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Socoliuc, V.; Peddis, D.; Petrenko, V.I.; Avdeev, M.V.; Susan-resiga, D.; Szabó, T.; Turcu, R.; Tombácz, E.; Vékás, L. Magnetic Nanoparticle Systems for Nanomedicine—A Materials Science Perspective. Magetochemistry 2019, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.; Nah, H.; Lee, J.-H.; Moon, S.H.; Kim, M.G.; Cheon, J. Critical Enhancements of MRI Contrast and Hyperthermic Effects by Dopant-Controlled Magnetic Nanoparticles. Angew. Chemie Int. Ed. 2009, 48, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Albino, M.; Fantechi, E.; Innocenti, C.; López-Ortega, A.; Bonanni, V.; Campo, G.; Pineider, F.; Gurioli, M.; Arosio, P.; Orlando, T.; et al. Role of Zn 2+ Substitution on the Magnetic, Hyperthermic, and Relaxometric Properties of Cobalt Ferrite Nanoparticles. J. Phys. Chem. C 2019, 123, 6148–6157. [Google Scholar] [CrossRef] [Green Version]
- Kozenkova, E.; Levada, K.; Efremova, M.V.; Omelyanchik, A.; Nalench, Y.A.; Garanina, A.S.; Pshenichnikov, S.; Zhukov, D.G.; Lunov, O.; Lunova, M.; et al. Multifunctional Fe3O4-Au Nanoparticles for the MRI Diagnosis and Potential Treatment of Liver Cancer. Nanomaterials 2020, 10, 1646. [Google Scholar] [CrossRef] [PubMed]
- Salvador, M.; Moyano, A.; Martínez-García, J.C.; Blanco-López, M.C.; Rivas, M. Synthesis of Superparamagnetic Iron Oxide Nanoparticles: SWOT Analysis Towards Their Conjugation to Biomolecules for Molecular Recognition Applications. J. Nanosci. Nanotechnol. 2019, 19, 4839–4856. [Google Scholar] [CrossRef] [PubMed]
- Levada, K.; Omelyanchik, A.; Rodionova, V.; Weiskirchen, R.; Bartneck, M. Magnetic-Assisted Treatment of Liver Fibrosis. Cells 2019, 8, 1279. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Cheng, C.A.; Zink, J.I. Spatial, Temporal, and Dose Control of Drug Delivery using Noninvasive Magnetic Stimulation. ACS Nano 2019, 13, 1292–1308. [Google Scholar] [CrossRef]
- Mulens, V.; Morales, M.; del, P.; Barber, D.F. Development of Magnetic Nanoparticles for Cancer Gene Therapy: A Comprehensive Review. ISRN Nanomater. 2013, 2013, 1–14. [Google Scholar] [CrossRef]
- Franco, O.; Lopez-Abarrategui, C.; Figueroa-Espi, V.; Lugo-Alvarez, M.; Pereira, C.; Garay, H.; Barbosa, J.; Jimenez-Hernandez, L.; Estevez-Hernandez, O.; Reguera-Ruiz, E.; et al. The intrinsic antimicrobial activity of citric acid-coated manganese ferrite nanoparticles is enhanced after conjugation with the antifungal peptide Cm-p5. Int. J. Nanomed. 2016, 11, 3849–3857. [Google Scholar] [CrossRef] [Green Version]
- Sanpo, N.; Wen, C.; Berndt, C.C.; Wang, J. Antibacterial properties of spinel ferrite nanoparticles. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2013; pp. 239–250. [Google Scholar]
- Hola, K.; Markova, Z.; Zoppellaro, G.; Tucek, J.; Zboril, R. Tailored functionalization of iron oxide nanoparticles for MRI, drug delivery, magnetic separation and immobilization of biosubstances. Biotechnol. Adv. 2015, 33, 1162–1176. [Google Scholar] [CrossRef]
