Surface Properties of Halloysite-Carbon Nanocomposites and Their Application for Adsorption of Paracetamol
Abstract
:1. Introduction
2. Materials and Reagents
2.1. Preparation of Halloysite-Carbon Nanocomposites
2.2. Halloysite-Carbon Composites Characterization
2.3. Adsorption Measurements
3. Results
3.1. Characterization of Halloysite-Carbon Nanocomposites
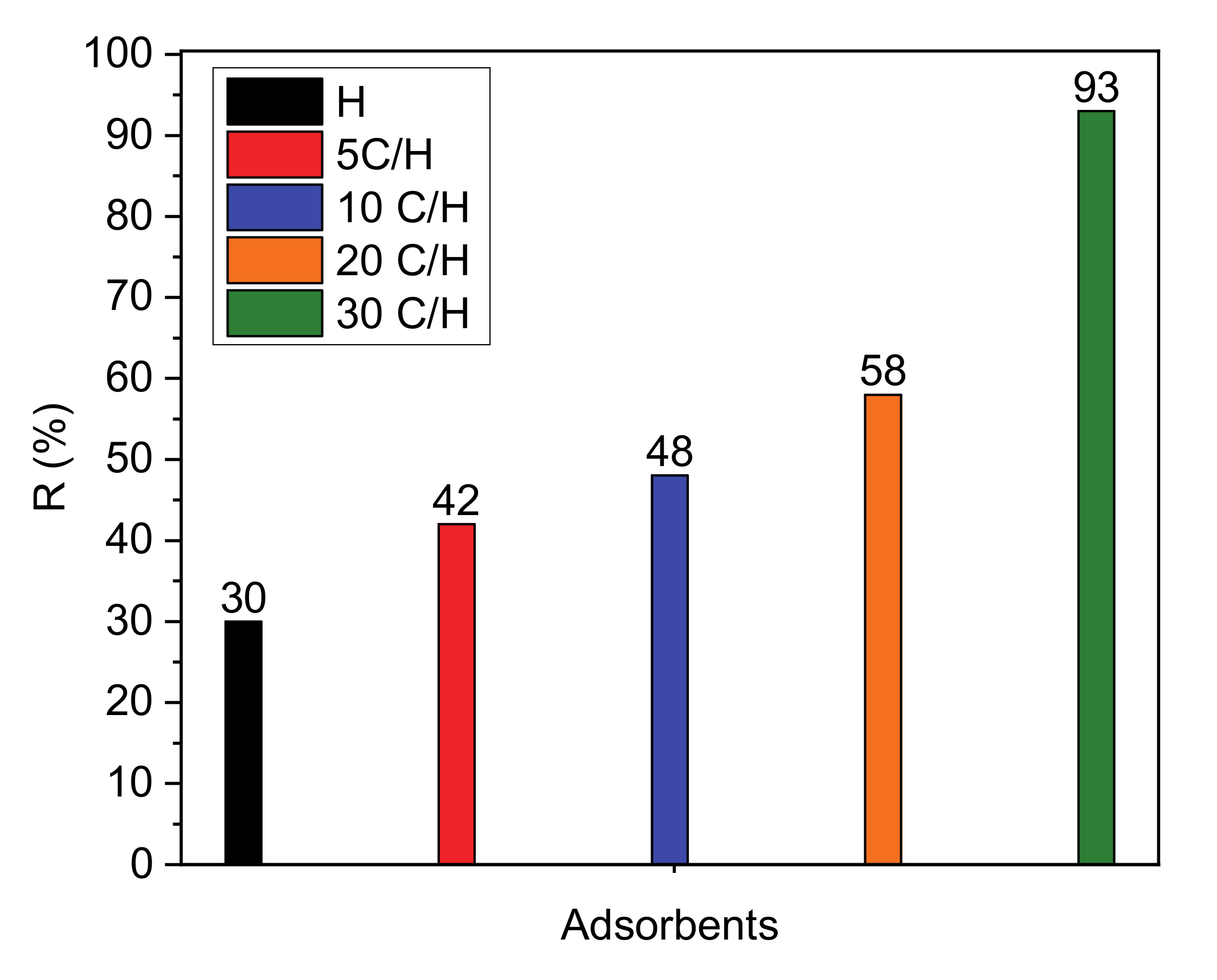
3.2. Adsorption of Paracetamol
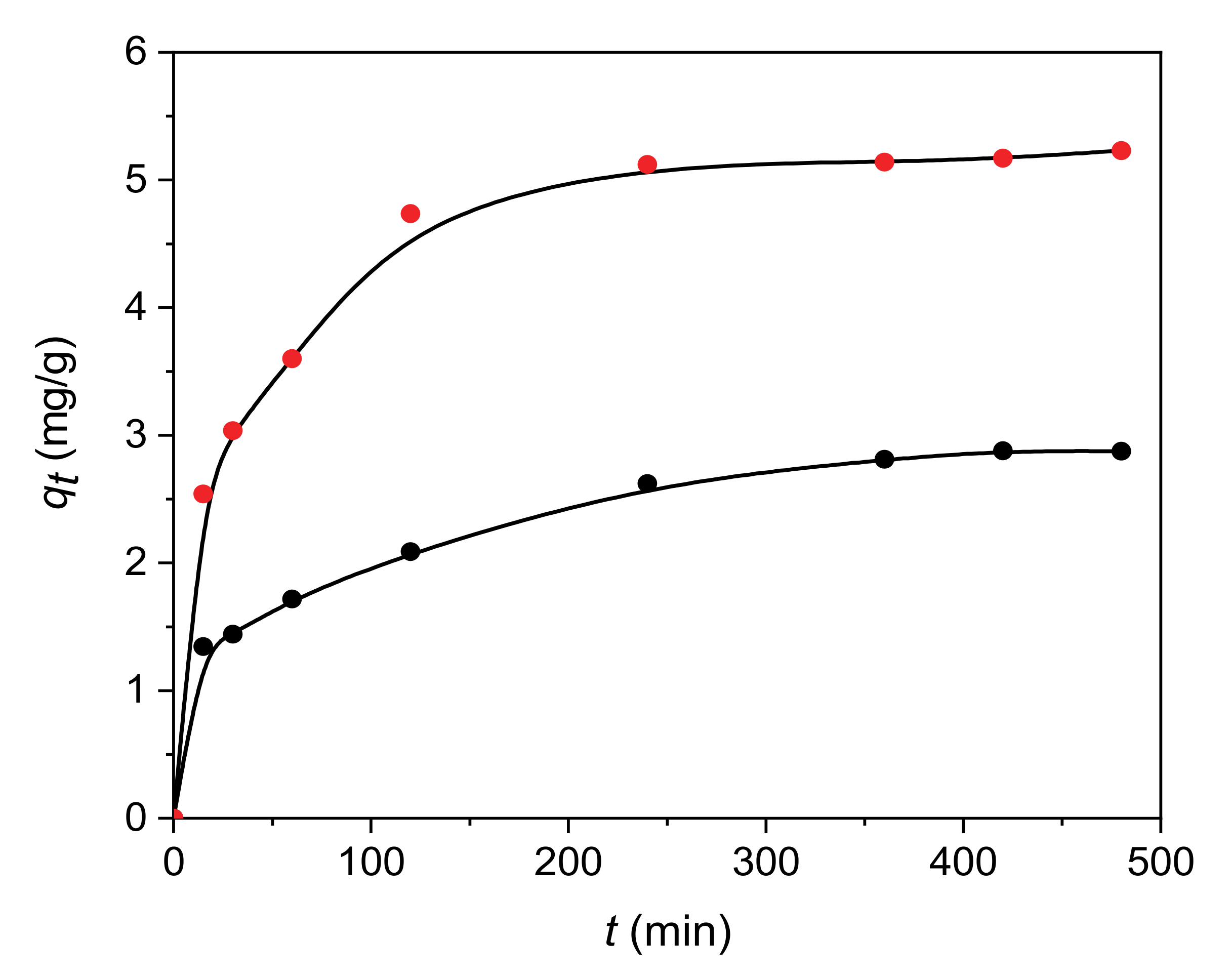
3.2.1. Kinetic Models
3.2.2. Adsorption Isotherms
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Joussein, E.; Petit, S.; Churchman, J.; Theng, B.; Righi, D.; Delvaux, B. Halloysite clay minerals—A review. Clay Miner. 2005, 40, 383–426. [Google Scholar] [CrossRef]
- Pasbakhsh, P.; Churchman, G.J.; Keeling, J.L. Characterisation of properties of various halloysites relevant to their use as nanotubes and microfibre fillers. Appl. Clay Sci. 2013, 74, 47–57. [Google Scholar] [CrossRef]
- Yuan, P.; Tan, D.; Annabi-Bergaya, F. Properties and applications of halloysite nanotubes: Recent research advances and future prospects. Appl. Clay Sci. 2015, 112–113, 75–93. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Mittal, A.; Usman, M.; Mittal, J.; Yu, G.; Núñez-Delgado, A.; Kornaros, M. A review on halloysite-based adsorbents to remove pollutants in water and wastewater. J. Mol. Liq. 2018, 269, 855–868. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Ania, C.O. Surface Chemistry of Activated Carbons and Its Characterization, Activated Carbons Surfaces in Environmental Remediation; Bandosz, T.J., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Moral-Rodríguez, A.I.; Leyva-Ramos, R.; Ania, C.O.; Ocampo-Pérez, R.; Isaacs-Páez, E.D.; Carrales-Alvarado, D.H.; Parra, J.B. Tailoring the textural properties of an activated carbon for enhancing its adsorption capacity towards diclofenac from aqueous solution. Environ. Sci. Pollut. Res. 2019, 26, 6141–6152. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, V.R.; Vairagade, V.S.; Kedar, A.P. Activated Carbon as Adsorbent In Advance Treatment of Wastewater. IOSR J. Mech. Civil Eng. 2017, 14, 36–40. [Google Scholar] [CrossRef]
