Strength Development and Elemental Distribution of Dolomite/Fly Ash Geopolymer Composite under Elevated Temperature
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Sample Preparation
2.3. Testing and Chracterization Method
3. Results and Discussions
3.1. Microstructure Analysis
H+ + OH− ↔ H2O
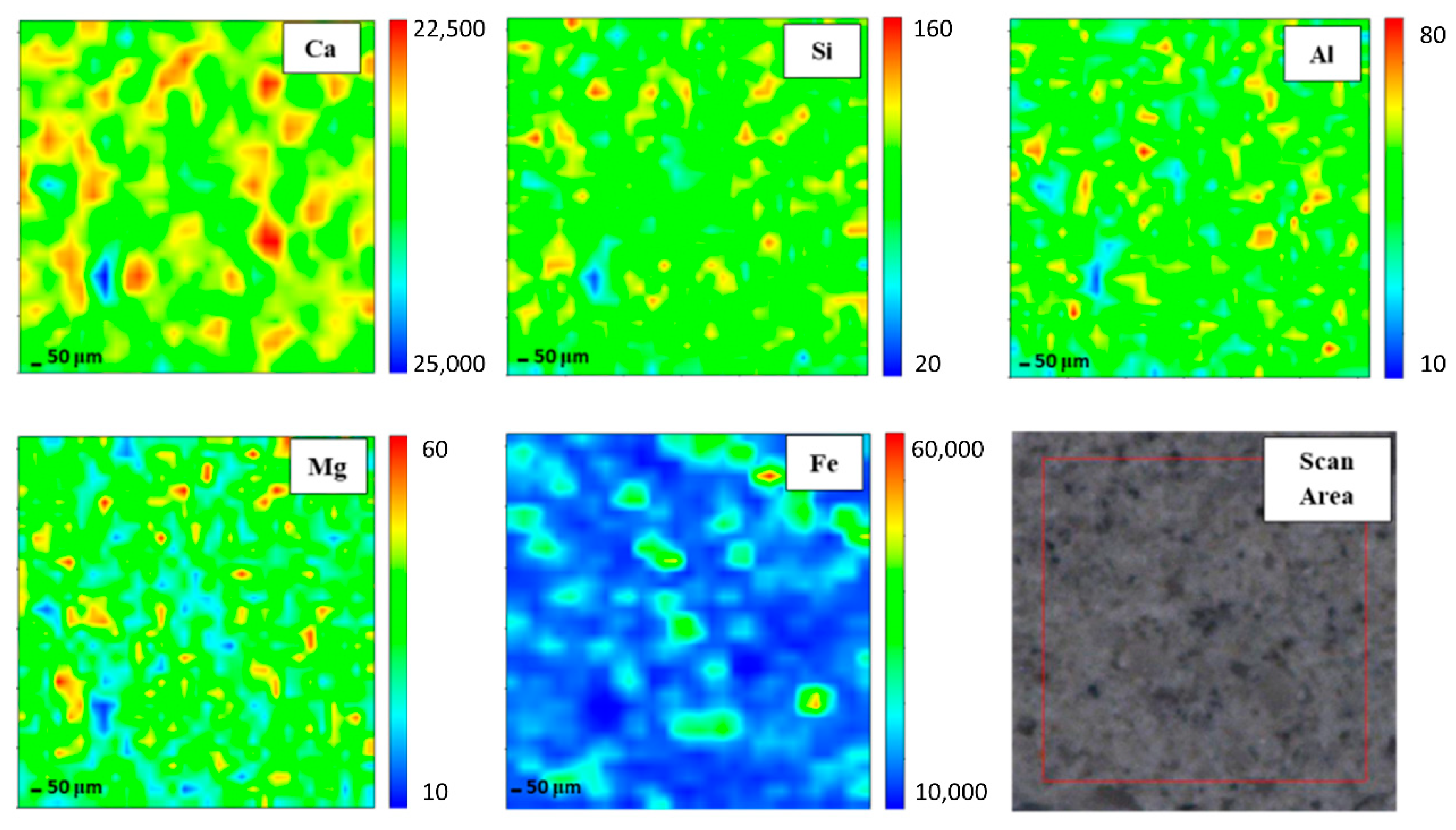
3.2. Elemental Distribution Analysis
3.3. Phase Analysis
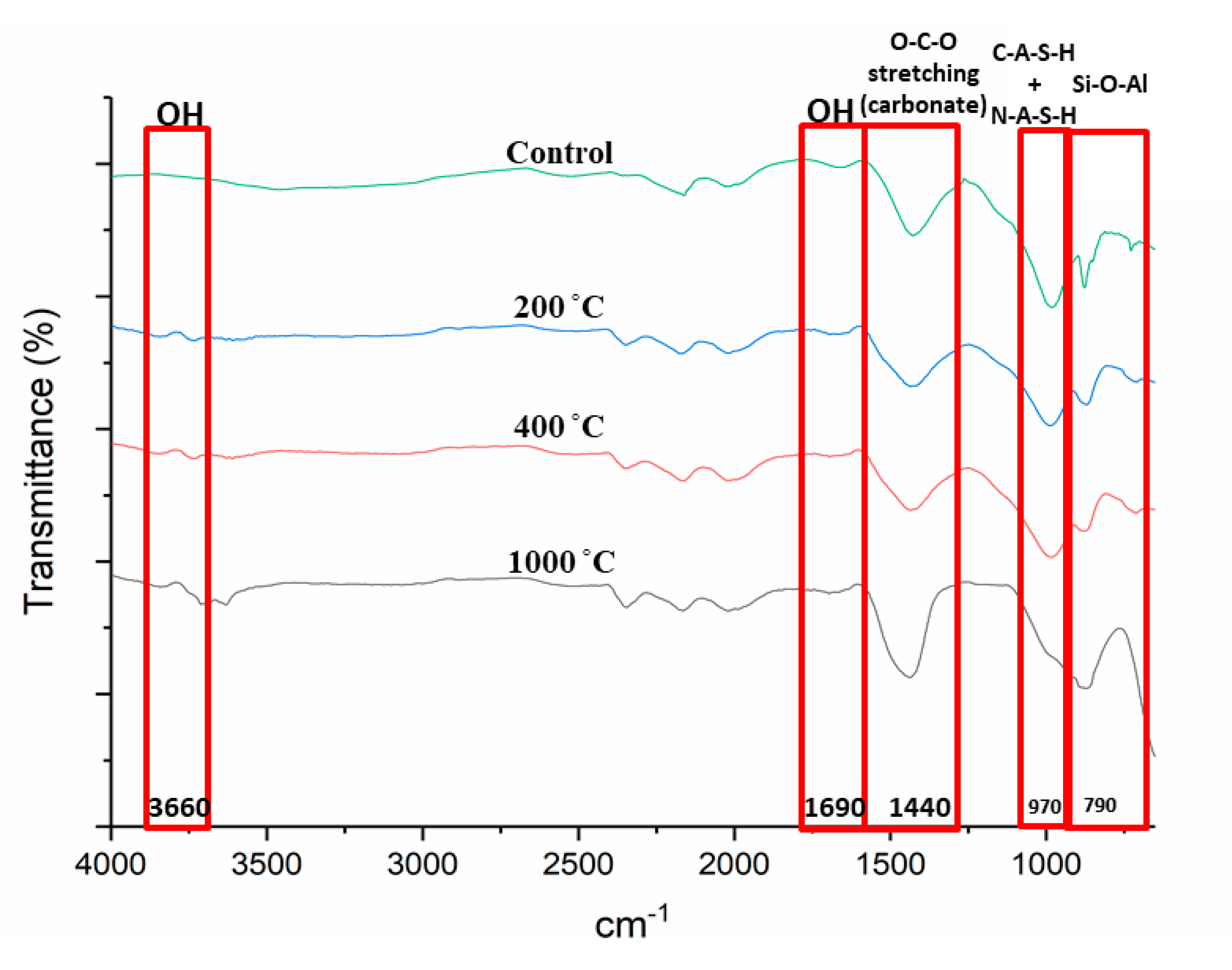
3.4. Functional Group Analysis
3.5. Compressive Strength
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sarker, P.K.; Kelly, S.; Yao, Z. Effect of fire exposure on cracking, spalling and residual strength of fly ash geopolymer concrete. Mater. Des. 2014, 63, 584–592. [Google Scholar] [CrossRef]
- Zeng, L.; Dan-yang, C.; Xu, Y.; Chun-wei, F.; Xiao-qin, P. Novel Method for Preparation of Calcined Kaolin Intercalation Compound Based Geopolymer. Appl. Clay Sci. 2014, 101, 637–642. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer Technology: The Current State of The Art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Application. Geopolym. Inst. 2008, 2, 585. [Google Scholar]
- Temuujin, J.; Rickard, W.; Lee, M.; van Riessen, A. Preparation and Thermal Properties of Fire Resistant Metakaolin Based Geopolymer Type Coatings. J. Non-Cryst. Solids 2011, 357, 1399–1404. [Google Scholar] [CrossRef]
- Coppola, L.; Coffetti, D.; Crotti, E. Pre-packed alkali activated cement-free mortars for repair of existinf masonry buildings and concrete structures. Constr. Build. Mater. 2018, 173, 111–117. [Google Scholar] [CrossRef]
- Burduhos Nergis, D.D.; Abdullah, M.M.A.B.; Sandu, A.; Vizureanu, P. XRD and TG-DTA Study of New Alkali Activated Materials Based on Fly Ash with Sand and Glass Powder. Materials 2020, 13, 343. [Google Scholar] [CrossRef] [Green Version]
- Ming, L.Y.; Sandu, A.V.; Yong, H.C.; Tajunnisa, Y.; Azzahran, S.F.; Bayuji, R.; Abdullah, M.M.A.B.; Vizureanu, P.; Hussin, K.; Jin, T.S.; et al. Compressive Strength and Thermal Conductivity of Fly Ash Geopolymer Concrete Incorporated with Lightweight Aggregate, Expanded Clay Aggregate and Foaming Agent. Rev. Chim. 2019, 70, 4021–4028. [Google Scholar] [CrossRef]
- Burduhos Nergis, D.D.; Vizureanu, P.; Corbu, O. Synthesis and Characteristics of Local Fly Ash Based Geopolymers Mixed with Natural Aggregates. Rev. Chim. 2019, 70, 1262–1267. [Google Scholar] [CrossRef]