- Jovanović, S.; Kumrić, K.; Bajuk-Bogdanović, D.; Jaňcar, B.; Spreitzer, M.; Trtićc-Petrović, T.; Suvorov, D. Cobalt Ferrite Nanospheres as a Potential Magnetic Adsorbent for Chromium(VI) Ions. J. Nanosci. Nanotechnol. 2019, 19, 5027–5034. [Google Scholar] [CrossRef]
- Campos, A.F.C.; de Oliveira, H.A.L.; da Silva, F.N.; da Silva, F.G.; Coppola, P.; Aquino, R.; Mezzi, A.; Depeyrot, J. Core-Shell Bimagnetic Nanoadsorbents for Hexavalent Chromium Removal from Aqueous Solutions. J. Hazard. Mater. 2019, 362, 82–91. [Google Scholar] [CrossRef]
- Baragaño, D.; Forján, R.; Welte, L.; Gallego, J.L.R. Nanoremediation of As and metals polluted soils by means of graphene oxide nanoparticles. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Moustafa, A.M.; Salah, L.M.; Salerno, M.; Abdellatif, M.H. Symmetry in magnetic and vibrational spectra of multi-element spinel ferrite. J. Magn. Magn. Mater. 2020, 513, 167267. [Google Scholar] [CrossRef]
- Abdellatif, M.H.; El-Komy, G.; Azab, A.A.; Moustafa, A.M.; Salerno, M. Crystal field deformation by Ce3+ doping in spinel Mn-Cr ferrite. J. Magn. Magn. Mater. 2020, 502, 166517. [Google Scholar] [CrossRef]
- Abdellatif, M.H.; El-Komy, G.M.; Azab, A.A.; Salerno, M. Crystal field distortion of La3+ ion-doped Mn-Cr ferrite. J. Magn. Magn. Mater. 2018, 447, 15–20. [Google Scholar] [CrossRef]
- Abdellatif, M.H.; Azab, A.A.; Salerno, M. Effect of rare earth doping on the vibrational spectra of spinel Mn-Cr ferrite. Mater. Res. Bull. 2018, 97, 260–264. [Google Scholar] [CrossRef]
- Cullity, B.D.; Graham, C.D. Introduction to magnetic materials. Mater. Today 2009, 12, 45. [Google Scholar]
- Liu, X.; Liu, J.; Zhang, S.; Nan, Z.; Shi, Q. Structural, Magnetic, and Thermodynamic Evolutions of Zn-Doped Fe3O4 Nanoparticles Synthesized Using a One-Step Solvothermal Method. J. Phys. Chem. C 2016, 120, 1328–1341. [Google Scholar] [CrossRef]
- Petrova, E.; Kotsikau, D.; Pankov, V. Structural characterization and magnetic properties of sol-gel derived ZnxFe3-xO4 nanoparticles. J. Magn. Magn. Mater. 2015, 378, 429–435. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Li, L.Y.; Wang, W.H.; Liu, H.; Ren, S.W.; Cui, X.Y.; Zheng, R.K. Tunable electrical and magnetic properties of half-metallic ZnxFe3−xO4 from first principles. Phys. Chem. Chem. Phys. 2011, 13, 21243. [Google Scholar] [CrossRef]
- Mameli, V.; Musinu, A.; Ardu, A.; Ennas, G.; Peddis, D.; Niznansky, D.; Sangregorio, C.; Innocenti, C.; Thanh, N.T.K.; Cannas, C. Studying the effect of Zn-substitution on the magnetic and hyperthermic properties of cobalt ferrite nanoparticles. Nanoscale 2016, 8, 10124–10137. [Google Scholar] [CrossRef] [Green Version]
