Superhydrophobic Methylated Silica Sol for Effective Oil–Water Separation
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
3. Results and Discussion
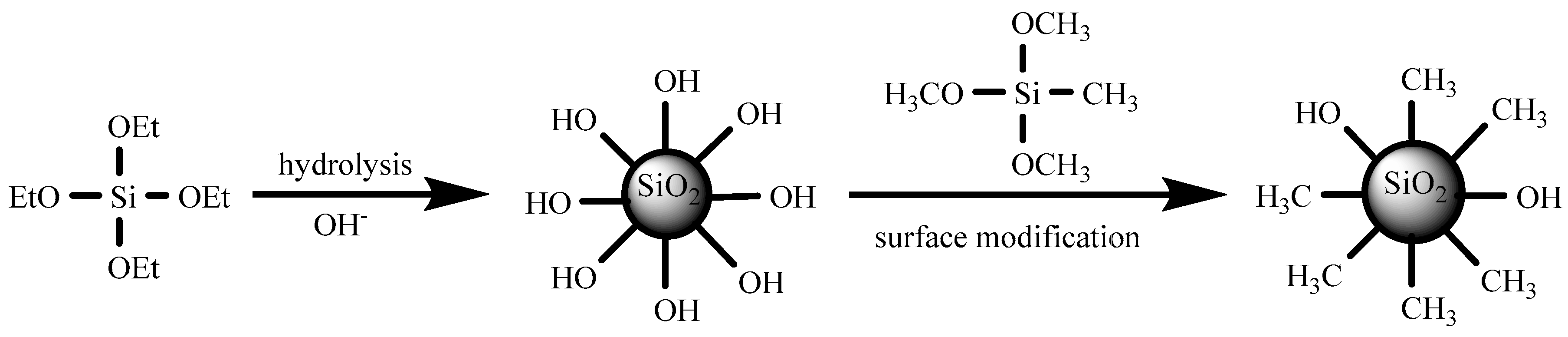
3.1. Reaction Mechanism of Superhydrophobic Silica Sol
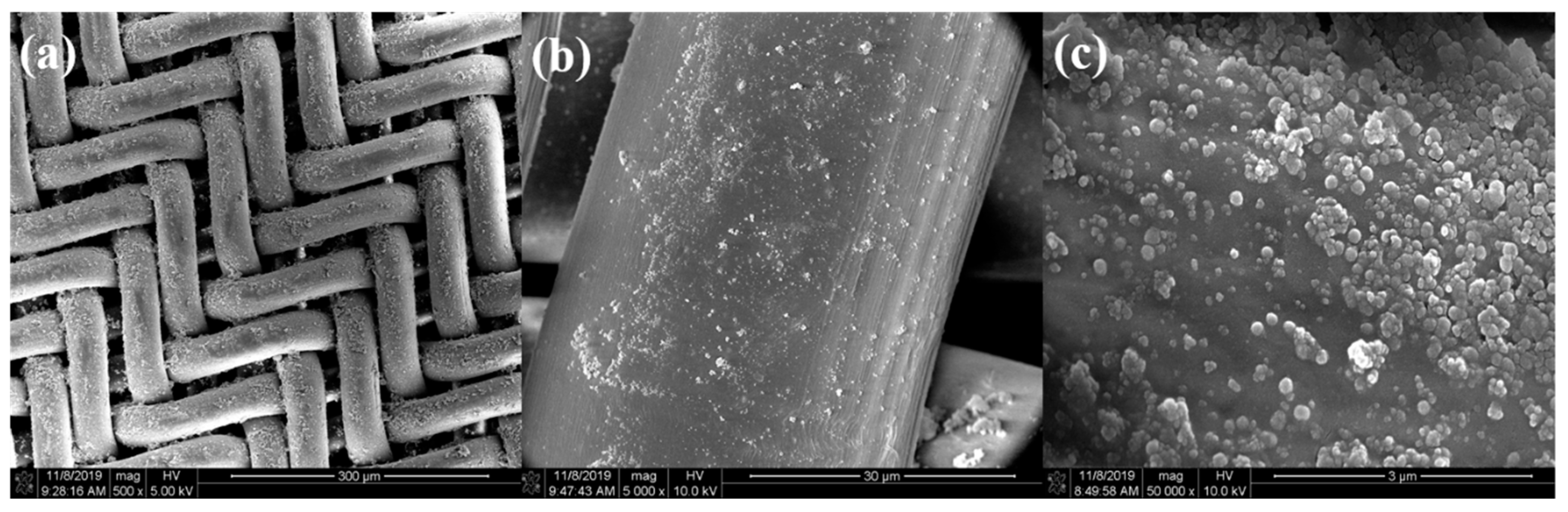
3.2. Morphology of the Pristine and Methylated Silica Coatings
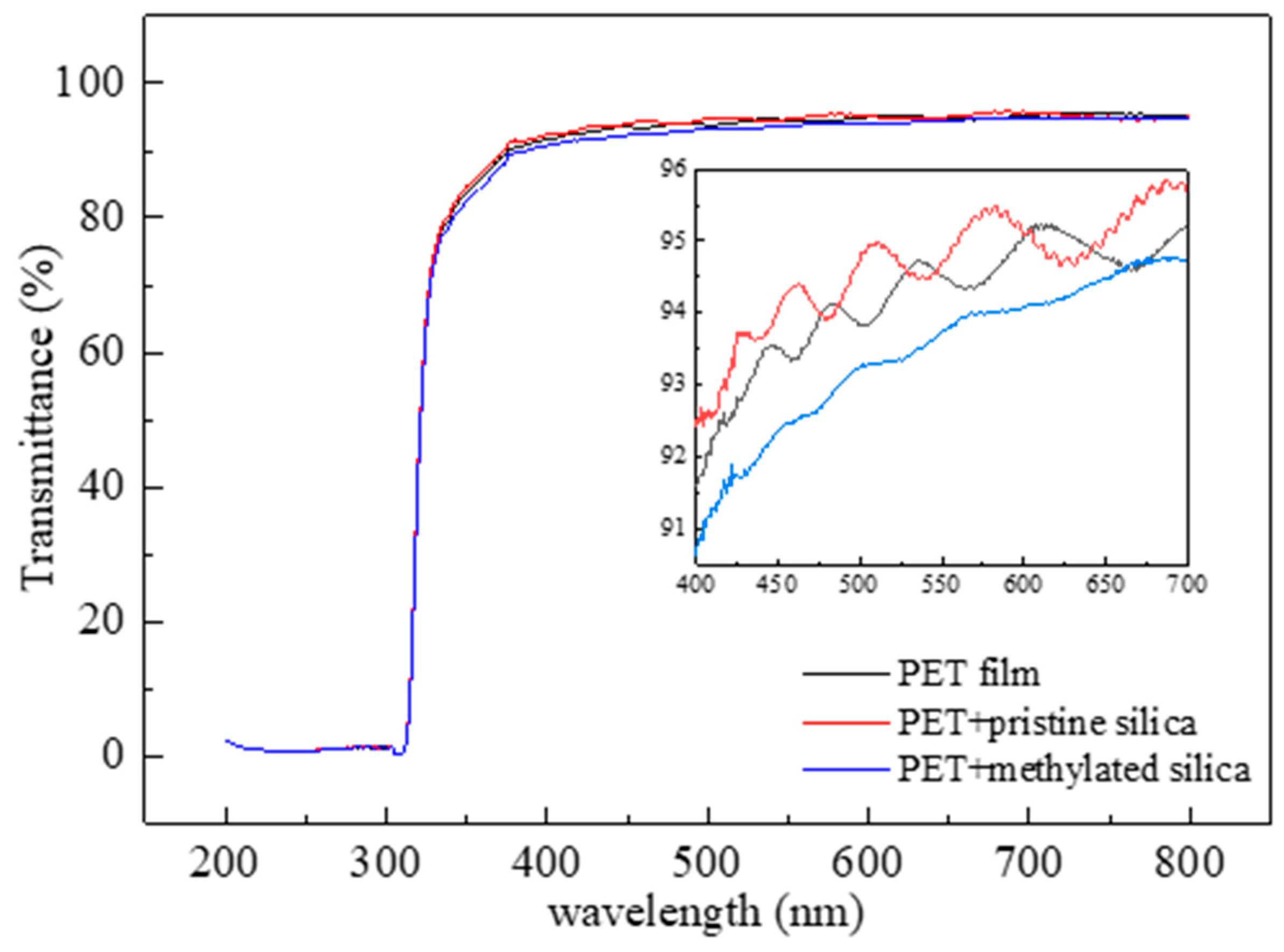
3.3. Wetting Behavior and Corresponding Application Demonstrations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-Hydrophobic Surfaces: From Natural to Artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Brown, P.S.; Bhushan, B. Bioinspired, roughness-induced, water and oil super-philic and super-phobic coatings prepared by adaptable layer-by-layer technique. Sci. Rep. 2015, 5, 14030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, N.; Wang, J.; Chen, Q.; Zhao, S.; Zhang, H.; Sun, H.; Jiang, L. Three-Level Biomimetic Rice-Leaf Surfaces with Controllable Anisotropic Sliding. Adv. Funct. Mater. 2011, 21, 2927–2932. [Google Scholar] [CrossRef]
- Kulinich, S.A.; Farzaneh, M. How Wetting Hysteresis Influences Ice Adhesion Strength on Superhydrophobic Surfaces. Langmuir 2009, 25, 8854–8856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Z.; Liu, K.; Jiang, L. Bioinspired Multifunctional Foam with Self-Cleaning and Oil/Water Separation. Adv. Funct. Mater. 2013, 23, 2881–2886. [Google Scholar] [CrossRef]
- Gui, X.; Wei, J.; Wang, K.; Cao, A.; Zhu, H.; Jia, Y.; Shu, Q.; Wu, D. Carbon Nanotube Sponges. Adv. Mater. 2010, 22, 617–621. [Google Scholar] [CrossRef]
- Lu, Y.; Sathasivam, S.; Song, J.; Crick, C.R.; Carmalt, C.; Parkin, I.P. Robust self-cleaning surfaces that function when exposed to either air or oil. Science 2015, 347, 1132–1135. [Google Scholar] [CrossRef]
- Gupta, R.K.; Dunderdale, G.J.; England, M.W.; Hozumi, A. Oil/water separation techniques: A review of recent progresses and future directions. J. Mater. Chem. A 2017, 5, 16025–16058. [Google Scholar] [CrossRef]