- Tang, J.; Mu, B.; Zong, L.; Zheng, M.; Wang, A. Facile and green fabrication of magnetically recyclable carboxyl-functionalized attapulgite/carbon nanocomposites derived from spent bleaching earth for wastewater treatment. Chem. Eng. J. 2017, 322, 102–114. [Google Scholar] [CrossRef]
- Anadao, P.; Pajolli, I.L.R.; Hildebrando, E.A.; Wiebeck, H. Preparation and characterization of carbon/montmorillonite composites and nanocomposites from waste bleaching sodium montmorillonite clay. Adv. Powder Technol. 2014, 25, 926–932. [Google Scholar] [CrossRef]
- Bakandritsos, A.; Kouvelos, E.; Steriotis, T.; Petridis, D. Aqueous and Gaseous Adsorption from Montmorillonite-Carbon Composites and from Derived Carbons. Langmuir 2005, 21, 2349–2355. [Google Scholar] [CrossRef]
- Chen, L.-F.; Liang, H.-W.; Lu, Y.; Cui, C.-H.; Yu, S.-H. Synthesis of an Attapulgite Clay@Carbon Nanocomposite Adsorbent by a Hydrothermal Carbonization Process and Their Application in the Removal of Toxic Metal Ions from Water. Langmuir 2011, 27, 8998–9004. [Google Scholar] [CrossRef]
- Liu, W.; Yang, T.; Xu, J.; Chen, Q.; Yao, C.; Zuo, S.; Kong, Y.; Fu, C. Preparation and Adsorption Property of Attapulgite/Carbon Nanocomposite. Environ. Prog. Sustain. Energy 2015, 34, 437–444. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, W.; Zhang, X.; Chen, T.; Frost, R.L. Catalytic deposition of nanocarbon onto palygorskite and its adsorption of phenol. Appl. Clay Sci. 2011, 52, 400–406. [Google Scholar] [CrossRef]
- Wu, X.; Gao, P.; Zhang, X.; Jin, G.; Xu, Y.; Wu, Y. Synthesis of clay/carbon adsorbent through hydrothermal carbonization of cellulose on palygorskite. Appl. Clay Sci. 2014, 95, 60–66. [Google Scholar] [CrossRef]
- Wu, X.; Xu, Y.; Zhang, X.; Wu, Y.; Gao, P. Adsorption of low-concentration methylene blue onto a palygorskite/carbon composite. New Carbon Mater. 2015, 30, 71–78. [Google Scholar] [CrossRef]
