Crystallization and Dielectric Properties of MWCNT /Poly(1-Butene) Composite Films by a Solution Casting Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation
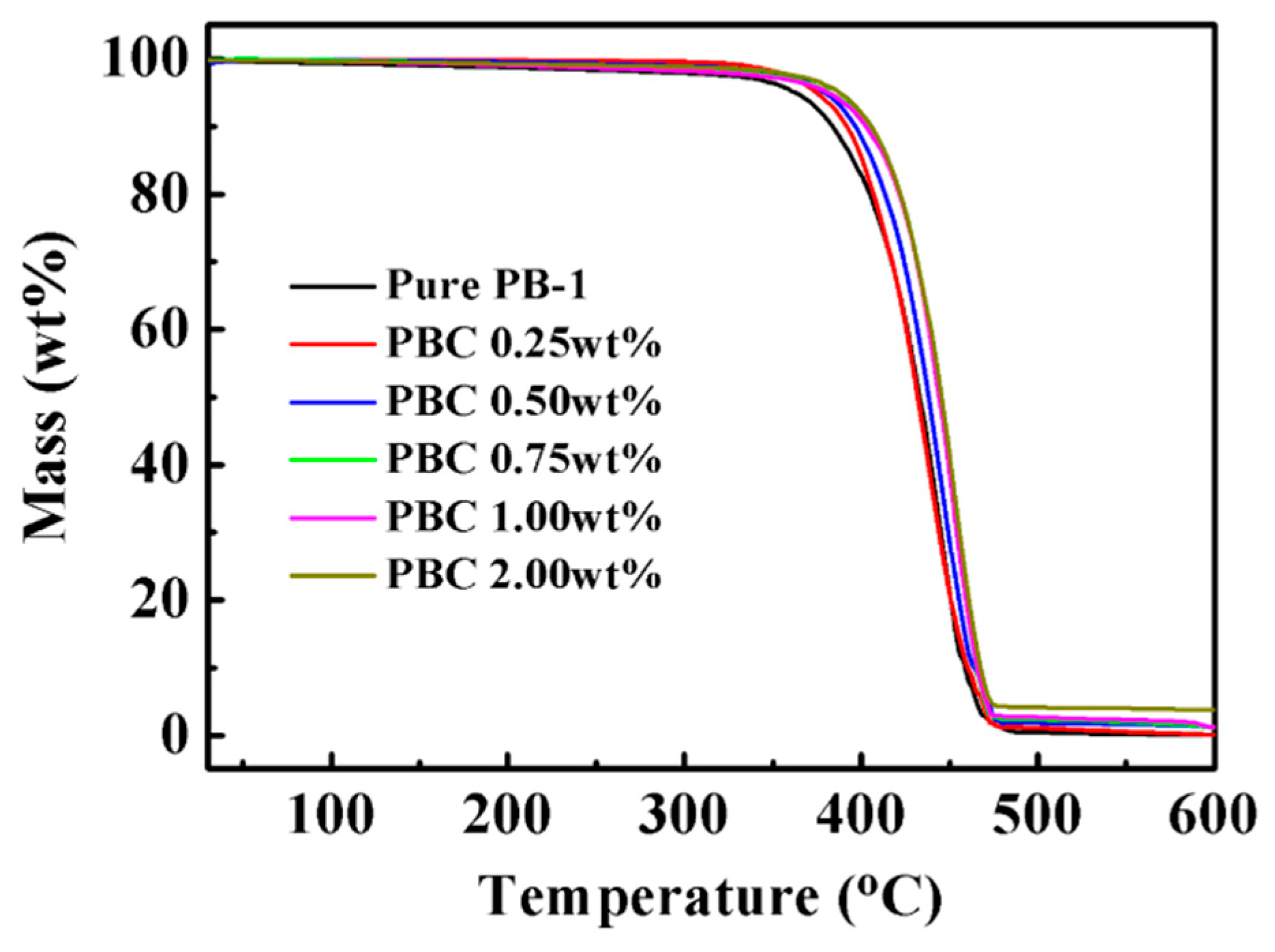
2.3. Characterization
3. Results and Discussion
3.1. Effect of MWCNT on Phase Transformation of PB-1 Thin Films
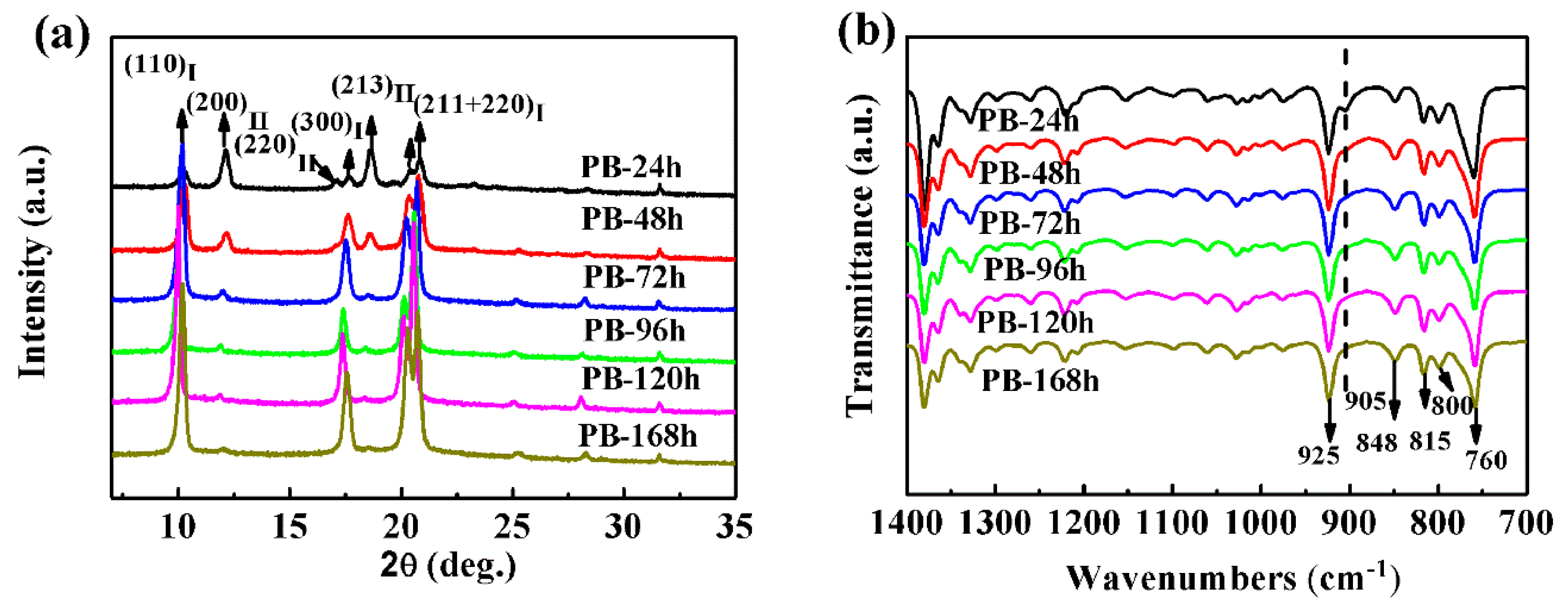
3.1.1. Effect of Phase Transition Time on the Crystal Form Transformation of Pure PB-1 Films
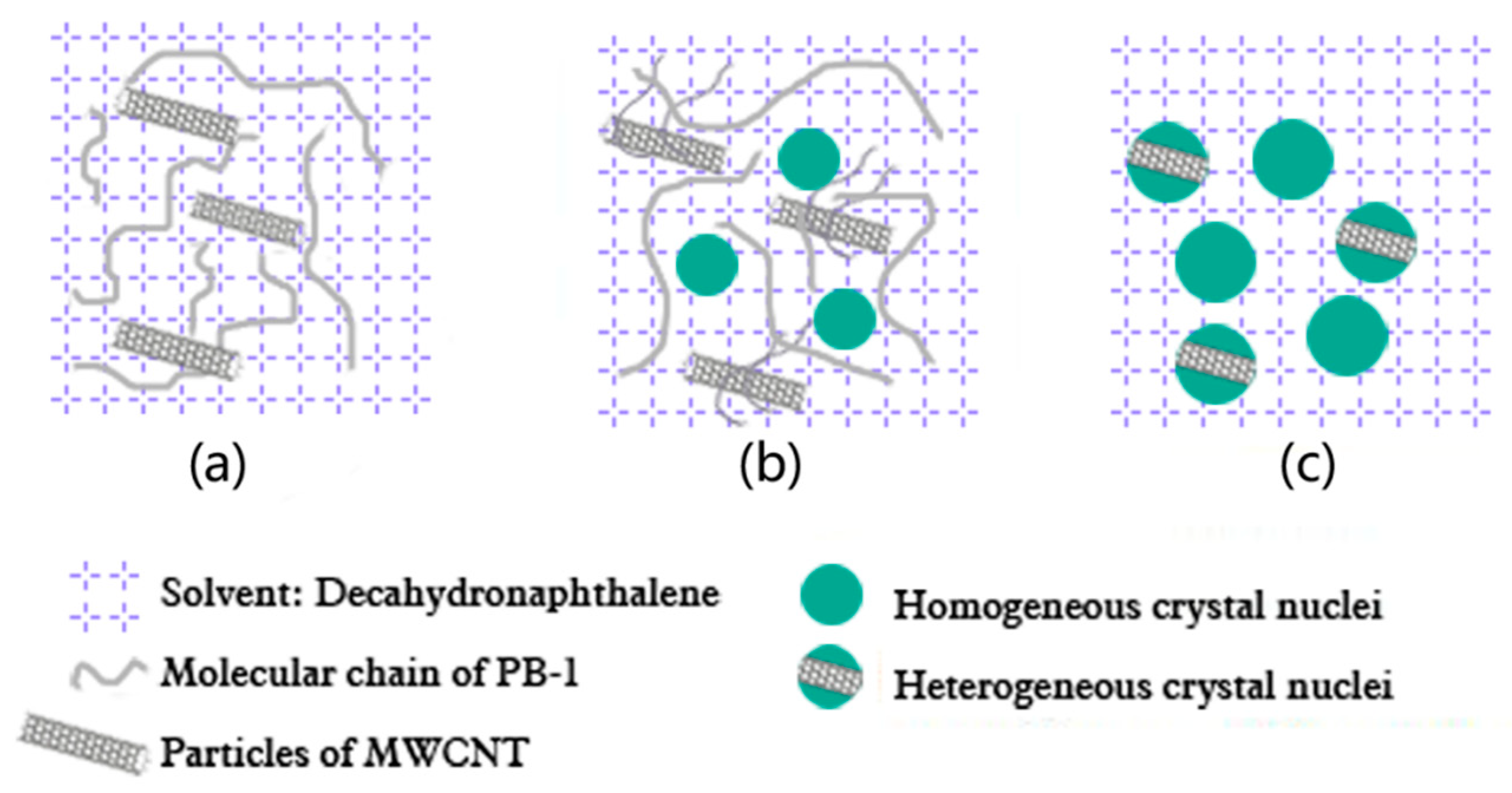
3.1.2. Effect of Phase Transition Time on Phase Transformation of MWCNT/PB-1 Composite Film
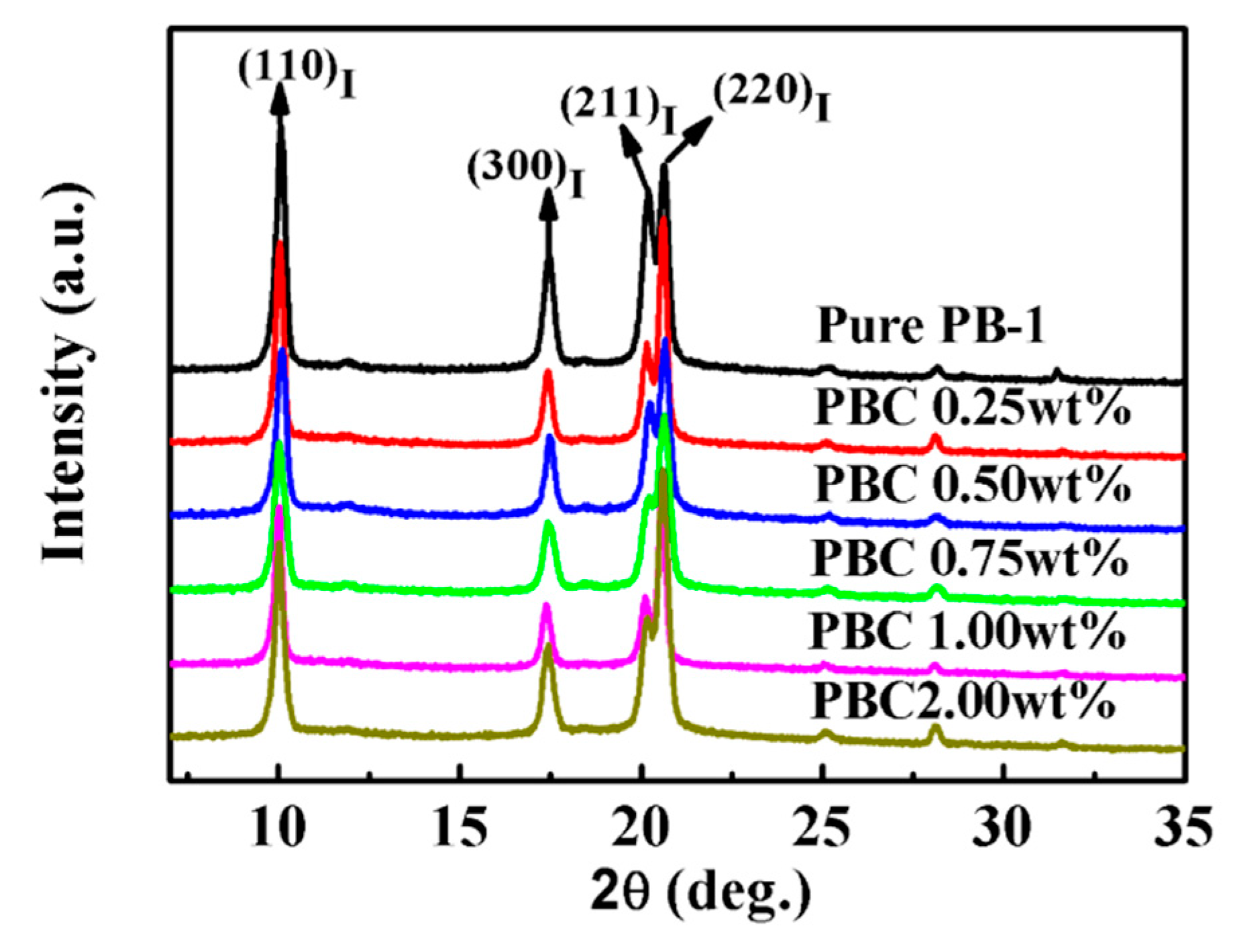
3.1.3. Effect of the MWCNT Contents on the Crystallization Properties
XRD Results
SEM Results
DSC Results
3.2. Influence of the Introduction of MWCNT on the Dielectric Properties of PB-1 Films
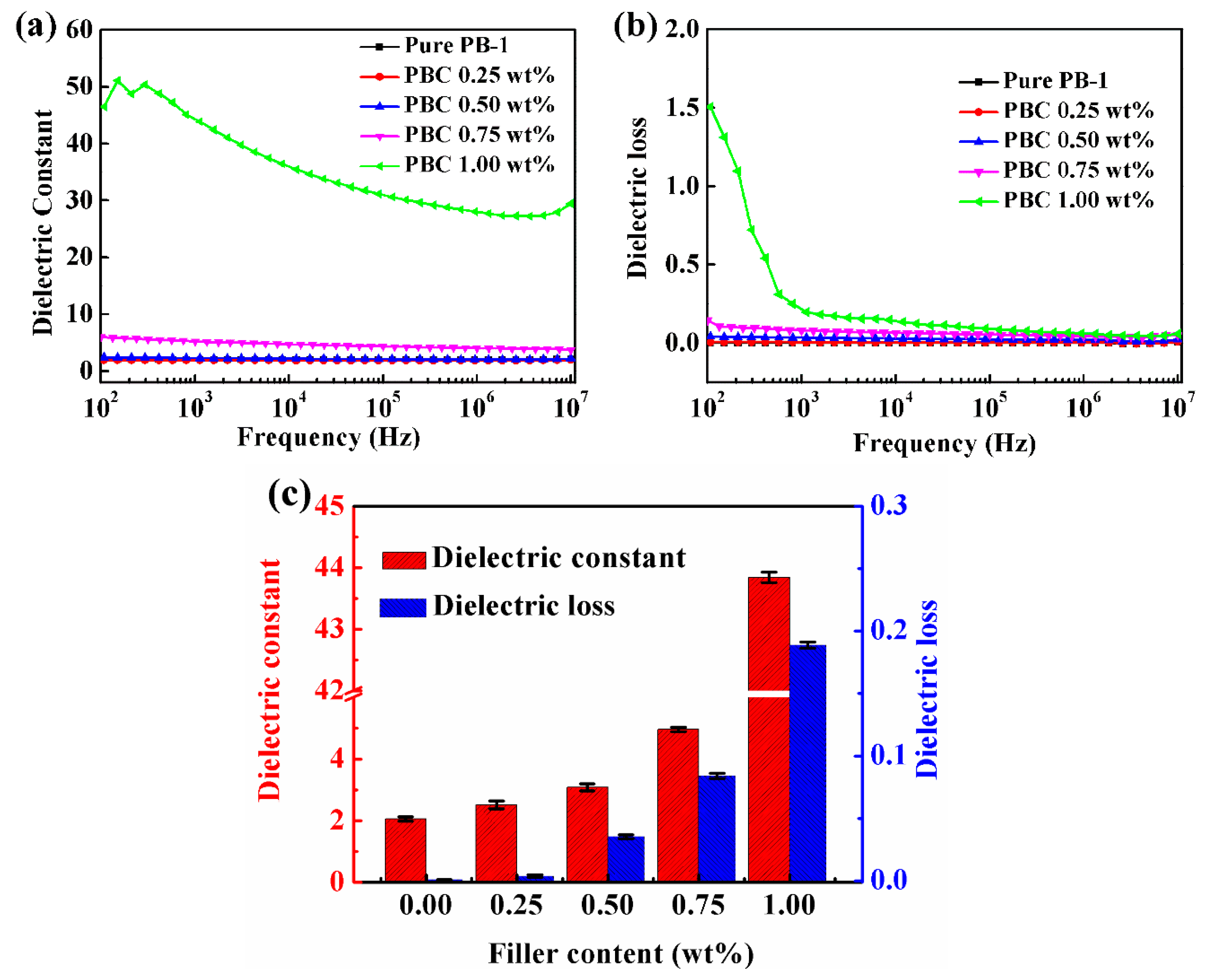
3.2.1. Effect of Phase Transition Time on Dielectric Properties
3.2.2. Effect of Phase Transition Time on Breakdown Strength
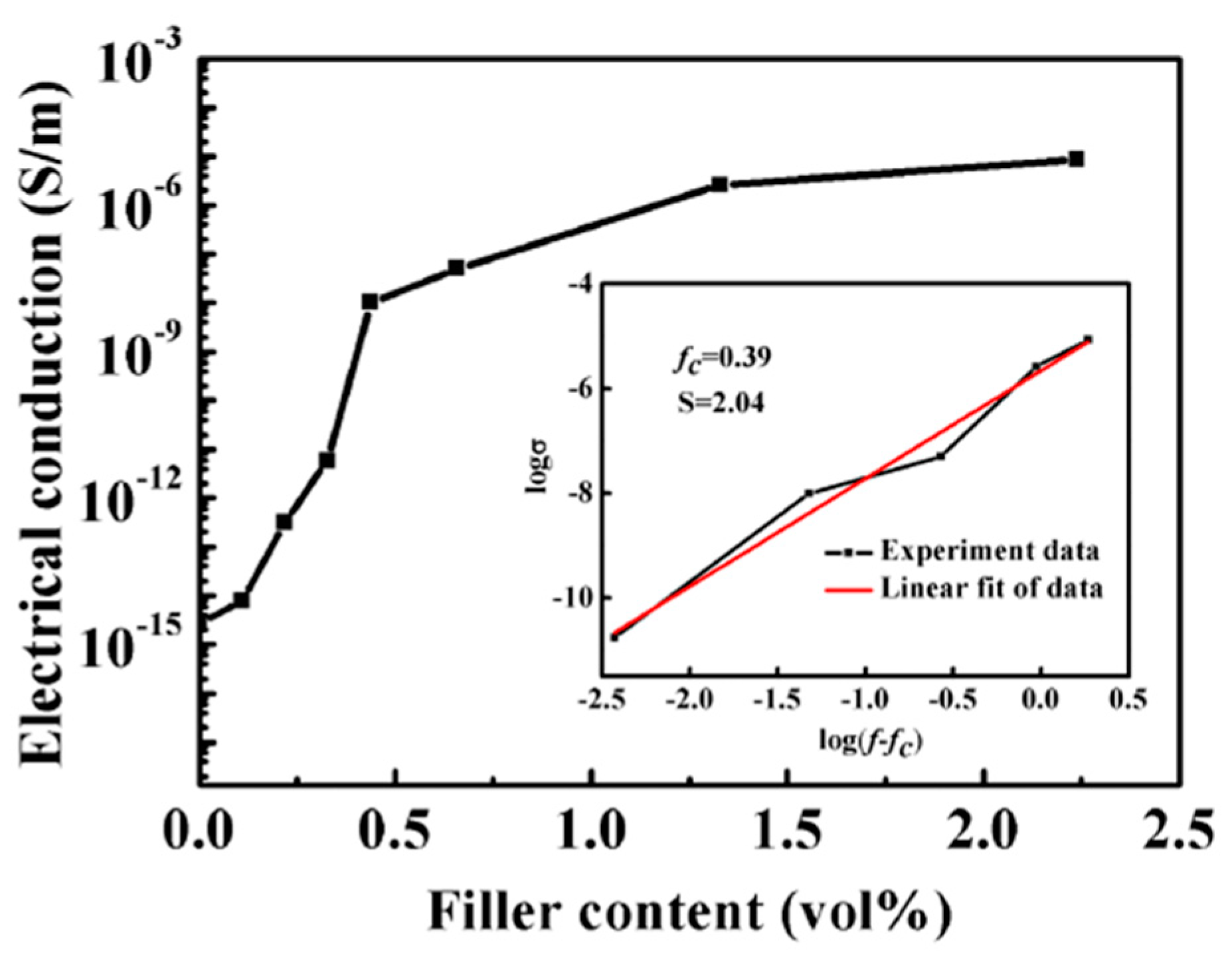
3.3. Determination of Percolation Threshold
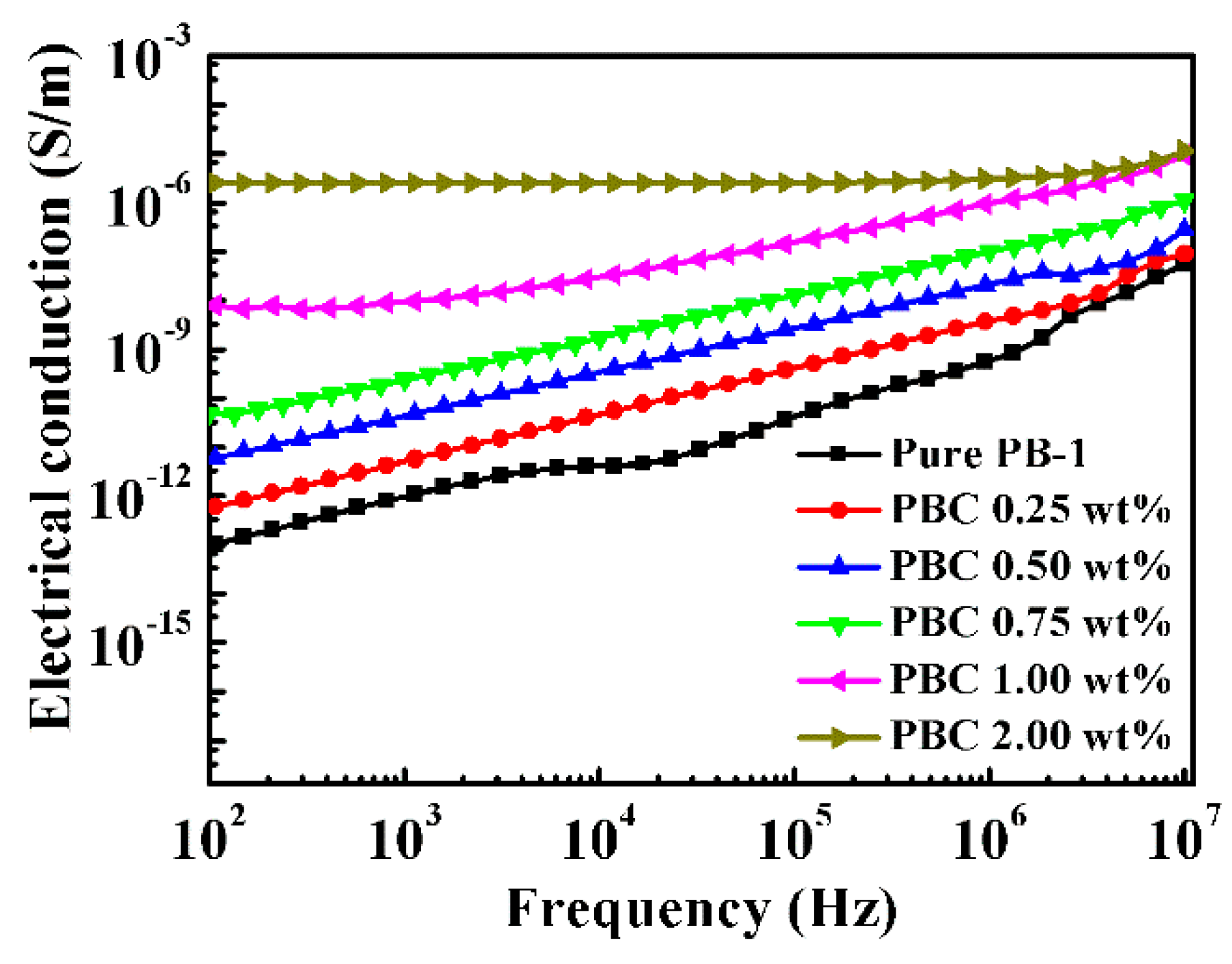
3.3.1. Conductivity of Modified PB-1 Films with Different Contents of MWCNT
3.3.2. Dielectric Properties of MWCNT/PB-1 Films with Different Contents of MWCNT
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pan, H.; Li, F.; Liu, Y.; Zhang, Q.H.; Wang, M.; Lan, S.; Zheng, Y.P.; Ma, J.; Gu, L.; Shen, Y.; et al. Ultrahigh–energy density lead-free dielectric films via polymorphic nano domain