Synthesis of ZnO-NPs Using a Convolvulus arvensis Leaf Extract and Proving Its Efficiency as an Inhibitor of Carbon Steel Corrosion
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Preparation of the Convolvulus Arvensis Extract
2.3. Synthesis of ZnO-NPs
2.4. Preparation of the Test Solution
2.5. Preparation of Carbon Steel Specimens
2.6. Characterization of ZnO-NPs
2.7. Surface Characterization
2.8. Weight Loss Method
2.9. Electrochemical Measurements
3. Results and Discussion
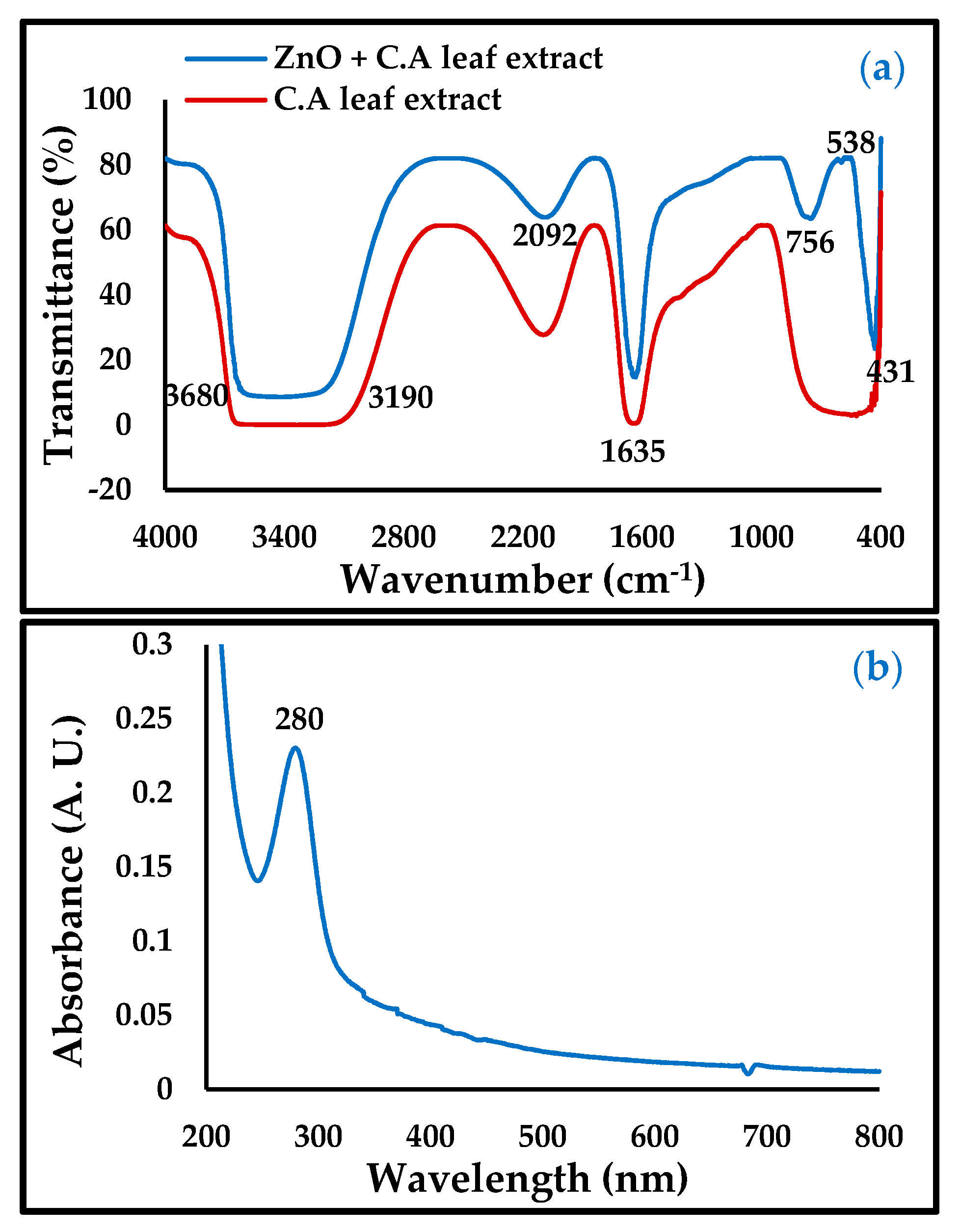
3.1. Characterization of ZnO-NPs