- Pilati, V.; Cabreira Gomes, R.; Gomide, G.; Coppola, P.; Silva, F.G.; Paula, F.L.O.; Perzynski, R.; Goya, G.F.; Aquino, R.; Depeyrot, J. Core/Shell Nanoparticles of Non-Stoichiometric Zn-Mn and Zn-Co Ferrites as Thermosensitive Heat Sources for Magnetic Fluid Hyperthermia. J. Phys. Chem. C 2018, 122, 3028–3038. [Google Scholar] [CrossRef] [Green Version]
- Barrera, G.; Coisson, M.; Celegato, F.; Raghuvanshi, S.; Mazaleyrat, F.; Kane, S.N.N.; Tiberto, P. Cation distribution effect on static and dynamic magnetic properties of Co1-xZnxFe2O4 ferrite powders. J. Magn. Magn. Mater. 2018, 456, 372–380. [Google Scholar] [CrossRef]
- Tovstolytkin, A.I.; Kulyk, M.M.; Kalita, V.M.; Ryabchenko, S.M.; Zamorskyi, V.O.; Fedorchuk, O.P.; Solopan, S.O.; Belous, A.G. Nickel-zinc spinel nanoferrites: Magnetic characterization and prospects of the use in self-controlled magnetic hyperthermia. J. Magn. Magn. Mater. 2019, 473, 422–427. [Google Scholar] [CrossRef]
- Viñas, S.L.; Simeonidis, K.; Li, Z.-A.; Ma, Z.; Myrovali, E.; Makridis, A.; Sakellari, D.; Angelakeris, M.; Wiedwald, U.; Spasova, M.; et al. Tuning the magnetism of ferrite nanoparticles. J. Magn. Magn. Mater. 2016, 415, 20–23. [Google Scholar] [CrossRef]
- López-Ortega, A.; Lottini, E.; Fernández, C.D.J.; Sangregorio, C. Exploring the Magnetic Properties of Cobalt-Ferrite Nanoparticles for the Development of a Rare-Earth-Free Permanent Magnet. Chem. Mater. 2015, 27, 4048–4056. [Google Scholar] [CrossRef]
- Jovanović, S.; Spreitzer, M.; Otoničar, M.; Jeon, J.-H.; Suvorov, D. pH control of magnetic properties in precipitation-hydrothermal-derived CoFe2O4. J. Alloy. Compd. 2014, 589, 271–277. [Google Scholar] [CrossRef]
- Tahar, L.B.; Basti, H.; Herbst, F.; Smiri, L.S.; Quisefit, J.P.; Yaacoub, N.; Grenèche, J.M.; Ammar, S. Co 1-xZn xFe 2O 4 (0 ≤ x ≤ 1) nanocrystalline solid solution prepared by the polyol method: Characterization and magnetic properties. Mater. Res. Bull. 2012, 47, 2590–2598. [Google Scholar] [CrossRef]
- Hanini, A.; Massoudi, M.E.; Gavard, J.; Kacem, K.; Ammar, S.; Souilem, O. Nanotoxicological study of polyol-made cobalt-zinc ferrite nanoparticles in rabbit. Environ. Toxicol. Pharmacol. 2016, 45, 321–327. [Google Scholar] [CrossRef]
- Gómez-Polo, C.; Recarte, V.; Cervera, L.; Beato-López, J.J.; López-García, J.; Rodríguez-Velamazán, J.A.; Ugarte, M.D.; Mendonça, E.C.; Duque, J.G.S. Tailoring the structural and magnetic properties of Co-Zn nanosized ferrites for hyperthermia applications. J. Magn. Magn. Mater. 2018, 465, 211–219. [Google Scholar] [CrossRef]
- Ben-Arfa, B.A.E.; Miranda Salvado, I.M.; Ferreira, J.M.F.; Pullar, R.C. Clove and cinnamon: Novel anti–oxidant fuels for preparing magnetic iron oxide particles by the sol–gel auto–ignition method. J. Alloy. Compd. 2019, 786, 71–76. [Google Scholar] [CrossRef]
- Makarova, L.A.; Alekhina, Y.A.; Omelyanchik, A.S.; Peddis, D.; Spiridonov, V.V.; Rodionova, V.V.; Perov, N.S. Magnetorheological foams for multiferroic applications. J. Magn. Magn. Mater. 2019, 485, 413–418. [Google Scholar] [CrossRef]