design. Science 2019, 365, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Baer, E.; Zhu, L. 50th Anniversary Perspective: Dielectric Phenomena in Polymers and Multilayered Dielectric Films. Macromolecules 2017, 50, 2239–2256. [Google Scholar] [CrossRef]
- Dang, Z.-M.; Zheng, M.-S.; Hu, P.-H.; Zha, J.-W. Dielectric Polymer Materials for Electrical Energy Storage and Dielectric Physics: A Guide. J. Adv. Phys. 2015, 4, 302–313. [Google Scholar] [CrossRef]
- Ning, N.; Bai, X.; Yang, D.; Zhang, L.; Lu, Y.; Nishi, T.; Tian, M. Dramatically improved dielectric properties of polymer composites by controlling the alignment of carbon nanotubes in matrix. RSC Adv. 2014, 4, 4543–4551. [Google Scholar] [CrossRef]
- Yao, J.; Hu, L.; Zhou, M.; You, F.; Jiang, X.; Gao, L.; Wang, Q.; Sun, Z.; Wang, J. Synergistic Enhancement of Thermal Conductivity and Dielectric Properties in Al2O3/BaTiO3/PP Composites. Materials 2018, 11, 1536. [Google Scholar] [CrossRef] [Green Version]
- Ho, J.; Ramprasad, R.; Boggs, S. Effect of Alteration of Antioxidant by UV Treatment on the Dielectric Strength of BOPP Capacitor Film. IEEE Trans. Dielectr. Electr. Insul. 2007, 14, 1295–1301. [Google Scholar] [CrossRef]
- Ho, J.; Jow, T.R. High field conduction in biaxially oriented polypropylene at elevated temperature. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 990–995. [Google Scholar] [CrossRef]