- Shahedan, N.F.; Abdullah, M.M.A.B.; Mahmed, N.; Kusbiantoro, A.; Hussin, K.; Sandu, A.V.; Naveed, A. Thermal Insulation Properties of Insulated Concrete. Rev. Chim. 2019, 70, 3027–3031. [Google Scholar] [CrossRef]
- Burduhos Nergis, D.D.; Abdullah, M.M.A.B.; Vizureanu, P. The Effect of Fly Ash/Alkaline Activator Ratio in Class F Fly Ash Based Geopolymers. Eur. J. Mater. Sci. Eng. 2017, 2, 111–118. [Google Scholar]
- Bouaissi, A.; LI, L.Y.; Moga, L.M.; Sandu, I.G.; Abdullah, M.M.A.B.; Sandu, A.V. A Review on Fly Ash as a Raw Cementitious Material for Geopolymer Concrete. Rev. Chim. 2018, 69, 1661–1667. [Google Scholar] [CrossRef]
- Burduhos Nergis, D.D.; Abdullah, M.M.A.B.; Vizureanu, P.; Faheem, M.T.M. Geopolymers and Their Uses: Review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 012019. [Google Scholar] [CrossRef]
- Burduhos Nergis, D.D.; Vizureanu, P.; Andrusca, L.; Achitei, D. Performance of local fly ash geopolymers under different types of acids. IOP Conf. Ser. Mater. Sci. Eng. 2019, 572, 012026. [Google Scholar] [CrossRef]
- Jun, N.H.; Minciuna, M.G.; Abdullah, M.M.A.; Jin, T.S.; Sandu, A.V.; Ming, L.Y. Mechanism of Cement Paste with Different Particle Sizes of Bottom Ash as Partial replacement in Portland Cement. Rev. Chim. 2017, 68, 2367–2372. [Google Scholar] [CrossRef]
- Al-Majidi, M.H.; Lampropoulos, A.; Cundy, A.; Meikle, S. Development of geopolymer mortar under ambient temperature for in situ applications. Constr. Build. Mater. 2016, 120, 198–211. [Google Scholar] [CrossRef] [Green Version]
- Talha Junaid, M.; Kayali, O.; Khennane, A. Response of alkali activated low calcium fly-ash based geopolymer concrete under compressive load at elevated temperatures. Mater. Struct. 2016, 50, 50. [Google Scholar] [CrossRef]
- Sitarz, M.; Hager, I.; Kochanek, J. Effect of High Temperature on Mechanical Properties of Geopolymer Mortar. MATEC Web Conf. 2018, 163, 06004. [Google Scholar] [CrossRef]
- Hamidi, R.M.; Man, Z.; Azizli, K.A. Concentration of NaOH and the Effect on the Properties of Fly Ash Based Geopolymer. Procedia Eng. 2016, 148, 189–193. [Google Scholar] [CrossRef] [Green Version]
- Morsy, M.; Alsayed, S.; Al-Salloum, Y.; Almusallam, T. Effect of Sodium Silicate to Sodium Hydroxide Ratios on Strength and Microstructure of Fly Ash Geopolymer Binder. Arab. J. Sci. Eng. 2014, 39, 4333–4339. [Google Scholar] [CrossRef]