- Wu, X.; Liu, C.; Qi, H.; Zhang, X.; Dai, J.; Zhang, Q.; Zhang, L.; Wu, Y.; Peng, X. Synthesis and adsorption properties of halloysite/carbon nanocomposites and halloysite-derived carbon nanotubes. Appl. Clay Sci. 2016, 119, 284–293. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, C.; Wei, J.; Tjiu, W.; Pan, J.; Chen, Y.; Liu, T. Surface Modifications of Halloysite Nanotubes with Superparamagnetic Fe3O4 Nanoparticles and Carbonaceous Layers for Efficient Adsorption of Dyes in Water Treatment. Chem. Res. Chin. Univ. 2014, 30, 971–977. [Google Scholar] [CrossRef]
- Szczepanik, B.; Rędzia, N.; Frydel, L.; Słomkiewicz, P.; Kołbus, A.; Styszko, K.; Dziok, T.; Samojeden, B. Synthesis and Characterization of Halloysite/Carbon Nanocomposites for Enhanced NSAIDs Adsorption from Water. Materials 2019, 12, 3754. [Google Scholar] [CrossRef] [Green Version]
- Stöcker, M. X-ray photoelectron spectroscopy on zeolites and related materials. Micropor. Mater. 1996, 6, 235–257. [Google Scholar] [CrossRef]
- Sánchez, R.T.; Basaldella, E.; Marco, J. The effect of thermal and mechanical treatments on kaolinite: Characterization by XPS and IEP measurements. J. Colloid Interface Sci. 1999, 215, 339–344. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, L.; Chen, J. Paracetamol in the environment and its degradation by microorganisms. Appl. Microbiol. Biotechnol. 2012, 96, 875–884. [Google Scholar] [CrossRef]
- Szczepanik, B.; Słomkiewicz, P.; Garnuszek, M.; Czech, K.; Banaś, D.; Kubala-Kukuś, A.; Stabrawa, I. The effect of chemical modification on the physico-chemical characteristics of halloysite: FTIR, XRF, and XRD studies. J. Mol. Struct. 2015, 1084, 16–22. [Google Scholar] [CrossRef]
- Kubala-Kukuś, A.; Szczepanik, B.; Stabrawa, I.; Banaś, D.; Szary, K.; Pajek, M.; Rogala, P.; Wójtowicz, K.; Słomkiewicz, P. X-ray photoelectron spectroscopy analysis of chemically modified halloysite. Radiat. Phys. Chem. 2020, 175, 108149. [Google Scholar] [CrossRef]