- Zhang, G.; Brannum, D.; Dong, D.; Tang, L.; Allahyarov, E.; Tang, S.; Kodweis, K.; Lee, J.-K.; Zhu, L. Interfacial Polarization-Induced Loss Mechanisms in Polypropylene/BaTiO3 Nanocomposite Dielectrics. Chem. Mater. 2016, 28, 4646–4660. [Google Scholar] [CrossRef]
- Iroh, J.O.; Mark, J.E. Polymer Data Handbook; Oxford University Press, Inc.: New York, NY, USA, 1999; pp. 345–346. [Google Scholar]
- Gedde, U.W.; Viebke, J.; Leijström, H.; Ifwarson, M. Long-term properties of hot-water polyolefin pipes—A review. Polym. Eng. Sci. 1994, 34, 1773–1787. [Google Scholar] [CrossRef]
- Luciani, L.; Seppala, J.; Löfgren, B. Poly-1-butene: Its preparation, properties and challenges. Prog. Polym. Sci. 1988, 13, 37–62. [Google Scholar] [CrossRef]
- Tashiro, K.; Hu, J.; Wang, H.; Hanesaka, M.; Saiani, A. Refinement of the Crystal Structures of Forms I and II of Isotactic Polybutene-1 and a Proposal of Phase Transition Mechanism between Them. Macromolecules 2016, 49, 1392–1404. [Google Scholar] [CrossRef]
- Su, F.; Li, X.; Zhou, W.; Chen, W.; Li, H.; Cong, Y.; Hong, Z.; Qi, Z.; Li, L. Accelerating crystal–crystal transition in poly(1-butene) with two-step crystallization: An in-situ microscopic infrared imaging and microbeam X-ray diffraction study. Polymer 2013, 54, 3408–3416. [Google Scholar] [CrossRef]
- Wanjale, S.D.; Jog, J.P. Crystallization and phase transformation kinetics of poly(1-butene)/MWCNT nanocomposites. Polymer 2006, 47, 6414–6421. [Google Scholar] [CrossRef]
- Zhao, Y.-X.; Xu, W.; Zhou, Y.-F.; Chen, J.-Y.; Han, L.; Li, D. Study on Crystal Form Transition and Non-Isothermal Crystallization of Glycidyl Methacrylate Grafted Isotactic Polybutene-1. Int. Polym. Process. 2017, 32, 26–33. [Google Scholar] [CrossRef]