- Zarina, Y.; Kamarudin, H.; Al Bakri, A.M.M.; Khairul Nizar, I.; Rafiza, A.R. Influence of Dolomite on The Mechanical Properties of Boiler Ash Geopolymer Paste. Key Eng. Mater. 2014, 594, 8–12. [Google Scholar] [CrossRef]
- Yip, C.K.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. Carbonate Mineral Addition to Metakaolin-Based Geopolymers. Cem. Concr. Compos. 2008, 30, 979–985. [Google Scholar] [CrossRef]
- Valencia Saavedra, W.G.; Mejía de Gutiérrez, R. Performance of Geopolymer Concrete Composed of Fly Ash After Exposure to Elevated Temperatures. Constr. Build. Mater. 2017, 154, 229–235. [Google Scholar] [CrossRef]
- Ahmari, S.; Zhang, L. The properties and durability of mine tailings-based geopolymeric masonry blocks. In Eco-Efficient Masonry Bricks and Blocks; Pacheco-Torgal, F., Lourenço, P.B., Labrincha, J.A., Kumar, S., Chindaprasirt, P., Eds.; Woodhead Publishing: Oxford, UK, 2015. [Google Scholar]
- Zulkifly, K.; Yong, H.; Abdullah, M.; Ming, L.; Panias, D.; Sakkas, K. Review of Geopolymer Behaviour in Thermal Environment. IOP Conf. Ser. Mater. Sci. Eng. 2017, 209, 012085. [Google Scholar] [CrossRef]
- Walkley, B.; San Nicolas, R.; Sani, M.-A.; Bernal, S.A.; van Deventer, J.S.J.; Provis, J.L. Structural Evolution of Synthetic Alkali Activated CaO-MgO-Na2O-Al2O3-SiO2 Materials is Influenced by Mg Content. Cem. Concr. Res. 2017, 99, 155–171. [Google Scholar] [CrossRef]
- Bernal, S.A.; San Nicolas, R.; Myers, R.J.; Mejía de Gutiérrez, R.; Puertas, F.; van Deventer, J.S.J.; Provis, J.L. MgO Content of Slag Controls Phase Evolution and Structural Changes Induced by Accelerated Carbonation in Alkali Activated Binders. Cem. Concr. Res. 2014, 57, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Sudbrink, B.; Khanzadeh Moradllo, M.; Hu, Q.; Ley, M.T.; Davis, J.M.; Materer, N.; Apblett, A. Imaging the presence of silane coatings in concrete with micro X-ray fluorescence. Cem. Concr. Res. 2017, 92, 121–127. [Google Scholar] [CrossRef]
- Heller-Kallai, L. Chapter 10.2—Thermally Modified Clay Minerals. In Developments in Clay Science; Bergaya, F., LAGALY, G., Eds.; Elsevier: Jerusalem, Israel, 2013. [Google Scholar]
- Clegg, F.; Breen, C.; Carter, M.A.; Ince, C.; Savage, S.D.; Wilson, M.A. Dehydroxylation and Rehydroxylation Mechanisms in Fired Clay Ceramic: A TG-MS and DRIFTS Investigation. J. Am. Ceram. Soc. 2012, 95, 416–422. [Google Scholar] [CrossRef]