- Artus, G.R.; Zimmermann, J.; Reifler, F.A.; Brewer, S.A.; Seeger, S. A superoleophobic textile repellent towards impacting drops of alkanes. Appl. Surf. Sci. 2012, 258, 3835–3840. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.-P.; Peng, J.; Liu, Y.; Wen, Y.; Zhang, X.; Jiang, L.; Wang, S. Nacre-Inspired Design of Mechanical Stable Coating with Underwater Superoleophobicity. ACS Nano 2013, 7, 5077–5083. [Google Scholar] [CrossRef]
- Li, D.; Guo, Z. Versatile superamphiphobic cotton fabrics fabricated by coating with SiO2 /FOTS. Appl. Surf. Sci. 2017, 426, 271–278. [Google Scholar] [CrossRef]
- Li, F.; Du, M.; Zheng, Q. Dopamine/Silica Nanoparticle Assembled, Microscale Porous Structure for Versatile Superamphiphobic Coating. ACS Nano 2016, 10, 2910–2921. [Google Scholar] [CrossRef] [PubMed]
- Saifaldeen, Z.S.; Khedir, Z.D.; Cansizoglu, K.R.; Demirkan, M.F.; Karabacak, T. Superamphiphobic aluminum alloy surfaces with micro and nanoscale hierarchical roughness produced by a simple and environmentally friendly technique. J. Mater. Sci. 2014, 49, 1839–1853. [Google Scholar] [CrossRef]
- Xu, W.; Song, J.; Sun, J.; Lu, Y.; Yu, Z. Rapid Fabrication of Large-Area, Corrosion-Resistant Superhydrophobic Mg Alloy Surfaces. ACS Appl. Mater. Interfaces 2011, 3, 4404–4414. [Google Scholar] [CrossRef] [PubMed]
- Amigoni, S.; Taffin de Givenchy, E.; Dufay, M.; Guittard, F.d.R. Covalent Layer-by-Layer Assembled Superhydrophobic Organic? Inorganic Hybrid Films. Langmuir 2009, 25, 11073–11077. [Google Scholar] [CrossRef] [PubMed]
- Rahmawan, Y.; Xu, L.; Yang, S. Self-assembly of nanostructures towards transparent, superhydrophobic surfaces. J. Mater. Chem. A 2013, 1, 2955–2969. [Google Scholar] [CrossRef]
- Genzer, J.; Efimenko, K. Creating Long-Lived Superhydrophobic Polymer Surfaces Through Mechanically Assembled Monolayers. Science 2000, 290, 2130–2133. [Google Scholar] [CrossRef] [Green Version]
- Su, C.; Li, Y.; Dai, Y.; Gao, F.; Tang, K.; Cao, H. Fabrication of three-dimensional superhydrophobic membranes with high porosity via simultaneous electrospraying and electrospinning. Mater. Lett. 2016, 170, 67–71. [Google Scholar] [CrossRef]
