High Velocity Suspension Flame Spraying (HVSFS) of Metal Suspensions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of the Metal Suspensions
2.2. Coating Deposition and Characterization
- mc: mass of coating
- mt: theoretically sprayed mass
3. Results and Discussion
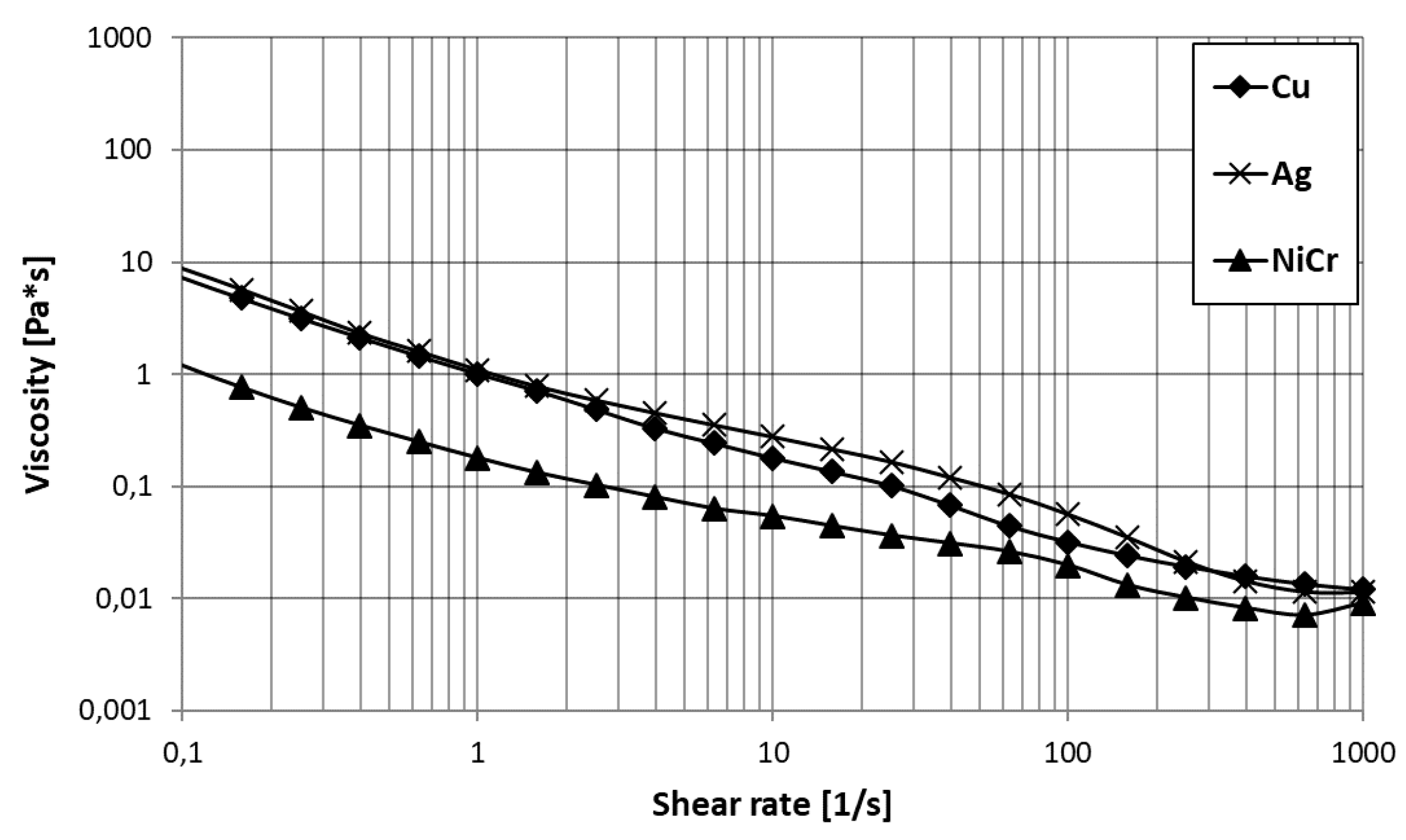
3.1. Suspension Characterization
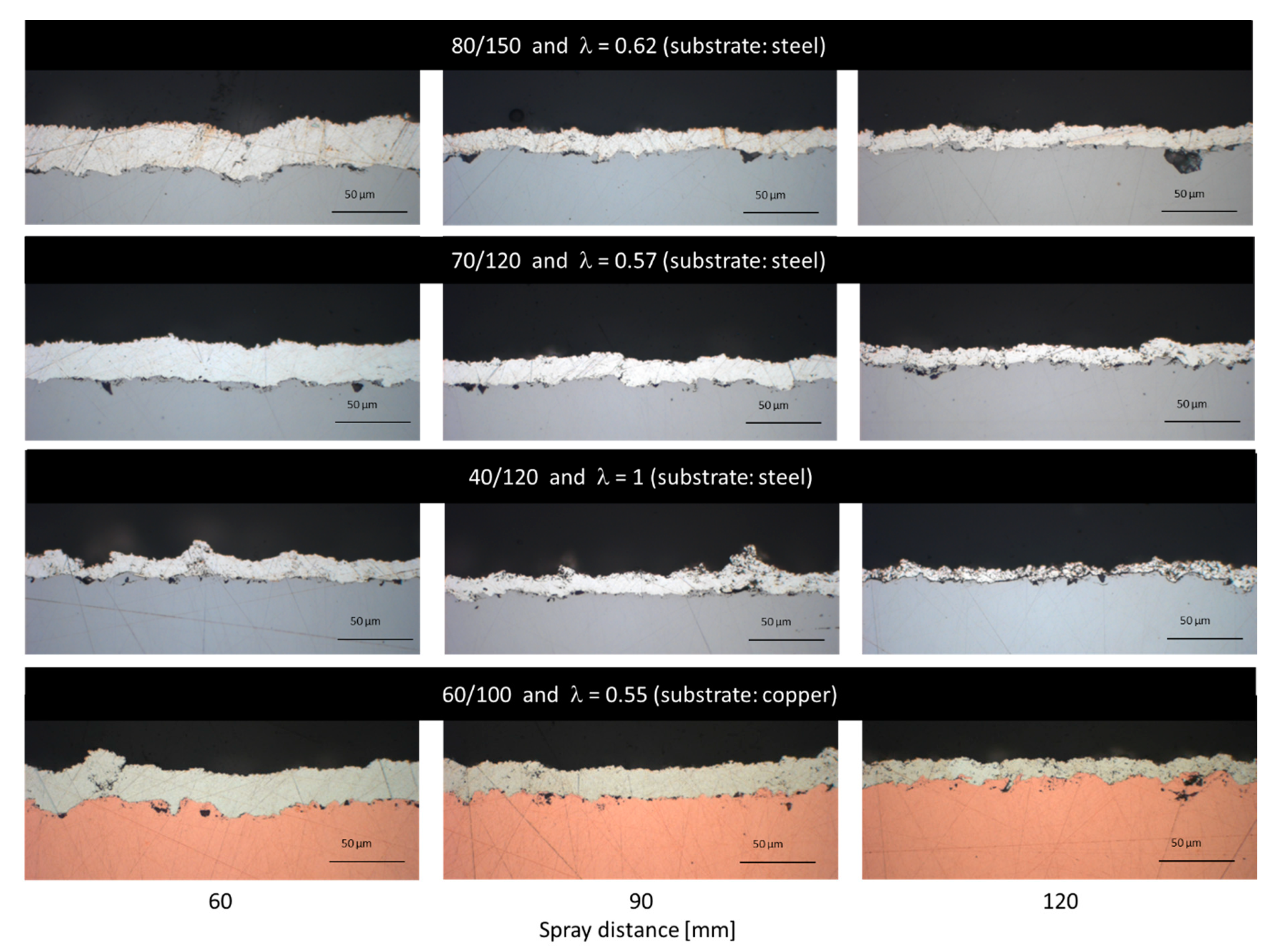
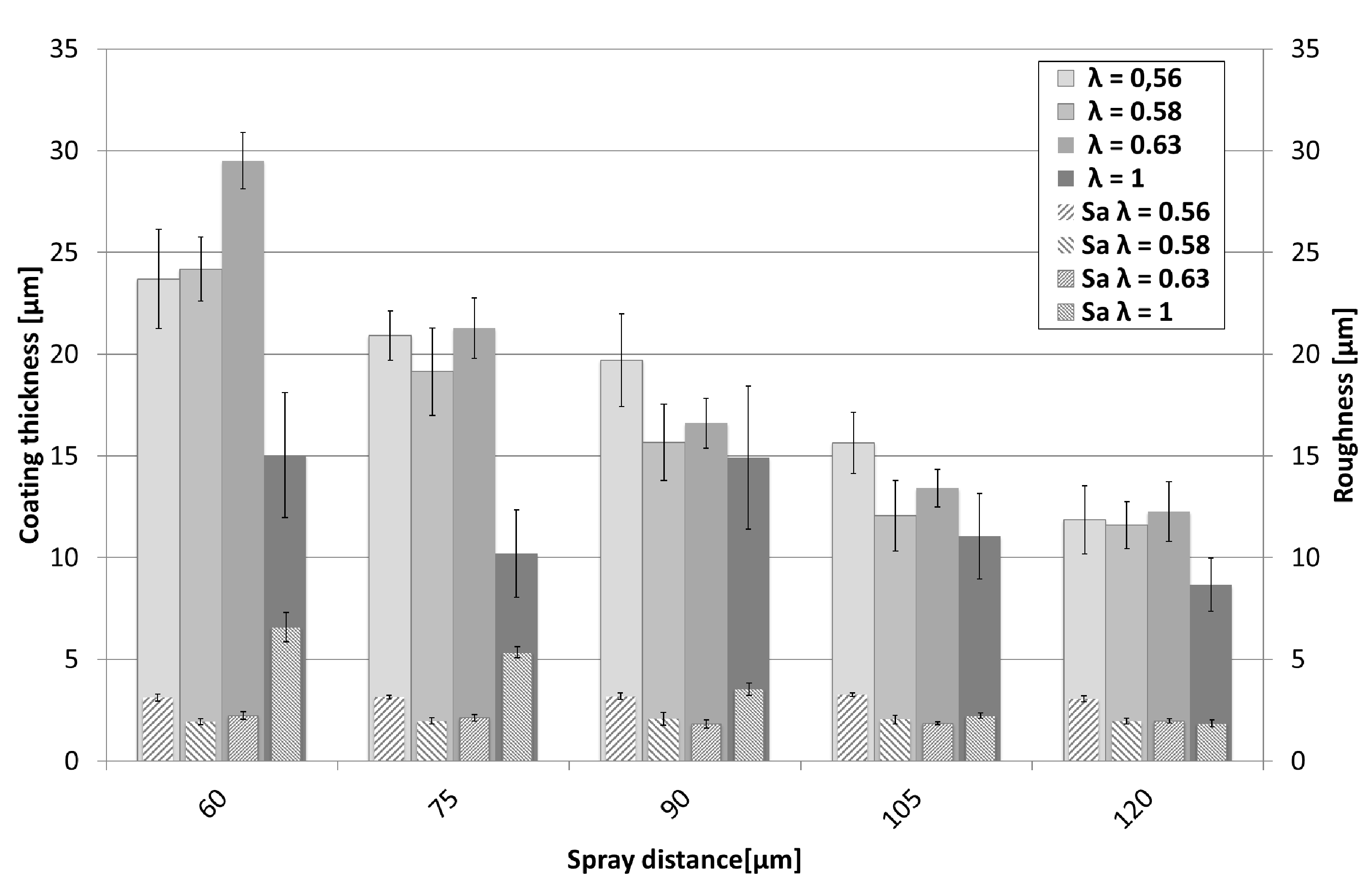
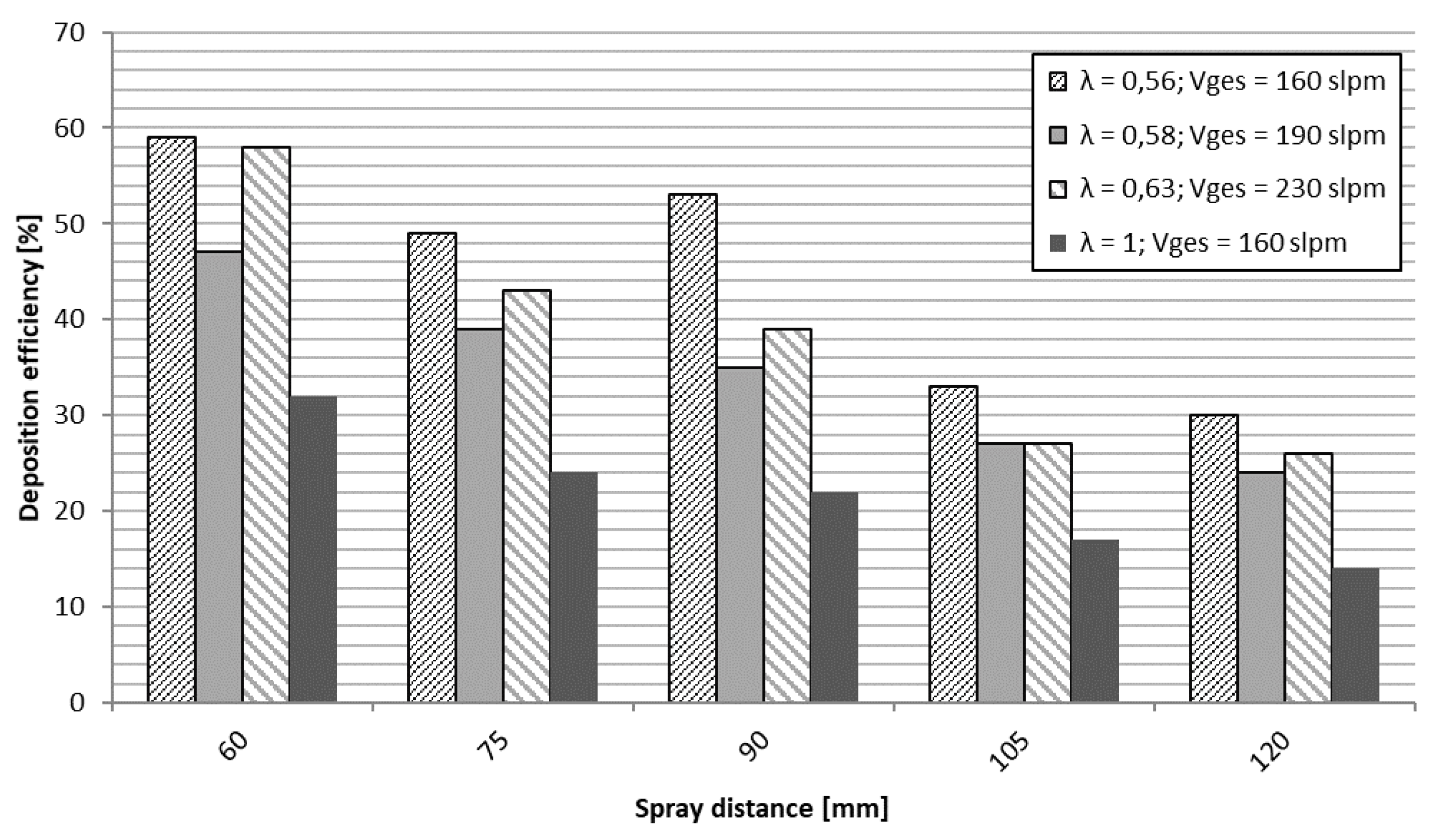
3.2. Coating Structure and Properties
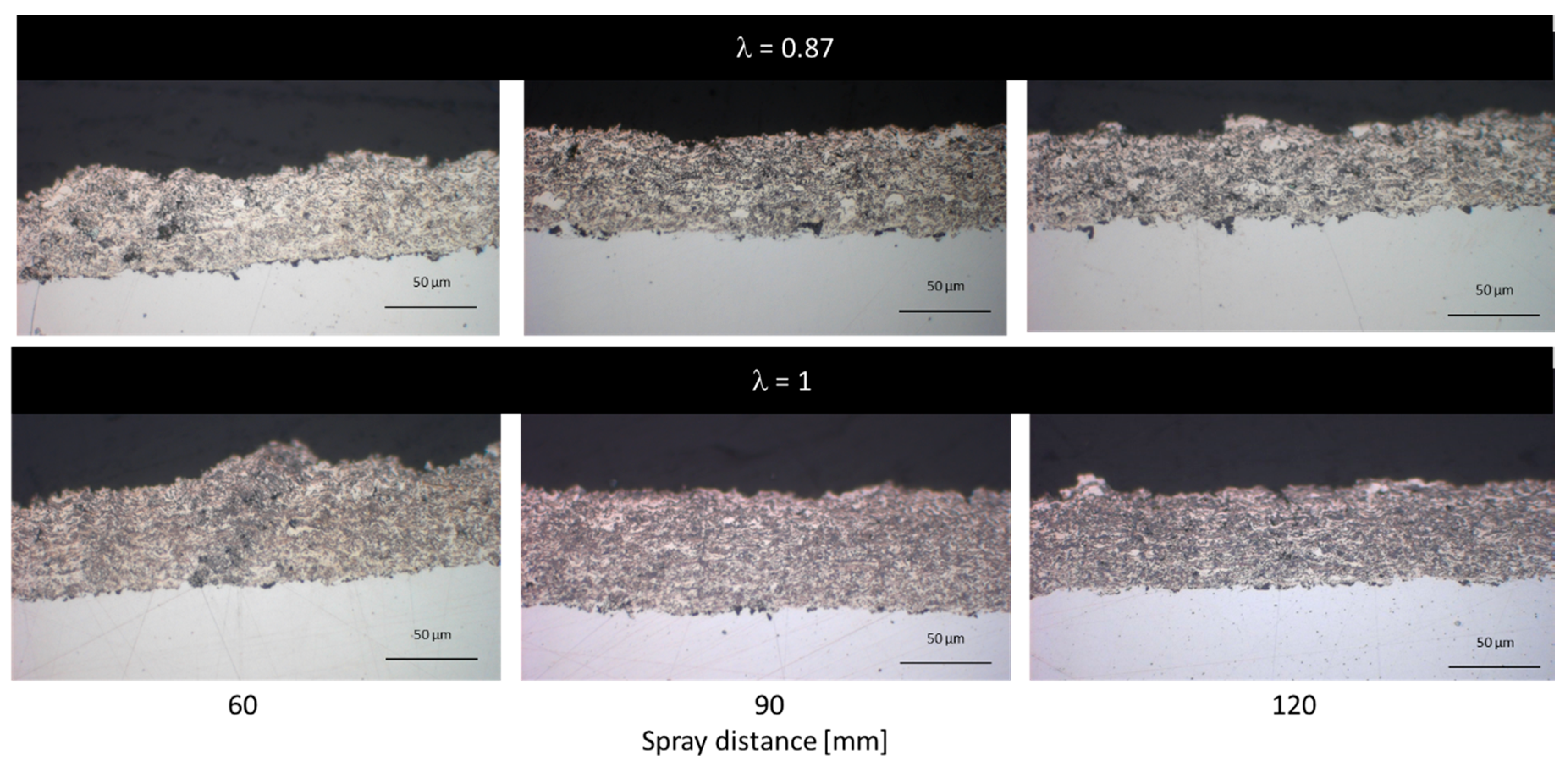
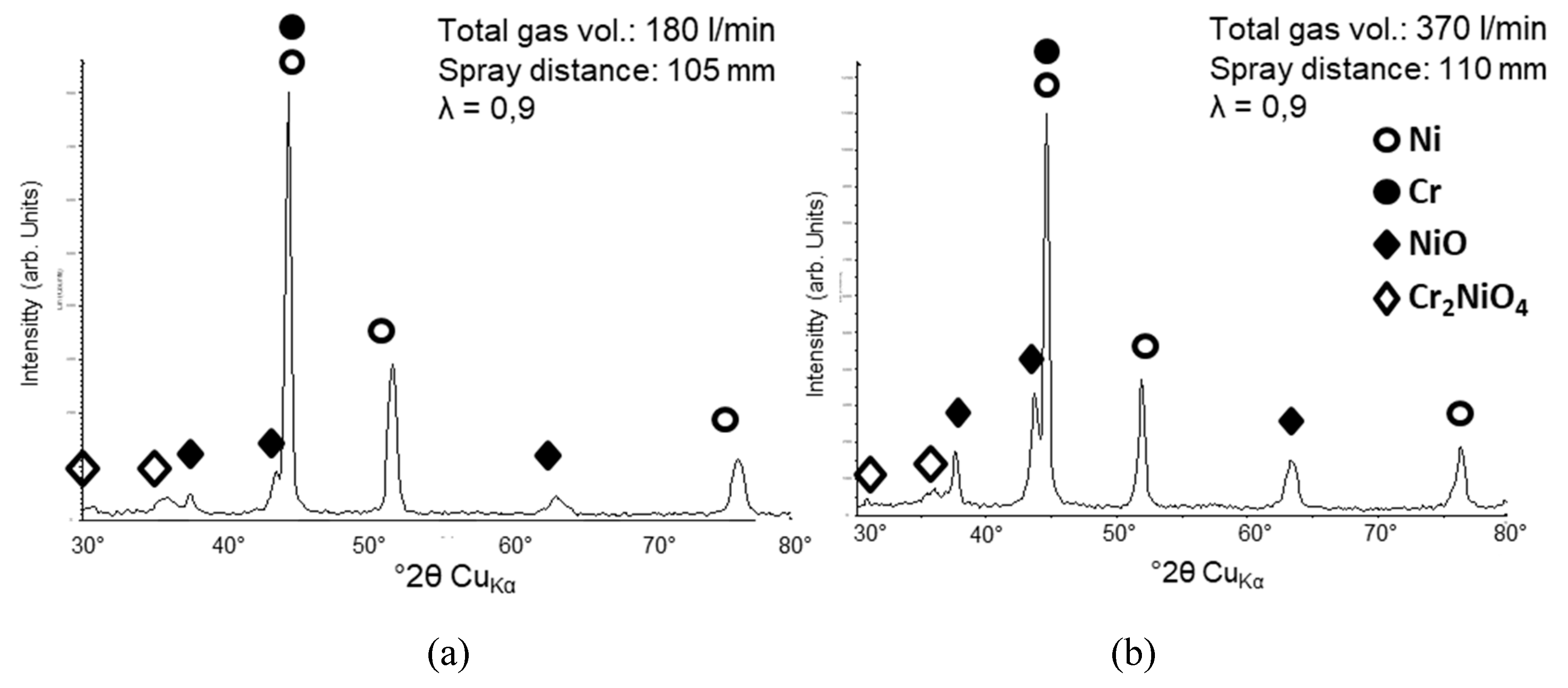
3.2.1. NiCr Coatings
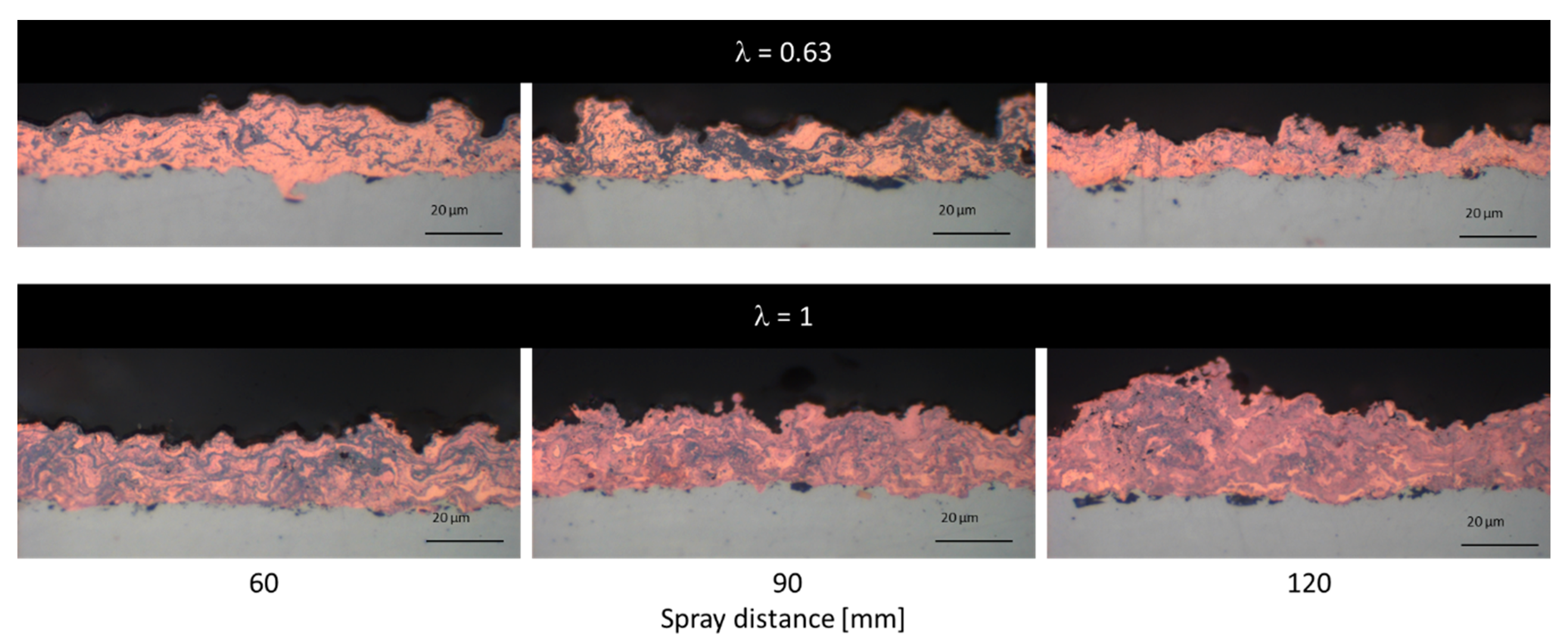
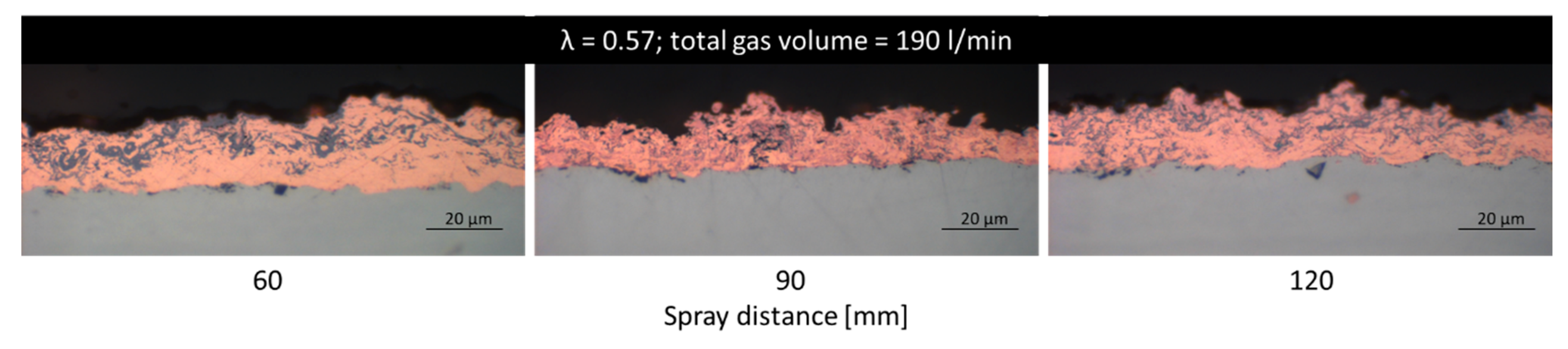
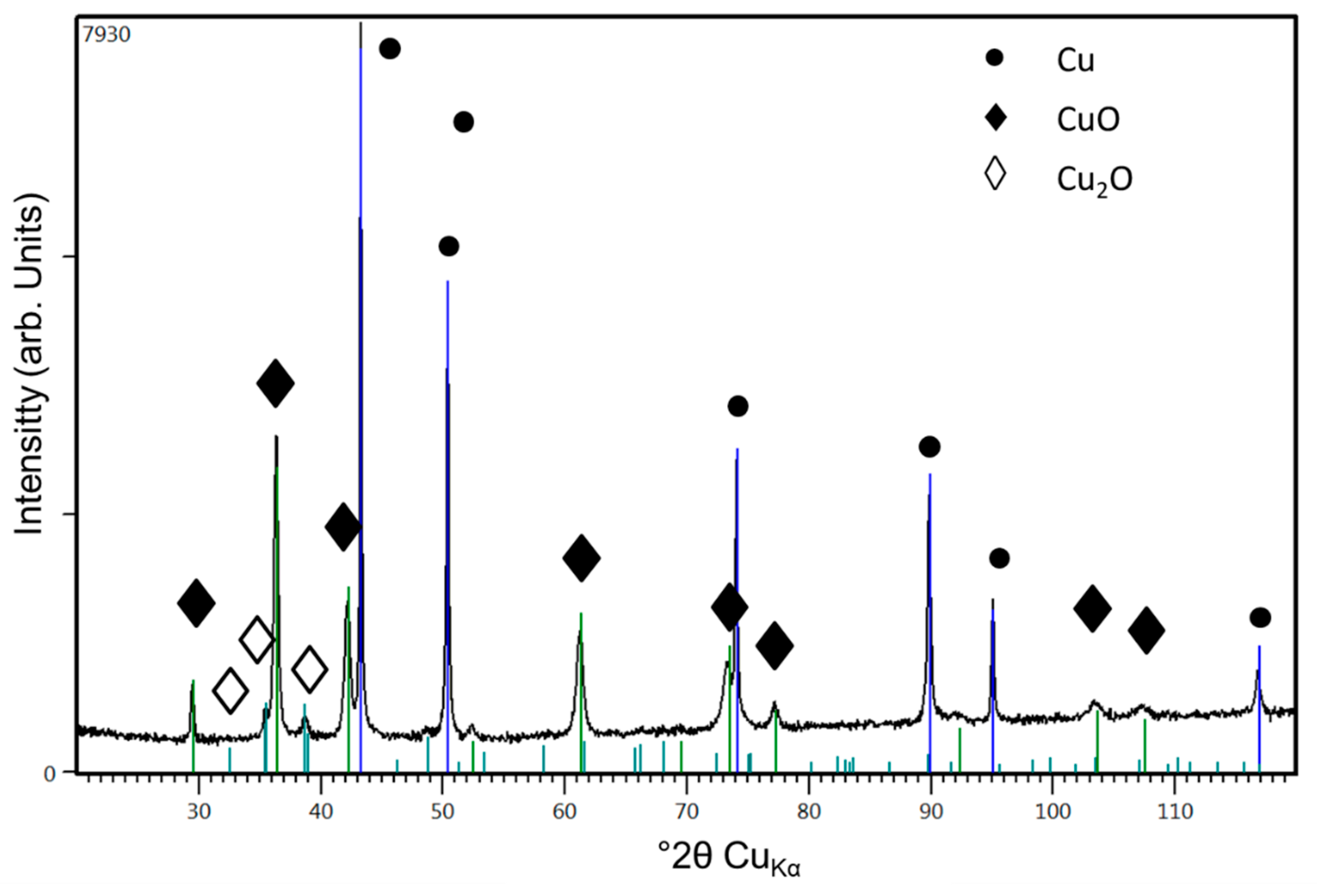
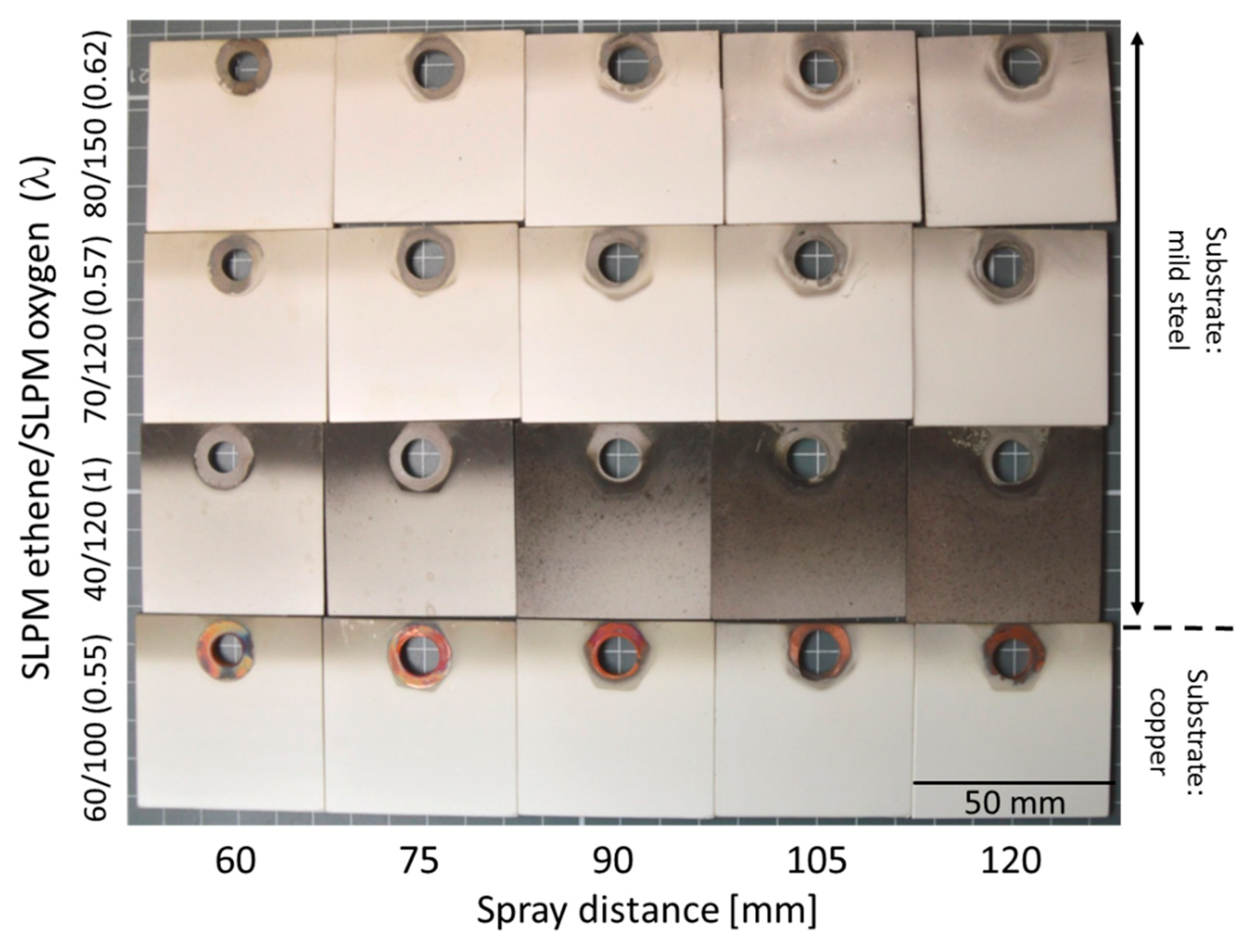
3.2.2. Cu Coatings
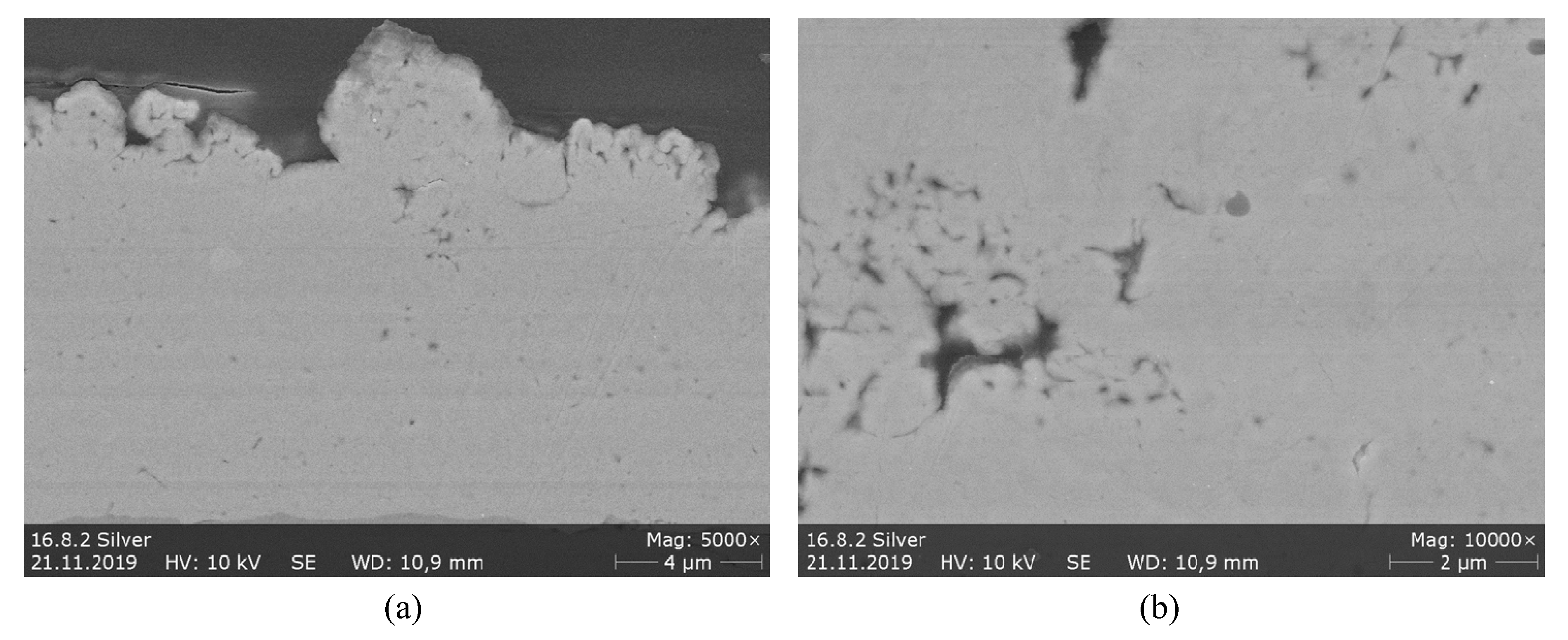
3.2.3. Ag Coatings
4. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Pawlowski, L. Suspension and Solution Thermal Spray Coatings. Surf. Coatings Tech. 2009, 203, 2807–2829. [Google Scholar] [CrossRef]
- Toma, F.L.; Berger, L.M.; Scheiz, S.; Langner, S.; Rödel, C.; Potthoff, A.; Sauchuk, V.; Kusnezoff, M. Comparison of the Microstructural Characteristics and Electrical Properties of Thermally Sprayed Al2O3 Coatings from Aqueous Suspensions and Feedstock Powders. J. Therm. Spray Tech. 2012, 21, 480–488. [Google Scholar] [CrossRef] [Green Version]