- Causin, V.; Marega, C.; Marigo, A.; Ferrara, G.; Idiyatullina, G.; Fantinel, F. Morphology, structure and properties of a poly(1-butene)/montmorillonite nanocomposite. Polymer 2006, 47, 4773–4780. [Google Scholar] [CrossRef]
- Marega, C. The effect of a synthetic double layer hydroxide on the rate of II→I phase transformation of poly(1-butene). Express Polym. Lett. 2011, 5, 1050–1061. [Google Scholar] [CrossRef]
- Ping, G.Z.; Zhang, J.Y.; Cheng, J.; Shi, L. Graphene nanosheets prepared by low-temperature exfoliation and reduction technique toward fabrication of high-performance poly (1-butene)/graphene films. Iran. Polym. J. 2017, 26, 55–69. [Google Scholar] [CrossRef]
- Wanjale, S.D.; Jog, J.P. Viscoelastic and dielectric behavior of poly (1-butene)/multi-walled carbon nanotube nanocomposites. J. Macromol. Sci. B 2006, 45, 1053–1064. [Google Scholar] [CrossRef]
- Cao, Y.; Irwin, P.; Younsi, K. The future of nanodielectrics in the electrical power industry. IEEE Trans. Dielectr. Electr. Insul. 2004, 11, 797–807. [Google Scholar]
- Chi, Q.; Ma, T.; Zhang, Y.; Chen, Q.; Zhang, C.; Cui, Y.; Zhang, T.; Lin, J.; Wang, X.; Lei, Q. Excellent energy storage of sandwich structured PVDF-Based composite at low electric field by introduction of the hybrid CoFe2O4@BZT–BCT nanofibers. ACS Sustain. Chem. Eng. 2018, 6, 403–412. [Google Scholar] [CrossRef]