- Omelyanchik, A.; Singh, G.; Volochaev, M.; Sokolov, A.; Rodionova, V.; Peddis, D. Tunable magnetic properties of Ni-doped CoFe2O4 nanoparticles prepared by the sol–gel citrate self-combustion method. J. Magn. Magn. Mater. 2019, 476, 387–391. [Google Scholar] [CrossRef]
- Jacobs, J.P.; Maltha, A.; Reintjes, J.G.H.; Drimal, J.; Ponec, V.; Brongersma, H.H. The surface of catalytically active spinels. J. Catal. 1994, 147, 294–300. [Google Scholar] [CrossRef]
- Vozniuk, O.; Tabanelli, T.; Tanchoux, N.; Millet, J.M.M.; Albonetti, S.; Di Renzo, F.; Cavani, F. Mixed-oxide catalysts with spinel structure for the valorization of biomass: The chemical-loop reforming of bioethanol. Catalysts 2018, 8, 332. [Google Scholar] [CrossRef] [Green Version]
- Antipov, S.; Turishchev, S.; Purtov, Y.; Shvyreva, U.; Sinelnikov, A.; Semov, Y.; Preobrazhenskaya, E.; Berezhnoy, A.; Shusharina, N.; Novolokina, N.; et al. The oligomeric form of the Escherichia coli dps protein depends on the availability of iron ions. Molecules 2017, 22, 1904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turishchev, S.Y.; Antipov, S.S.; Novolokina, N.V.; Chuvenkova, O.A.; Melekhov, V.V.; Ovsyannikov, R.; Senkovskii, B.V.; Timchenko, A.A.; Ozoline, O.N.; Domashevskaya, E.P. A soft X-ray synchrotron study of the charge state of iron ions in the ferrihydrite core of the ferritin Dps protein in Escherichia coli. Biophys. Russ. Fed. 2016, 61, 705–710. [Google Scholar] [CrossRef]
- Cannas, C.; Falqui, A.; Musinu, A.; Peddis, D.; Piccaluga, G. CoFe2O4 nanocrystalline powders prepared by citrate-gel methods: Synthesis, structure and magnetic properties. J. Nanoparticle Res. 2006, 8, 255–267. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the ‘ Debye—Scherrer equation. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Hu, Y.; Li, X.; Li, J.; Wang, L.; Li, H. Tuning of magnetic properties and hyperfine interaction by the substitution of Ni2+ for cobalt ferrite nanoparticles. J. Mater. Sci. Mater. Electron. 2019, 30, 19647–19653. [Google Scholar] [CrossRef]
- Smit, J.; Wijn, H.P.J. Ferrites; Philips’ Technical Library: Eindhoven, The Netherlands, 1959; p. 384. [Google Scholar]
- Vegard, L. Die Konstitution der Mischkristalle und die Raumfüllung der Atome. Z. Phys. 1921, 5, 17–26. [Google Scholar] [CrossRef]
- Muscas, G.; Jovanović, S.; Vukomanović, M.; Spreitzer, M.; Peddis, D. Zn-doped cobalt ferrite: Tuning the interactions by chemical composition. J. Alloy. Compd. 2019, 796, 203–209. [Google Scholar] [CrossRef]
- Morrish, A.H. The Physical Principles of Magnetism; Wiley-IEEE Press: Hoboken, NJ, USA, 1965; Volume 1, ISBN 0-7803-6029-X. [Google Scholar]