- Bolelli, G.; Cannillo, V.; Gadow, R.; Killinger, A.; Lusvarghi, L.; Rauch, J. Properties of High Velocity Suspension Flame Sprayed (HVSFS) TiO2 coatings. Surf. Coatings Tech. 2009, 203, 1722–1732. [Google Scholar] [CrossRef]
- Toma, F.L.; Berger, L.M.; Jacquet, D.; Wicky, D.; Villaluenga, I.; de Miguel, Y.R.; Lindeløv, J.S. Comparative study on the photocatalytic behaviour of titanium oxide thermal sprayed coatings from powders and suspensions. Surf. Coatings Tech. 2009, 203, 2150–2156. [Google Scholar] [CrossRef]
- Oberste Berghaus, J.; Legoux, J.G.; Moreau, C.; Hui, R.; Decès-Petit, C.; Qu, W.; Yick, S.; Wang, Z.; Maric, R.; Ghosh, D. Suspension HVOF Spraying of Reduced Temperature Solid Oxide Fuel Cell Electrolytes. J. Therm. Spray Tech. 2008, 17, 700–707. [Google Scholar] [CrossRef] [Green Version]
- Bolelli, G.; Rauch, J.; Cannillo, V.; Killinger, A.; Lusvarghi, L.; Gadow, R. Investigation of High-Velocity Suspension Flame Sprayed (HVSFS) glass coatings. Mater. Lett. 2008, 62, 2772–2775. [Google Scholar] [CrossRef]