- Słomkiewicz, P.M.; Szczepanik, B.; Garnuszek, M. Determination of adsorption isotherms of aniline and 4-chloroaniline on halloysite adsorbent by inverse liquid chromatography. Appl. Clay Sci. 2015, 114, 221–228. [Google Scholar] [CrossRef]
- Lech, A. Computer Software KSPD; Metroster: Toruń, Poland, 2011. [Google Scholar]
- Soma, M.; Churchman, G.J.; Theng, B.K.G. X-ray photoelectron spectroscopic analysis of halloysites with different composition and particle morphology. Clay Miner. 1992, 27, 413–421. [Google Scholar] [CrossRef]
- Kang, H.; Liu, X.; Zhang, S.; Li, J. Functionalization of halloysite nanotubes (HNTs) via mussel-inspired surface modification and silane grafting for HNTs/soy protein isolate nanocomposite film preparation. RSC Adv. 2017, 7, 24140–24148. [Google Scholar] [CrossRef] [Green Version]
- Ratner, B.D.; Castner, D.G. Electron spectroscopy for chemical analysis. In Surface Analysis: The Principal Techniques; Vickerman, J.C., Ed.; Wiley: Chichester, UK, 2009; p. 56. [Google Scholar]
- Schultz, J.; Lavielle, L.; Martin, C. The role of the interface in carbon fibre-epoxy composites. J. Adhes. 1987, 23, 45–60. [Google Scholar] [CrossRef]
- Schultz, J.; Lavielle, L. Interfacial properties of carbon fiber-epoxy matrix composites. Inverse gas chromatography characterisation of polymers and other materials. ACS Symp. Ser. 1989, 391, 185–202. [Google Scholar]
- Gutmann, V. The Donor-Acceptor Approach to Molecular Interactions; Plenum Press: New York, NY, USA, 1978. [Google Scholar]
- Riddle, F.L.; Fowkes, F.M. Spectral Shifts in Acid-Base Chemistry. 1. Van der Waals Contributions to Acceptor Numbers. J. Am. Chem. Soc. 1990, 112, 3259–3264. [Google Scholar] [CrossRef]
- Fowkes, F.M. Molecular Forces at Interfaces in Surface and Coating Related to Paper and Wood; Marchessault, R.H., Skaar, C., Eds.; Syracuse University Press: Syracuse, NY, USA, 1967; Volume 5, pp. 99–125. [Google Scholar]
- The Data Base of Cirrus Plus Software; Surface Measurement Systems, Ltd.: Alperton, UK, 2013.