- Wu, J.; Li, X.; Wu, Y.; Liao, G.; Johnston, P.; Topham, P.D.; Wang, L. Rinse-resistant superhydrophobic block copolymer fabrics by electrospinning, electrospraying and thermally-induced self-assembly. Appl. Surf. Sci. 2017, 422, 769–777. [Google Scholar] [CrossRef] [Green Version]
- Gengec, N.A.; Cengiz, U.; Erbil, H. Superhydrophobic perfluoropolymer/polystyrene blend films induced by nonsolvent. Appl. Surf. Sci. 2016, 383, 33–41. [Google Scholar] [CrossRef]
- Rao, A.V.; Latthe, S.S.; Nadargi, D.Y.; Hirashima, H.; Ganesan, V. Preparation of MTMS based transparent superhydrophobic silica films by sol–gel method. J. Colloid Interface Sci. 2009, 332, 484–490. [Google Scholar]
- Mahadik, S.A.; Mahadik, D.B.; Kavale, M.S.; Parale, V.G.; Wagh, P.B.; Barshilia, H.C.; Gupta, S.C.; Hegde, N.D.; Rao, A.V. Thermally stable and transparent superhydrophobic sol–gel coatings by spray method. J. Sol-Gel Sci. Technol. 2012, 63, 580–586. [Google Scholar] [CrossRef]
- Kumar, D.; Wu, X.; Fu, Q.; Ho, J.W.C.; Kanhere, P.D.; Li, L.; Chen, Z. Development of durable self-cleaning coatings using organic–inorganic hybrid sol–gel method. Appl. Surf. Sci. 2015, 344, 205–212. [Google Scholar] [CrossRef]
- Van Blaaderen, A.; Vrij, A. Synthesis and Characterization of Monodisperse Colloidal Organo-silica Spheres. J. Colloid Interface Sci. 1993, 156, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Van Blaaderen, A.; Kentgens, A. Particle morphology and chemical microstructure of colloidal silica spheres made from alkoxysilanes. J. Non-Cryst. Solids 1992, 149, 161–178. [Google Scholar] [CrossRef]
- Wang, S.; Liu, K.; Yao, X.; Jiang, L. Bioinspired Surfaces with Superwettability: New Insight on Theory, Design, and Applications. Chem. Rev. 2015, 115, 8230–8293. [Google Scholar] [CrossRef]