- Stiegler, N.; Bellucci, D.; Bolelli, G.; Cannillo, V.; Gadow, R.; Killinger, A.; Lusvarghi, L.; Sola, A. High-Velocity Suspension Flame Sprayed (HVSFS) Hydroxyapatite Coatings for Biomedical Applications. J. Therm. Spray Tech. 2012, 21, 275–287. [Google Scholar] [CrossRef]
- Díaz, L.A.; Cabal, B.; Prado, C.; Moya, J.S.; Torrecillas, R.; Fernández, A.; Arhire, I.; Krieg, P.; Killinger, A.; Gadow, R. High-velocity suspension flame sprayed (HVSFS) soda-lime glass coating on titanium substrate: Its bactericidal behaviour. J. Eur. Ceram. Soc. 2016, 36, 2653–2658. [Google Scholar] [CrossRef]
- Aghasibeig, M.; Mousavi, M.; Ben Ettouill, F.; Moreau, C.; Wuthrich, R.; Dolatabadi, A. Electrocatalytically Active Nickel-Based Electrode Coatings Formed by Atmospheric and Suspension Plasma Spraying. J. Therm. Spray Tech. 2014, 23, 220–226. [Google Scholar] [CrossRef]
- Oberste Berghaus, J.; Marple, B.; Moreau, C. Suspension Plasma Spraying of Nanostructured WC-12Co Coatings. J. Therm. Spray Tech. 2006, 15, 676–681. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.Q.; Roth, J.; Gandy, D.W.; Frederick, G.J. A New High-Velocity Oxygen Fuel Process for Making Finely Structured and Highly Bonded Inconel Alloy Layers from Liquid Feedstock. J. Therm. Spray Tech. 2006, 15, 670–675. [Google Scholar] [CrossRef]