- Cobos, M.A.; de la Presa, P.; Llorente, I.; Alonso, J.M.; García-Escorial, A.; Marín, P.; Hernando, A.; Jiménez, J.A. Magnetic Phase Diagram of Nanostructured Zinc Ferrite as a Function of Inversion Degree δ. J. Phys. Chem. C 2019, 123, 17472–17482. [Google Scholar] [CrossRef]
- Concas, G.; Spano, G.; Cannas, C.; Musinu, A.; Peddis, D.; Piccaluga, G. Inversion degree and saturation magnetization of different nanocrystalline cobalt ferrites. J. Magn. Magn. Mater. 2009, 321, 1893–1897. [Google Scholar] [CrossRef]
- Andersen, H.L.; Granados-Miralles, C.; Saura-Múzquiz, M.; Stingaciu, M.; Larsen, J.; Søndergaard-Pedersen, F.; Ahlburg, J.V.; Keller, L.; Frandsen, C.; Christensen, M. Enhanced intrinsic saturation magnetization of Zn x Co 1-x Fe 2 O 4 nanocrystallites with metastable spinel inversion. Mater. Chem. Front. 2019, 3, 668–679. [Google Scholar] [CrossRef]
- Yaseneva, P.; Bowker, M.; Hutchings, G. Structural and magnetic properties of Zn-substituted cobalt ferrites prepared by co-precipitation method. Phys. Chem. Chem. Phys. 2011, 13, 18609–18614. [Google Scholar] [CrossRef]
- Apostolov, A.; Apostolova, I.; Wesselinowa, J. Specific absorption rate in Zn-doted ferrites for self-controlled magnetic hyperthermia. Eur. Phys. J. B 2019, 92, 58. [Google Scholar] [CrossRef]
- Chaudhary, V.; Wang, Z.; Ray, A.; Sridhar, I.; Ramanujan, R.V. Self pumping magnetic cooling. J. Phys. D. Appl. Phys. 2017, 50, 03LT03. [Google Scholar] [CrossRef]
- Skokov, K.P.; Gutfleisch, O. Viewpoint on the letter ‘Self pumping magnetic cooling’ by V Chaudhary et al (2017 J. Phys. D: Appl. Phys. 50 03LT03). J. Phys. D. Appl. Phys. 2017, 50, 131001. [Google Scholar] [CrossRef]
- Mørup, S.; Hansen, M.F.; Frandsen, C. 1.04 Magnetic Nanoparticles. In Comprehensive Nanoscience and Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 89–140. ISBN 9780128035818. [Google Scholar]
- Kodama, R.H.; Berkowitz, A.E.; McNiff, E.J.; Foner, S. Surface spin disorder in NiFe2O4 nanoparticles. Phys. Rev. Lett. 1996, 77, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Muscas, G.; Yaacoub, N.; Concas, G.; Sayed, F.; Sayed Hassan, R.; Greneche, J.M.; Cannas, C.; Musinu, A.; Foglietti, V.; Casciardi, S.; et al. Evolution of the magnetic structure with chemical composition in spinel iron oxide nanoparticles. Nanoscale 2015, 7, 13576–13585. [Google Scholar] [CrossRef]
- Peddis, D.; Cannas, C.; Musinu, A.; Ardu, A.; Orru, F.; Fiorani, D.; Laureti, S.; Rinaldi, D.; Muscas, G.; Concas, G.; et al. Beyond the Effect of Particle Size: Influence of CoFe2O4 Nanoparticle Arrangements on Magnetic Properties. Chem. Mater. 2013, 25, 2005–2013. [Google Scholar] [CrossRef]
- Sharifi, I.; Shokrollahi, H.; Amiri, S. Ferrite-based magnetic nanofluids used in hyperthermia applications. J. Magn. Magn. Mater. 2012, 324, 903–915. [Google Scholar] [CrossRef]
- Frtús, A.; Smolková, B.; Uzhytchak, M.; Lunova, M.; Jirsa, M.; Kubinová, Š.; Dejneka, A.; Lunov, O. Analyzing the mechanisms of iron oxide nanoparticles interactions with cells: A road from failure to success in clinical applications. J. Control. Release 2020, 328, 59–77. [Google Scholar] [CrossRef]