- Wanjale, S.D.; Jog, J.P. Poly(1-butene)/clay nanocomposites: Preparation and properties. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 1014–1021. [Google Scholar] [CrossRef]
- Lotz, B.; Thierry, A. Spherulite Morphology of Form III Isotactic Poly(1-butene). Macromolecules 2003, 36, 286–290. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, D.; Yan, S. Direct formation of form I poly(1-butene) single crystals from melt crystallization in ultrathin films. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 2641–2645. [Google Scholar] [CrossRef]
- Jones, A.T. Polybutene-1—Type II crystalline form. J. Polym. Sci. Part B Polym. Lett. 1963, 1, 455–456. [Google Scholar] [CrossRef]
- Natta, G.; Corradini, P.; Bassi, I.W. 22-The crystalline structure of isotactic poly-α-butene. In Stereoregular Polymers and Stereospecific Polymerizations; Natta, G., Danusso, F., Eds.; Pergamon Press Ltd.: Pergamon, UK, 1967; p. 138. [Google Scholar]
- Ukita, M. The Vibrational Spectra and Vibrational Assignments of Isotactic Polybutene-1. Bull. Chem. Soc. Jpn. 1966, 39, 742–749. [Google Scholar] [CrossRef] [Green Version]
- Mathieu, F.; Patricia, P.; Matthias, G.; Philippe, O.; Éric, D. Thermal, rheological and electrical analysis of MWCNT/epoxy matrices. Compos. Sci. Technol. 2015, 110, 118–125. [Google Scholar]
- Chae, H.G.; Choi, Y.H.; Minus, M.L.; Kumar, S. Carbon nanotube reinforced small diameter polyacrylonitrile based carbon fiber. Compos. Sci. Technol. 2009, 69, 406–413. [Google Scholar] [CrossRef]