- Toma, F.L.; Berger, L.M.; Naumann, T. Suspensionsspritzen—Das Potential einer neuen Spritztechnologie, Suspension Spraying—The Potential of a New Spray Technology. Therm. Spray Bull. 2010, 62, 24–29. [Google Scholar]
- Ak, N.F.; Tekmen, C.; Ozdemir, I.; Soykan, H.S.; Celik, E. NiCr coatings on stainless steel by HVOF technique. Surf. Coatings Tech. 2003, 174–175, 1070–1073. [Google Scholar] [CrossRef]
- Higuera, V.; Belzunce, F.J.; Carriles, A.; Poveda, S. Influence of the thermal-spray procedure on the properties of a nickel-chromium coating. J. Mater. Sci. 2002, 37, 649–654. [Google Scholar] [CrossRef]
- Donner, K.R.; Gaertner, F.; Klassen, T. Metallization of Thin Al2O3 Layers in Power Electronics Using Cold Gas Spraying. J. Therm. Spray Tech. 2011, 20, 299–306. [Google Scholar] [CrossRef]
- Chavan, N.M.; Ramakrishna, M.; Phani, P.S.; Rao, D.S.; Sundararajan, G. The influence of process parameters and heat treatment on the properties of cold sprayed silver coatings. Surf. Coatings Tech. 2011, 205, 4798–4807. [Google Scholar] [CrossRef]