- Rodrigues, G.R.; López-Abarrategui, C.; de la Serna Gómez, I.; Dias, S.C.; Otero-González, A.J.; Franco, O.L. Antimicrobial magnetic nanoparticles based-therapies for controlling infectious diseases. Int. J. Pharm. 2019, 555, 356–367. [Google Scholar] [CrossRef]
- Sutton, S. Measurement of microbial cells by optical density. J. Valid. Technol. 2011, 17, 46–49. [Google Scholar]
- Stevenson, K.; McVey, A.F.; Clark, I.B.N.; Swain, P.S.; Pilizota, T. General calibration of microbial growth in microplate readers. Sci. Rep. 2016, 6, 38828. [Google Scholar] [CrossRef] [Green Version]
- Mamonova, I.A.; Babushkina, I.V.; Norkin, I.A.; Gladkova, E.V.; Matasov, M.D.; Puchin’yan, D.M. Biological activity of metal nanoparticles and their oxides and their effect on bacterial cells. Nanotechnol. Russ. 2015, 10, 128–134. [Google Scholar] [CrossRef]
- Makumire, S.; Revaprasadu, N.; Shonhai, A. DnaK Protein Alleviates Toxicity Induced by Citrate-Coated Gold Nanoparticles in Escherichia coli. PLoS ONE 2015, 10, e0121243. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
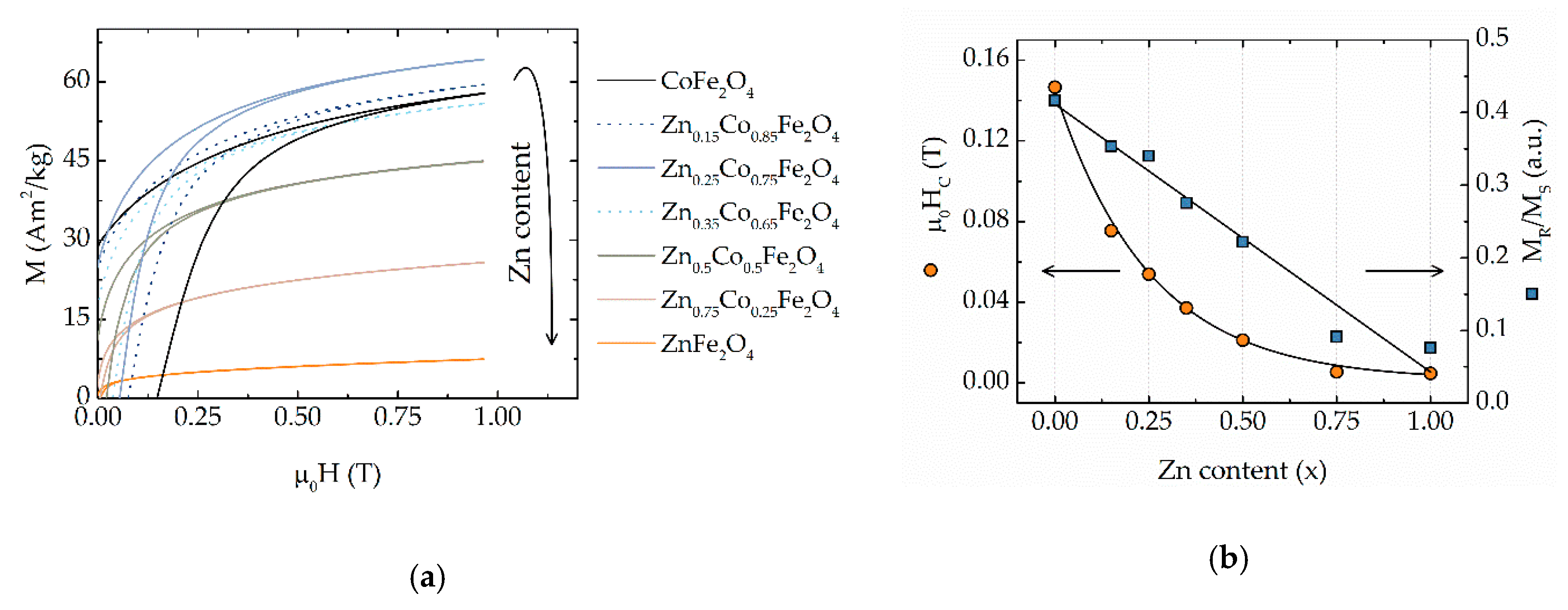

| Sample Composition | DXRD, nm | MS,** Am2/kg | MR/MS, a.u. | μ0HC, mT | Keff, ×104 J/m3 |
|---|---|---|---|---|---|
| CoFe2O4 | 18(2) * | 69(2) | 0.42(2) | 140(4) | 14(2) |
| Zn0.15Co0.85Fe2O4 | 15(2) | 71(2) | 0.35(1) | 76(3) | 12(2) |
| Zn0.25Co0.75Fe2O4 | 16(2) | 74(2) | 0.34(1) | 54(2) | 9.5(4) |
| Zn0.35Co0.65Fe2O4 | 14(1) | 65(2) | 0.27(1) | 37(2) | 7.5(3) |
| Zn0.50Co0.50Fe2O4 | 15(2) | 52(2) | 0.22(1) | 21(1) | 3.3(1) |
| Zn0.75Co0.25Fe2O4 | 15(1) | 32(1) | 0.09(1) | 5.5(2) | 0.72(4) |
| ZnFe2O4 | 14(1) | 10(1) | 0.08(1) | 4.8(2) | 0.064(4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omelyanchik, A.; Levada, K.; Pshenichnikov, S.; Abdolrahim, M.; Baricic, M.; Kapitunova, A.; Galieva, A.; Sukhikh, S.; Astakhova, L.; Antipov, S.; et al. Green Synthesis of Co-Zn Spinel Ferrite Nanoparticles: Magnetic and Intrinsic Antimicrobial Properties. Materials 2020, 13, 5014. https://doi.org/10.3390/ma13215014
Omelyanchik A, Levada K, Pshenichnikov S, Abdolrahim M, Baricic M, Kapitunova A, Galieva A, Sukhikh S, Astakhova L, Antipov S, et al. Green Synthesis of Co-Zn Spinel Ferrite Nanoparticles: Magnetic and Intrinsic Antimicrobial Properties. Materials. 2020; 13(21):5014. https://doi.org/10.3390/ma13215014
Chicago/Turabian StyleOmelyanchik, Alexander, Kateryna Levada, Stanislav Pshenichnikov, Maryam Abdolrahim, Miran Baricic, Anastasiya Kapitunova, Alima Galieva, Stanislav Sukhikh, Lidiia Astakhova, Sergey Antipov, and et al. 2020. "Green Synthesis of Co-Zn Spinel Ferrite Nanoparticles: Magnetic and Intrinsic Antimicrobial Properties" Materials 13, no. 21: 5014. https://doi.org/10.3390/ma13215014