- Rubio, J.; Rubio, F.; Fierro, J.L.; Gutierrez, M.C.; Oteo, J.L. Surface characterization of carbon fibers by inverse gas chromatography at low pressures. J. Mater. Res. 2002, 17, 413–422. [Google Scholar] [CrossRef]
- Lagregren, S. About the theory of so-called adsorption of soluble substances. Kungl. Sven. Veten. Akad. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second-order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon solution. J. Sanit. Eng. Div. Am. Soc. Civ. Eng. 1963, 89, 31–59. [Google Scholar]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H.M.F. Über die adsorption in lősungen. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Bernal, V.; Erto, A.; Giraldo, L.; Moreno-Piraján, J.C. Effect of solution pH on the adsorption of paracetamol on chemically modified activated carbons. Molecules 2017, 22, 1032. [Google Scholar] [CrossRef]
- Terzyk, A.P. The influence of activated carbon surface chemical composition on the adsorption of acetaminophen (paracetamol) in vitro: Part II. TG, FTIR, and XPS analysis of carbons and the temperature dependence of adsorption kinetics at the neutral pH. Colloids Surf. 2001, 177, 23–45. [Google Scholar] [CrossRef]





| Element Sample | O (at%) | C (at%) | Si (at%) | Al (at%) | Fe (at%) |
|---|---|---|---|---|---|
| H | 62.8 | 7.34 | 16.3 | 13.0 | 0.56 |
| 5C/H | 59.5 | 9.92 | 16.4 | 13.8 | 0.47 |
| 10C/H | 55.2 | 12.4 | 17.4 | 14.4 | 0.63 |
| 20C/H | 54.7 | 15.9 | 15.3 | 13.0 | 1.15 |
| 30C/H | 51.1 | 20.4 | 16.5 | 11.4 | 0.65 |
| Component | FWHM (eV) | Position (eV) | Area | Area Uncertainty |
|---|---|---|---|---|
| H sample | ||||
| O-C=O | 1.95 | 290.45 | 552.77 | 78.79 |
| C=O | 2.00 | 288.42 | 851.59 | 114.34 |
| C-O-C (C-O-H) | 2.00 | 286.83 | 1092.47 | 142.30 |
| C-C | 2.00 | 284.93 | 4499.66 | 517.05 |
| ~282.6 eV peak | 2.20 | 282.64 | 915.42 | 121.80 |
| 5C/H sample | ||||
| O-C=O | 2.00 | 289.51 | 407.51 | 60.94 |
| C=O | 2.01 | 287.97 | 1126.27 | 146.19 |
| C-O-C (C-O-H) | 2.01 | 286.17 | 3324.27 | 390.08 |
| C-C | 2.08 | 284.63 | 4453.26 | 512.06 |
| ~282.6 eV peak | 2.12 | 282.63 | 2366.74 | 285.32 |