- Rolland, G.; Sallamand, P.; Guipont, V.; Jeandin, M.; Boller, E.; Bourda, C. Damage Study of Cold-Sprayed Composite Materials for Application to Electrical Contacts. J. Therm. Spray Tech. 2012, 21, 758–772. [Google Scholar] [CrossRef]
- Krieg, P.; Killinger, A.; Gadow, R.; Burtscher, S.; Bernstein, A. High velocity suspension flame spraying (HVSFS) of metal doped bioceramic coatings. Bioact. Mater. 2017, 2, 162–169. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, X.; Xie, Y.; Ding, C.; Ruan, H.; Fan, C. Anti-bacterial and cytotoxic properties of plasma sprayed silver-containing HA coatings. J. Mater. Sci. Mater. Med. 2008, 19, 3603–3609. [Google Scholar] [CrossRef]
- Trenkle, F.; Winkelmann, M.; Wüst, F.; Luth, J. Thermal spraying of thin metallic coatings. Therm. Spray Bull. 2017, 69, 22–27. [Google Scholar]
- Krieg, P. Dissertation Peter Valentin Krieg, Hochgeschwindigkeitssuspensionsflamm¬spritzen von Metallen und metalldotierten Biokeramiken. In Forschungsberichte des Instituts für Fertigungs—technologie keramischer Bauteile (IFKB); Gadow, R., Ed.; Shaker Verlag: Aachen, Germany, 2018; ISBN 978-3-8440-6091-1. [Google Scholar]
- Fauchais, P.L.; Heberlein, J.V.R.; Boulos, M.I. Thermal Spray Fundamentals: From Powder to Part; Springer: Boston, MA, USA, 2014; pp. 1028–1029. [Google Scholar] [CrossRef]