- Salleh, M.M.; McDonald, S.; Terada, Y.; Yasuda, H.; Nogita, K. Development of a microwave sintered TiO2 reinforced Sn–0.7 wt% Cu–0.05 wt% Ni alloy. Mater. Des. 2015, 82, 136–147. [Google Scholar] [CrossRef]
- Giergiczny, Z. Fly ash and slag. Cem. Concr. Res. 2019, 124, 105826. [Google Scholar] [CrossRef]
- Phoo-ngernkham, T.; Maegawa, A.; Mishima, N.; Hatanaka, S.; Chindaprasirt, P. Effects of Sodium Hydroxide and Sodium Silicate Solutions on Compressive and Shear Bond Strengths of FA–GBFS Geopolymer. Constr. Build. Mater. 2015, 91, 1–8. [Google Scholar] [CrossRef]
- Machner, A.; Zajac, M.; Ben Haha, M.; Kjellsen, K.O.; Geiker, M.R.; De Weerdt, K. Chloride-binding capacity of hydrotalcite in cement pastes containing dolomite and metakaolin. Cem. Concr. Res. 2018, 107, 163–181. [Google Scholar] [CrossRef]
- Ke, X.; Bernal, S.A.; Provis, J.L. Controlling the reaction kinetics of sodium carbonate-activated slag cements using calcined layered double hydroxides. Cem. Concr. Res. 2016, 81, 24–37. [Google Scholar] [CrossRef] [Green Version]
- Kuenzel, C.; Grover, L.M.; Vandeperre, L.; Boccaccini, A.R.; Cheeseman, C.R. Production of Nepheline/Quartz Ceramics from Geopolymer Mortars. J. Eur. Ceram. Soc. 2014, 33, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.J.; Li, S.; Wang, Y.C.; Xu, D.L. Microstructural and Strength Evolutions of Geopolymer Composite Reinforced by Resin Exposed to Elevated Temperature. J. Non-Cryst. Solids 2012, 358, 620–624. [Google Scholar] [CrossRef]
- Abo Sawan, S.E.; Zawrah, M.F.; Khattab, R.M.; Abdel-Shafi, A.A. Fabrication, sinterabilty and characterization of non-colored and colored geopolymers with improved properties. Mater. Res. Express 2019, 6, 075205. [Google Scholar] [CrossRef]
- Zaharaki, D.; Komnitsas, K.; Perdikatsis, V. Use of Analytical Techniques for Identification of Inorganic Polymer Gel Composition. J. Mater. Sci. 2010, 45, 2715–2724. [Google Scholar] [CrossRef]
- Assaedi, H.; Shaikh, F.U.A.; Low, I.M. Effect of Nano-clay on Mechanical and Thermal Properties of Geopolymer. J. Asian Ceram. Soc. 2016, 4, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Peyne, J.; Gautron, J.; Doudeau, J.; Rossignol, S. Development of Low Temperature Lightweight Geopolymer Aggregate, From Industrial Waste, in Comparison With High Temperature Processed Aggregates. J. Clean. Prod. 2018, 189, 47–58. [Google Scholar] [CrossRef]