- Sandler, J.; Shaffer, M.; Prasse, T.; Bauhofer, W.; Schulte, K.; Windle, A. Development of a dispersion process for carbon nanotubes in an epoxy matrix and the resulting electrical properties. Polymer 1999, 40, 5967–5971. [Google Scholar] [CrossRef]
- Qian, Z.; Wang, Y.; Li, J.; Wu, D. Development of sustainable polyoxymethylene-based composites with recycled carbon fibre mechanical enhancement, morphology, and crystallization kinetics. J. Reinf. Plast. Comp. 2014, 33, 294–309. [Google Scholar] [CrossRef]
- Feng, H.; Wang, X.; Wu, D. Fabrication of Spirocyclic Phosphazene Epoxy-Based Nanocomposites with Graphene via Exfoliation of Graphite Platelets and Thermal Curing for Enhancement of Mechanical and Conductive Properties. Ind. Eng. Chem. Res. 2013, 52, 10160–10171. [Google Scholar] [CrossRef]
- Tomer, V.; Manias, E.; Randall, C.A. High field properties and energy storage in nanocomposite dielectrics of poly(vinylidene fluoride-hexafluoropropylene). J. Appl. Phys. 2011, 110, 044107. [Google Scholar] [CrossRef] [Green Version]
- Rinne, H. The Weibull Distribution: A handbook; Chapman and Hall/CRC: New York, NY, USA, 2008; pp. 15–24. [Google Scholar]
- Zhou, Y.; Hu, J.; Dang, B.; He, J. Mechanism of highly improved electrical properties in polypropylene by chemical modification of grafting maleic anhydride. J. Phys. D Appl. Phys. 2016, 49, 415301. [Google Scholar] [CrossRef]