| 10C/H sample | ||||
| O-C=O | 2.11 | 290.32 | 592.12 | 83.55 |
| C=O | 2.07 | 288.03 | 1503.63 | 189.14 |
| C-O-C (C-O-H) | 2.10 | 285.79 | 4809.26 | 550.27 |
| C-C | 2.07 | 284.53 | 8427.77 | 934.58 |
| ~282.6 eV peak | 2.01 | 282.41 | 3968.63 | 459.86 |
| 20C/H sample | ||||
| O-C=O | 2.11 | 290.61 | 795.15 | 107.71 |
| C=O | 2.10 | 288.39 | 1379.57 | 175.10 |
| C-O-C (C-O-H) | 2.07 | 286.39 | 2483.15 | 298.15 |
| C-C | 2.18 | 285.08 | 13,555.25 | 1471.95 |
| ~282.6 eV peak | 2.05 | 282.72 | 4332.91 | 499.12 |
| 30C/H sample | ||||
| O-C=O | 2.10 | 290.93 | 607.53 | 85.40 |
| C=O | 2.05 | 288.67 | 1429.30 | 180.74 |
| C-O-C (C-O-H) | 2.12 | 286.40 | 2690.53 | 320.92 |
| C-C | 2.17 | 284.82 | 14,335.89 | 1553.32 |
| ~282.6 eV peak | 2.17 | 282.81 | 7829.35 | 871.42 |
| Solvent Name | Cross-Sectional Area (m2) | Dispersive Energy of Probe Molecule (mJ/m−2) | AN* (kcal/mol) | DN (kcal/mol) |
|---|---|---|---|---|
| Hexane | 5.15 × 10−19 | 0.0184 | - | - |
| Heptane | 5.73 × 10−19 | 0.0203 | - | - |
| Octane | 6.3 × 10−19 | 0.0213 | - | - |
| Nonane | 6.9 × 10−19 | 0.0227 | - | - |
| Acetone | 3.4 × 10−19 | 0.0165 | 2.5 | 17 |
| Acetonitrile | 2.14 × 10−19 | 0.0275 | 4.7 | 14.1 |
| Dichloromethane | 2.45 × 10−19 | 0.0245 | 3.9 | 0 |
| Ethyl Acetate | 3.3 × 10−19 | 0.0196 | 1.5 | 17.1 |
| Methanol | 2.41 × 10−19 | 0.0181 | 12 | 20 |
| Adsorbent | Specific Component of Free Energy Adsorption (kcal/mol) | Ka | Kb | Kb/Ka | ||||
|---|---|---|---|---|---|---|---|---|
| Acetone | Acetonitrile | Dichloro-Methane | Ethyl Acetate | Methanol | ||||
| H | 3.9 | 2.7 | 1.1 | 2.9 | 4.8 | 0.16 | 0.22 | 1.38 |
| 5C/H | 4.2 | 3.5 | 0.6 | 3.9 | 7.5 | 0.21 | 0.18 | 0.86 |
| 10C/H | 2.6 | 1.9 | 0.8 | 3.2 | 6.5 | 0.16 | 0.11 | 0.68 |
| 20C/H | 1.9 | 1.0 | 0.8 | 2.5 | 5.7 | 0.13 | 0.09 | 0.69 |
| 30C/H | 1.7 | 0.9 | 0.7 | 2.2 | 4.3 | 0.11 | 0.06 | 0.54 |
| Adsorbent | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||
|---|---|---|---|---|
| k1 (min−1) | R2 | k2 (g mg−1 min−1) | R2 | |
| H | 0.0136 | 0.8792 | 0.0552 | 0.9879 |