- Brousse, E.; Montavon, G.; Fauchais, P.; Denoirjean, A.; Rat, V.; Coudert, J.-F.; Ageorges, H. Thin and dense yttria-partially stabalized zirconia electrolytes for IT-SOFC manufactured by suspension plasma spraying. Therm. Spray Crossing Bord. (DVS), 2008; 547–552. [Google Scholar]
- Racek, O. The effect of HVOF particle-substrate interactions on local variations in the coating microstructure and the corrosion resistence. J. Therm. Spray Tech. 2010, 19, 841–851. [Google Scholar] [CrossRef]
- Förg, A.; Blum, M.; Killinger, A.; Nicolás, J.A.M.; Gadow, R. Deposition of chromium oxide-chromium carbide coatings via high velocity suspension flame spraying (HVSFS). Surf. Coatings Tech. 2018, 351, 171–176. [Google Scholar] [CrossRef]
- Luth, J.; Winkelmann, M.; Wüst, F.; Hartmann, S.; Trenkle, F.; Krieg, P.; Killinger, A. Dünne, metallische Heizschichten, hergestellt durch Suspensionsspritzen - Thin, metallic heating coatings, manufactured by means of suspension spraying. Therm. Spray Bull. 2019, 12, 26. [Google Scholar]








| NiCr 80/20 | Cu | Ag | |
|---|---|---|---|
| Manufacturer | H.C. Starck | Evochem GmbH | Metalor Technologies SA |
| Notation | Amperit 251.051 | 14/200 | P747-35 |