- Part, W.K.; Ramli, M.; Cheah, C.B. An overview on the influence of various factors on the properties of geopolymer concrete derived from industrial by-products. Constr. Build. Mater. 2015, 77, 370–395. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, S.; Singh, L.P. Studies on Enhanced Thermally Stable High Strength Concrete Incorporating Silica Nanoparticles. Constr. Build. Mater. 2017, 153, 506–513. [Google Scholar] [CrossRef]
- Yuan, P. Chapter 7—Thermal-Treatment-Induced Deformations and Modifications of Halloysite. In Developments in Clay Science; Yuan, P., Thill, A., Bergaya, F., Eds.; Elsevier: Guangzhou, China, 2016. [Google Scholar]
- Kong, D.L.Y.; Sanjayan, J.G. Effect of Elevated Temperatures on Geopolymer Paste, Mortar and Concrete. Cem. Concr. Res. 2010, 40, 334–339. [Google Scholar] [CrossRef]





| Chemical Compound | Composition (wt %) |
|---|---|
| CaO | 80.21 |
| Al2O3 | 1.52 |
| SiO2 | 2.50 |
| Fe2O3 | 0.15 |
| MgO | 15.50 |
| CuO | 0.07 |
| MnO | 0.02 |
| Chemical Compound | Composition (wt %) |
|---|---|
| SiO2 | 55.3 |
| Al2O3 | 25.8 |
| Fe2O3 | 5.5 |
| CaO | 2.9 |
| MgO | 0.8 |
| SO3 | 0.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azimi, E.A.; Abdullah, M.M.A.B.; Vizureanu, P.; Salleh, M.A.A.M.; Sandu, A.V.; Chaiprapa, J.; Yoriya, S.; Hussin, K.; Aziz, I.H. Strength Development and Elemental Distribution of Dolomite/Fly Ash Geopolymer Composite under Elevated Temperature. Materials 2020, 13, 1015. https://doi.org/10.3390/ma13041015
Azimi EA, Abdullah MMAB, Vizureanu P, Salleh MAAM, Sandu AV, Chaiprapa J, Yoriya S, Hussin K, Aziz IH. Strength Development and Elemental Distribution of Dolomite/Fly Ash Geopolymer Composite under Elevated Temperature. Materials. 2020; 13(4):1015. https://doi.org/10.3390/ma13041015
Chicago/Turabian StyleAzimi, Emy Aizat, Mohd Mustafa Al Bakri Abdullah, Petrica Vizureanu, Mohd Arif Anuar Mohd Salleh, Andrei Victor Sandu, Jitrin Chaiprapa, Sorachon Yoriya, Kamarudin Hussin, and Ikmal Hakem Aziz. 2020. "Strength Development and Elemental Distribution of Dolomite/Fly Ash Geopolymer Composite under Elevated Temperature" Materials 13, no. 4: 1015. https://doi.org/10.3390/ma13041015