| 30C/H | 0.0121 | 0.8665 | 0.0721 | 0.9992 |
| Adsorbent | kd1 (mg g−1 min−1/2) | c1 (mg g−1) | R12 | kd2 (mg g−1 min−1/2) | c2 (mg g−1) | R22 |
|---|---|---|---|---|---|---|
| H | 0.3263 | 0.91 | 0.8762 | 0.0341 | 1.77 | 0.9532 |
| 30C/H | 0.5012 | 2.01 | 0.8456 | 0.0632 | 4.07 | 0.9651 |
| Isotherm | Parameter | Adsorbent | |||||
|---|---|---|---|---|---|---|---|
| H | 30C/H | ||||||
| Temperature (K) | |||||||
| 298 | 303 | 313 | 298 | 303 | 313 | ||
| Freundlich | KF (mg∙g−1) (dm−3∙mg−1)1/n | 0.0048 | 0.0021 | 0.0006 | 0.8061 | 0.3730 | 0.2773 |
| n | 1.72 | 1.87 | 2.12 | 0.57 | 0.66 | 0.69 | |
| R2 | 0.8233 | 0.9245 | 0.9414 | 0.8945 | 0.8956 | 0.8922 | |
| Langmuir one-center | KL (dm3∙mg−1) | 0.0673 | 0.0488 | 0.0352 | 0.0889 | 0.0684 | 0.0543 |
| qm (mg∙g−1) | 7.8 | 6.0 | 5.7 | 8.7 | 6.8 | 6.1 | |
| R2 | 0.9513 | 0.9321 | 0.9461 | 0.8734 | 0.8922 | 0.9432 | |
| Langmuir multi-center | KML (dm3∙mg−1)1/n | 0.0021 | 0.0018 | 0.0007 | 0.0487 | 0.0357 | 0.0291 |
| qm (mg∙g−1) | 12.1 | 9.8 | 4.2 | 30.7 | 14.7 | 8.1 | |
| n | 0.50 | 0.56 | 0.55 | 1.6 | 1.28 | 1.20 | |
| R2 | 0.9907 | 0.9984 | 0.9991 | 0.9962 | 0.9985 | 0.9994 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczepanik, B.; Banaś, D.; Kubala-Kukuś, A.; Szary, K.; Słomkiewicz, P.; Rędzia, N.; Frydel, L. Surface Properties of Halloysite-Carbon Nanocomposites and Their Application for Adsorption of Paracetamol. Materials 2020, 13, 5647. https://doi.org/10.3390/ma13245647
Szczepanik B, Banaś D, Kubala-Kukuś A, Szary K, Słomkiewicz P, Rędzia N, Frydel L. Surface Properties of Halloysite-Carbon Nanocomposites and Their Application for Adsorption of Paracetamol. Materials. 2020; 13(24):5647. https://doi.org/10.3390/ma13245647
Chicago/Turabian StyleSzczepanik, Beata, Dariusz Banaś, Aldona Kubala-Kukuś, Karol Szary, Piotr Słomkiewicz, Nina Rędzia, and Laura Frydel. 2020. "Surface Properties of Halloysite-Carbon Nanocomposites and Their Application for Adsorption of Paracetamol" Materials 13, no. 24: 5647. https://doi.org/10.3390/ma13245647