- Wu, D.F.; Wu, L.; Sun, Y.R.; Zhang, M. Rheological properties and crystallization behavior of multi-walled carbon nanotube/poly(ε-caprolactone) composites. J. Polym. Sci. Pol. Phys. 2007, 45, 3137–3147. [Google Scholar] [CrossRef]
- Nan, C.-W. Physics of inhomogeneous inorganic materials. Prog. Mater. Sci. 1993, 37, 1–116. [Google Scholar] [CrossRef]
- Da Silva, A.B.; Arjmand, M.; Sundararaj, U.; Bretas, R.E.S. Novel composites of copper nanowire/PVDF with superior dielectric properties. Polymer 2014, 55, 226–234. [Google Scholar] [CrossRef]
- Paleo, A.; Zille, A.; Van Hattum, F.; Ares-Pernas, A.; Moreira, J.A. Dielectric relaxation of near-percolated carbon nanofiber polypropylene composites. Phys. B Condens. Matter 2017, 516, 41–47. [Google Scholar] [CrossRef]
- Prashantha, K.; Soulestin, J.; Lacrampe, M.; Krawczak, P.; Dupin, G.; Claes, M.; Tewari, A. Electrical and Dielectric Properties of Multi-Walled Carbon Nanotube Filled Polypropylene Nanocomposites. Polym. Polym. Compos. 2010, 18, 489–494. [Google Scholar] [CrossRef]
- Baji, A.; Mai, Y.-W.; Abtahi, M.; Wong, S.-C.; Liu, Y.; Li, Q. Microstructure development in electrospun carbon nanotube reinforced polyvinylidene fluoride fibers and its influence on tensile strength and dielectric permittivity. Compos. Sci. Technol. 2013, 88, 1–8. [Google Scholar] [CrossRef]



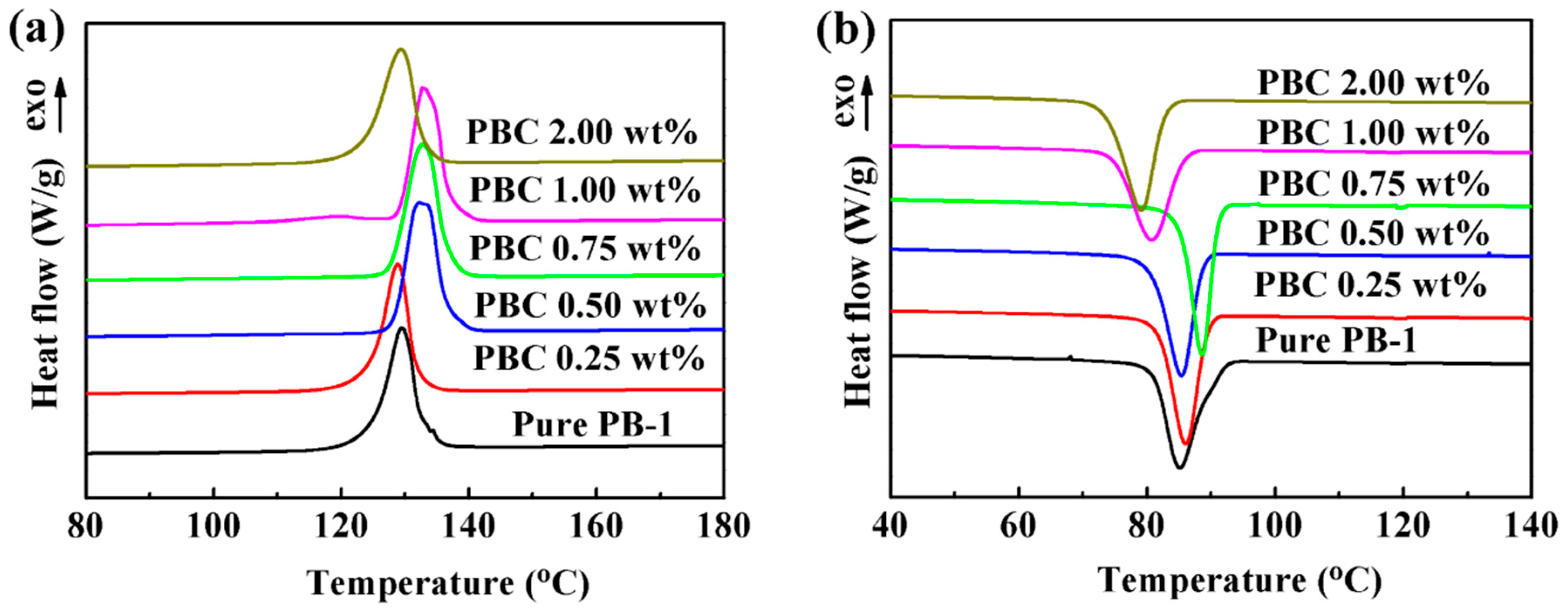



| Samples | Tm (°C) | ΔHm (J/g) | Tc (°C) | ΔHc (J/g) | Xc (%) |
|---|---|---|---|---|---|
| Pure PB-1 | 129.54 | 71.09 | 85.11 | 40.39 | 56.7 |
| PBC-0.25 wt% | 128.86 | 73.49 | 85.40 | 38.58 | 58.8 |