- Tian, H.; Gao, X.; Yang, T.; Li, D.; Chen, Y. Fabrication and characterization of superhydrophobic silica nanotrees. J. Sol-Gel Sci. Technol. 2008, 48, 277–282. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Wang, H.-J.; Liang, Y.-H.; Li, X.-J.; Ren, L.-Q.; Cui, Z.-Q.; Luo, C. One-step fabrication of robust superhydrophobic and superoleophilic surfaces with self-cleaning and oil/water separation function. Sci. Rep. 2018, 8, 3869. [Google Scholar] [CrossRef] [Green Version]
- Pilotek, S.; Schmidt, H. Wettability of Microstructured Hydrophobic Sol-Gel Coatings. J. Sol-Gel Sci. Technol. 2003, 26, 789–792. [Google Scholar] [CrossRef]
- Shang, H.M.; Wang, Y.; Takahashi, K.; Cao, G.Z.; Li, D.; Xia, Y.N. Nanostructured superhydrophobic surfaces. J. Mater. Sci. 2005, 40, 3587–3591. [Google Scholar] [CrossRef]
- Tadanaga, K.; Kitamuro, K.; Matsuda, A.; Minami, T. Formation of Superhydrophobic Alumina Coating Films with High Transparency on Polymer Substrates by the Sol-Gel Method. J. Sol-Gel Sci. Technol. 2003, 26, 705–708. [Google Scholar] [CrossRef]
- Shang, H.; Wang, Y.; Limmer, S.; Chou, T.; Takahashi, K.; Cao, G. Optically transparent superhydrophobic silica-based films. Thin Solid Films 2005, 472, 37–43. [Google Scholar] [CrossRef]
- Akamatsu, Y.; Makita, K.; Inaba, H.; Minami, T. Water-repellent coating films on glass prepared from hydrolysis and polycondensation reactions of fluoroalkyltrialkoxylsilane. Thin Solid Films 2001, 389, 138–145. [Google Scholar] [CrossRef]
- Kron, J.; Schottner, G.; Deichmann, K.-J. Glass design via hybrid sol–gel materials. Thin Solid Films 2001, 392, 236–242. [Google Scholar] [CrossRef]
- Satoh, K.; Nakazumi, H.; Morita, M. Preparation of Super-Water-Repellent Fluorinated Inorganic-Organic Coating Films on Nylon 66 by the Sol-Gel Method Using Microphase Separation. J. Sol-Gel Sci. Technol. 2003, 27, 327–332. [Google Scholar] [CrossRef]
- Gu, G.; Dang, H.; Zhang, Z.; Wu, Z. Fabrication and characterization of transparent superhydrophobic thin films based on silica nanoparticles. Appl. Phys. A 2006, 83, 131–132. [Google Scholar] [CrossRef]