| Density [g/cm³] | 8.31 | 8.93 | 10.49 |
| D10 [µm] | 4 | - | 1.8 |
| D50 [µm] | 7.8 | - | 6.2 |
| D90 [µm] | 13.6 | - | 20 |
| Powder type | Gas atomized | Chemically derived | Agglomerated and sintered |
| Morphology | spherical and dense | irregular dendritic | spherical |
| Parameter | NiCr | Cu | Ag | |||
|---|---|---|---|---|---|---|
| Normalized ethene-oxygen ratio λ | 0.87 | 1 | 0.57/0.63 | 1 | 0.63 | 1 |
| *TGFR (oxygen + ethene) (slpm) | 180 | 200 | 190/230 | 230 | 230 | 160 |
| **GFR oxygen (slpm) | 130 | 150 | 120/150 | 58 | 150 | 120 |
| **GFR ethene (slpm) | 50 | 50 | 70/80 | 172 | 80 | 40 |
| Spray distance variations (mm) | For all three materials: 60; 75; 90; 105; 120 | |||||
| Meander offset (mm) | For all three materials: 3 | |||||
| Torch speed (mm/s) | For all three materials: 500 | |||||
| Length of combustion chamber (mm) | 22 | 22 | 22 | 22 | 12 | 12 |
| Suspension feed rate (g/min) | 55 | 43 | 52 | |||
| Number of torch passes | 8 | 8 | 8 | 8 | 4 | 4 |
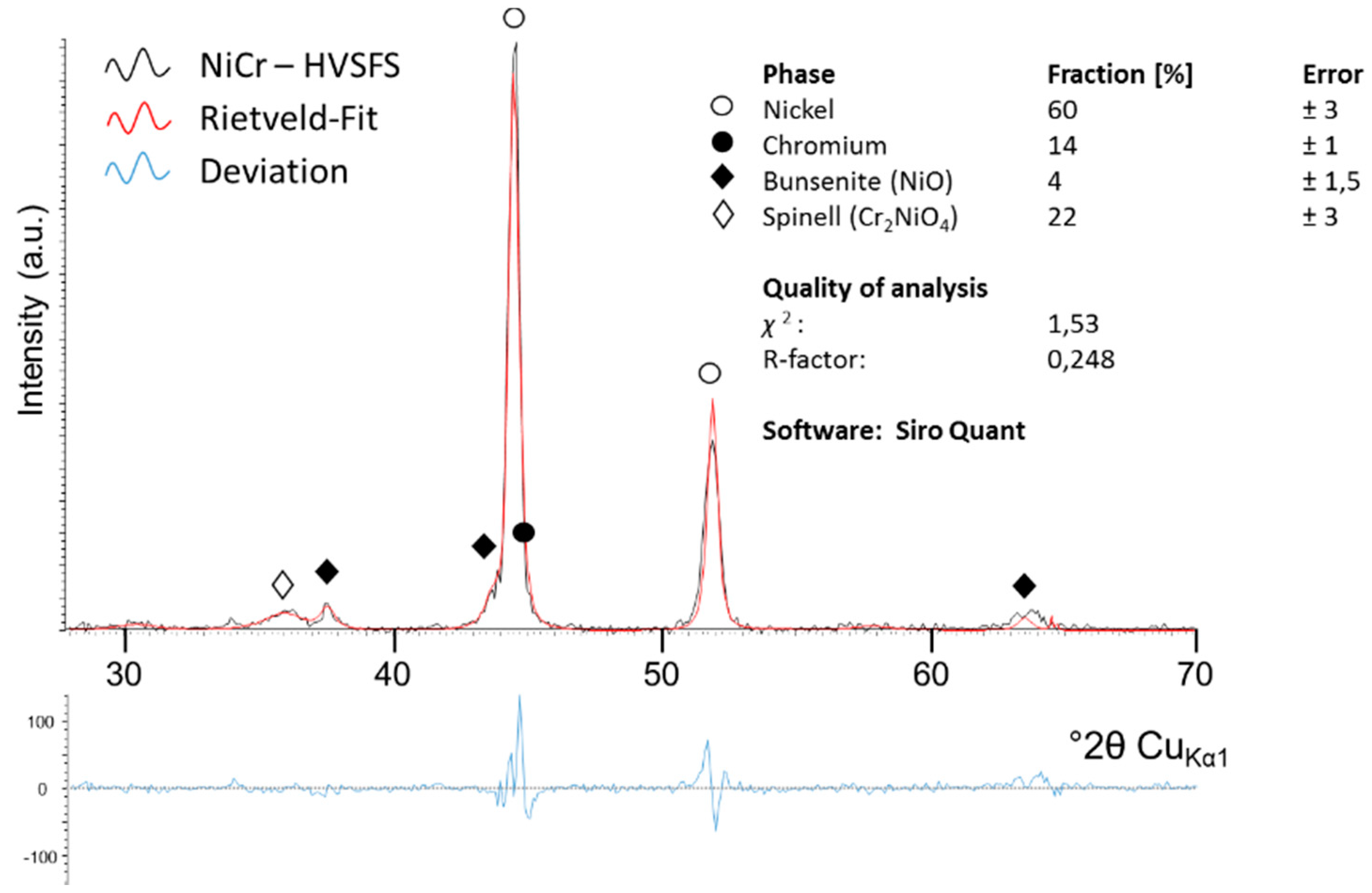
| Phase (Ref. No.) | Fraction (wt%) | Error (wt%) |
|---|---|---|
| Nickel (000-04-0850) | 60 | ±3 |
| Chrome (000-01-1250) | 14 | ±1 |
| Spinell Cr2NiO4 (000-47-1049) | 22 | ±3 |