| PBC-0.50 wt% | 132.22 | 77.79 | 86.00 | 39.10 | 62.3 |
| PBC-0.75 wt% | 132.73 | 89.32 | 88.57 | 38.03 | 71.8 |
| PBC-1.00 wt% | 132.70 | 89.31 | 80.59 | 36.62 | 71.9 |
| PBC-2.00 wt% | 129.28 | 85.53 | 79.02 | 40.29 | 69.6 |
| Polymer | Filler | Filler Content | Experimental Method | ε’ | tan δ | Ref |
|---|---|---|---|---|---|---|
| Polypropylene | CNFs | 1.9 vol% | Melt hot pressing | 8.7 | 0.16 | [39] |
| MWCNT | 2.0 wt% | Melt hot pressing | 28 | 0.005 | [40] | |
| Polyvinylidene fluoride | MWCNT | 3.0 wt% | Electrospinning | 16.1 | 0.025 | [41] |
| poly (1-butene) | Graphene nanosheets | 2.0 wt% | Melt hot pressing | 33.9 | 1.5 | [18] |
| MWCNT | 7.0 wt% | Melt hot pressing | 70.0 | – | [19] | |
| MWCNT | 1.0 wt% | Solution casting | 43.9 | 0.19 | our work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Sun, Q.; Chen, X.; Xu, Y.; Jiang, Z. Crystallization and Dielectric Properties of MWCNT /Poly(1-Butene) Composite Films by a Solution Casting Method. Materials 2020, 13, 755. https://doi.org/10.3390/ma13030755
Li L, Sun Q, Chen X, Xu Y, Jiang Z. Crystallization and Dielectric Properties of MWCNT /Poly(1-Butene) Composite Films by a Solution Casting Method. Materials. 2020; 13(3):755. https://doi.org/10.3390/ma13030755
Chicago/Turabian StyleLi, Lingfei, Qiu Sun, Xiangqun Chen, Yongjun Xu, and Zhaohua Jiang. 2020. "Crystallization and Dielectric Properties of MWCNT /Poly(1-Butene) Composite Films by a Solution Casting Method" Materials 13, no. 3: 755. https://doi.org/10.3390/ma13030755