- Bossi, R.; Rigét, F.F.; Dietz, R.; Sonne, C.; Fauser, P.; Dam, M.; Vorkamp, K. Preliminary screening of perfluorooctane sulfonate (PFOS) and other fluorochemicals in fish, birds and marine mammals from Greenland and the Faroe Islands. Environ. Pollut. 2005, 136, 323–329. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, Z.; Zhang, F.; Liu, X.; Jin, J.; Jiang, L. Superhydrophobic and Superoleophilic PVDF Membranes for Effective Separation of Water-in-Oil Emulsions with High Flux. Adv. Mater. 2013, 25, 2071–2076. [Google Scholar] [CrossRef]
- Li, K.; Zeng, X.; Li, H.; Lai, X. Facile fabrication of a robust superhydrophobic/superoleophilic sponge for selective oil absorption from oily water. RSC Adv. 2014, 4, 23861. [Google Scholar] [CrossRef]
- Yang, H.; Pi, P.; Cai, Z.-Q.; Wen, X.; Wang, X.; Cheng, J.; Yang, Z.-r. Facile preparation of super-hydrophobic and super-oleophilic silica film on stainless steel mesh via sol–gel process. Appl. Surf. Sci. 2010, 256, 4095–4102. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, N.; Jiang, L.; Jin, J. Recent progress in developing advanced membranes for emulsified oil/water separation. NPG Asia Mater. 2014, 6, e101. [Google Scholar] [CrossRef]
- Wang, J.; Wang, A.; Wang, W. Robustly superhydrophobic/superoleophilic kapok fiber with ZnO nanoneedles coating: Highly efficient separation of oil layer in water and capture of oil droplets in oil-in-water emulsions. Ind. Crops Prod. 2017, 108, 303–311. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Lin, J.; Chen, H.; Fei, T.; Zhang, J. Highly transparent superhydrophobic organic–inorganic nanocoating from the aggregation of silica nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2013, 421, 51–62. [Google Scholar] [CrossRef]
- Nakamura, M.; Ozaki, S.; Abe, M.; Doi, H.; Matsumoto, T.; Ishimura, K. Size-controlled synthesis, surface functionalization, and biological applications of thiol-organosilica particles. Colloids Surf. B Biointerfaces 2010, 79, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Xu, J.; Xie, L. Ultra low-dielectric-constant methylated mesoporous silica films with high hydrophobicity and stability. Mater. Chem. Phys. 2011, 129, 1195–1200. [Google Scholar] [CrossRef]