| Bunsenit NiO (000-75-0198) | 4 | ±1,5 |
| Ref. No (Refer to Figure 13) | Cu (wt%) | O (wt%) | Other (wt%) |
|---|---|---|---|
| 1 | 95.32 ± 0.6 | 0.71 ± 0.13 | 3.97 ± 0.31 |
| 2 | 87.79 ± 0.45 | 7.46 ± 0.10 | 5.74 ± 0.19 |
| 3 | 95.76 ± 0.56 | 0.69 ± 0.07 | 3.55 ± 0.27 |
| 4 | 92.66 ± 0.44 | 0.85 ± 0.04 | 6.49 ± 0.42 |
| 5 | 93.09 ± 0.47 | 6.91 ± 0.11 | - |
| 6 | 95.11 ± 0.47 | 4.63 ± 0.1 | 0.25 ± 0.18 |
| 7 | 97.09 ± 0.45 | 2.64 ± 0.09 | 0.27 ± 0.04 |
| 8 | 95.11 ± 0.48 | 4.89 ± 0.10 | - |
| 9 | 97.06 ± 0.48 | 2.94 ± 0.09 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blum, M.; Krieg, P.; Killinger, A.; Gadow, R.; Luth, J.; Trenkle, F. High Velocity Suspension Flame Spraying (HVSFS) of Metal Suspensions. Materials 2020, 13, 621. https://doi.org/10.3390/ma13030621
Blum M, Krieg P, Killinger A, Gadow R, Luth J, Trenkle F. High Velocity Suspension Flame Spraying (HVSFS) of Metal Suspensions. Materials. 2020; 13(3):621. https://doi.org/10.3390/ma13030621
Chicago/Turabian StyleBlum, Matthias, Peter Krieg, Andreas Killinger, Rainer Gadow, Jan Luth, and Fabian Trenkle. 2020. "High Velocity Suspension Flame Spraying (HVSFS) of Metal Suspensions" Materials 13, no. 3: 621. https://doi.org/10.3390/ma13030621