- Mahadik, S.A.; Kavale, M.S.; Mukherjee, S.; Rao, A.V. Transparent Superhydrophobic silica coatings on glass by sol–gel method. Appl. Surf. Sci. 2010, 257, 333–339. [Google Scholar] [CrossRef]
- Chu, Z.; Seeger, S. Superamphiphobic surfaces. Chem. Soc. Rev. 2014, 43, 2784–2798. [Google Scholar] [CrossRef]
- Liu, M.; Wang, S.; Jiang, L. Nature-inspired superwettability systems. Nat. Rev. Mater. 2017, 2, 17036. [Google Scholar] [CrossRef]
- Tuteja, A.; Choi, W.; Ma, M.; Mabry, J.M.; Mazzella, S.A.; Rutledge, G.C.; McKinley, G.H.; Cohen, R.E. Designing Superoleophobic Surfaces. Science 2007, 318, 1618–1622. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Wang, Z.; Huang, S.; Pan, Y.; Zhao, X. Flexible, Durable, and Unconditioned Superoleophobic/Superhydrophilic Surfaces for Controllable Transport and Oil-Water Separation. Adv. Funct. Mater. 2018, 28, 1706867. [Google Scholar] [CrossRef]
- Metın, D.; Tihminlioğlu, F.; Balkose, D.; Ülkü, S.; Métin, D.; Tıhmınlıoğlu, F. The effect of interfacial interactions on the mechanical properties of polypropylene/natural zeolite composites. Compos. Part A Appl. Sci. Manuf. 2004, 35, 23–32. [Google Scholar] [CrossRef] [Green Version]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Ding, H.; Zhang, H.; Guo, C.; Hong, X.; Sun, L.; Ding, F. Superhydrophobic Methylated Silica Sol for Effective Oil–Water Separation. Materials 2020, 13, 842. https://doi.org/10.3390/ma13040842
Li J, Ding H, Zhang H, Guo C, Hong X, Sun L, Ding F. Superhydrophobic Methylated Silica Sol for Effective Oil–Water Separation. Materials. 2020; 13(4):842. https://doi.org/10.3390/ma13040842
Chicago/Turabian StyleLi, Jiao, Hao Ding, Heqiang Zhang, Chunlin Guo, Xiaoyan Hong, Luyi Sun, and Fuchuan Ding. 2020. "Superhydrophobic Methylated Silica Sol for Effective Oil–Water Separation" Materials 13, no. 4: 842. https://doi.org/10.3390/ma13040842