3.2. Weight Loss Method
3.3. Electrochemical Measurements
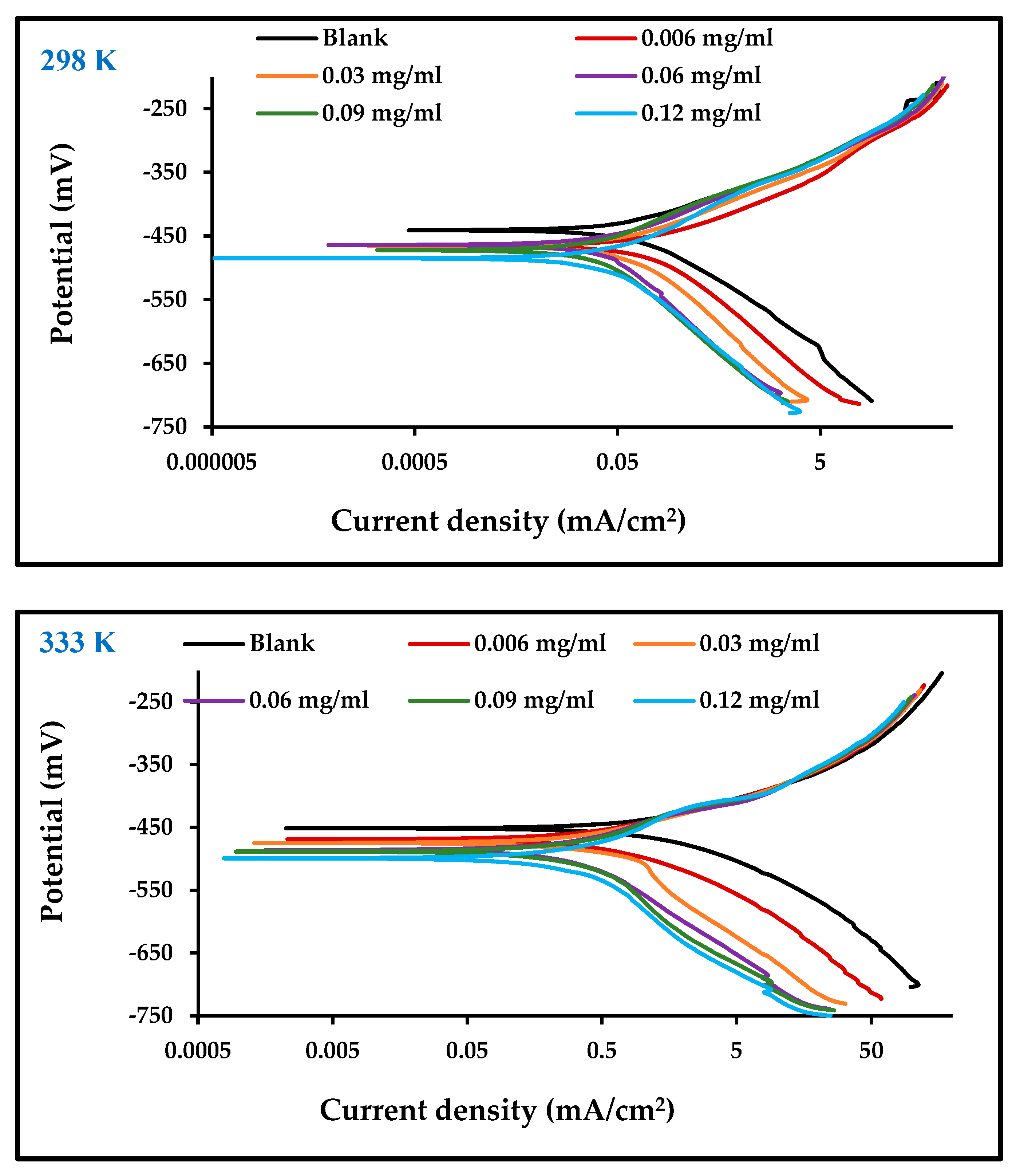
3.3.1. Potentiodynamic Polarization Measurements
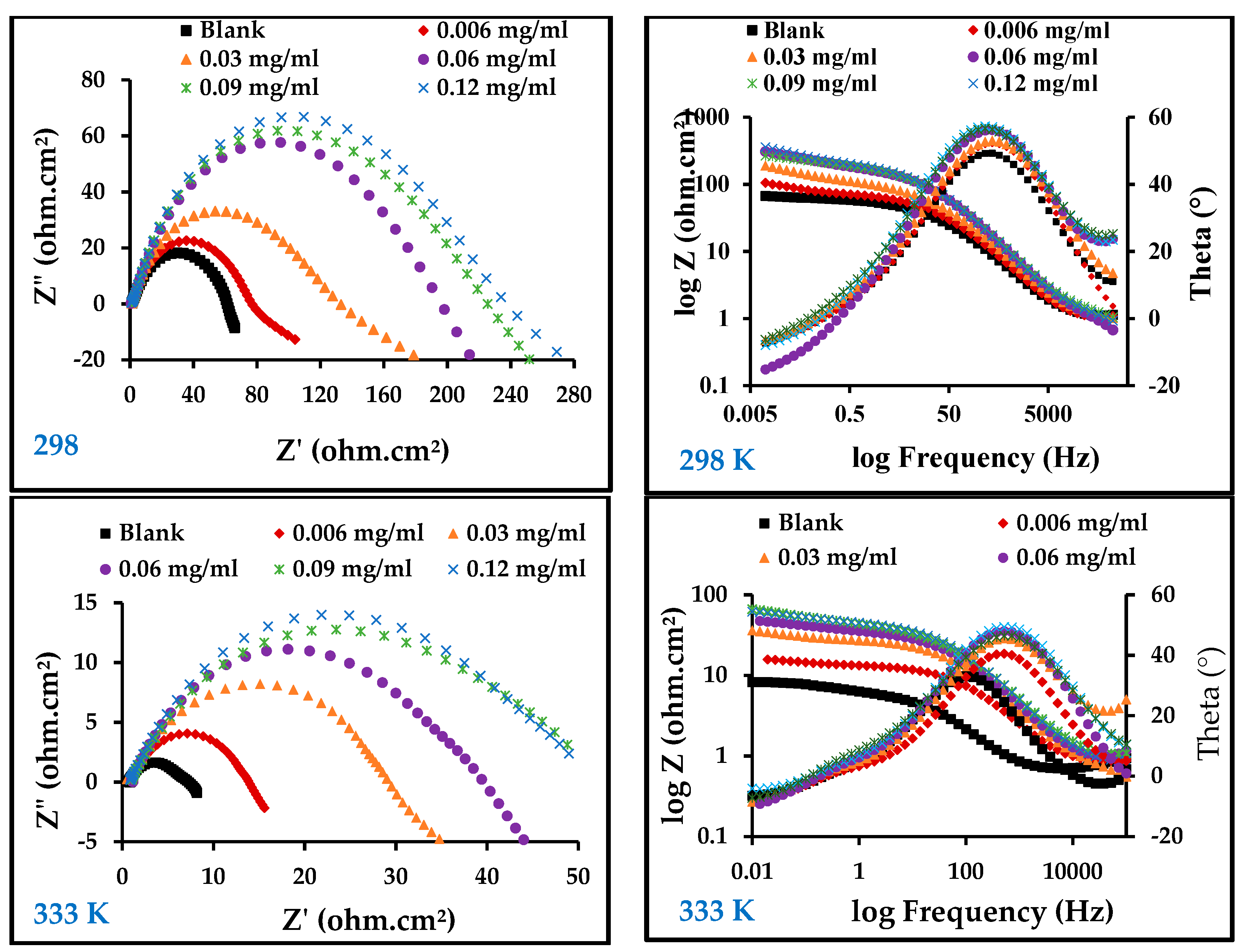
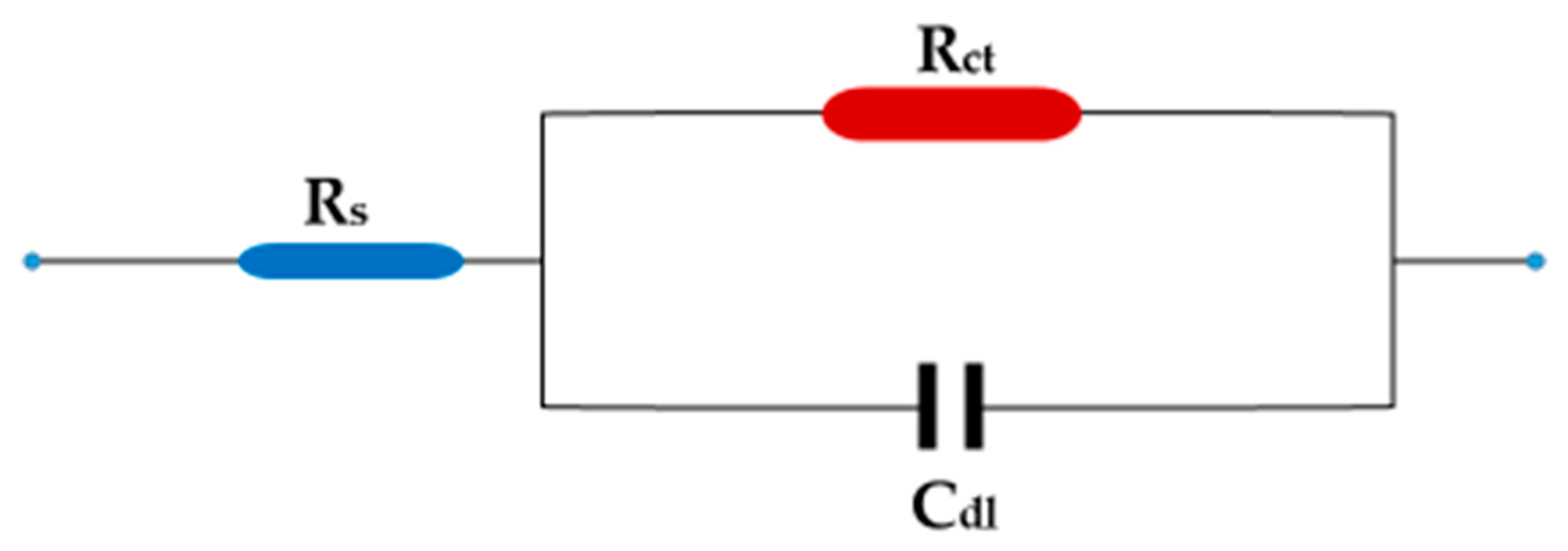
3.3.2. Electrochemical Impedance Measurements
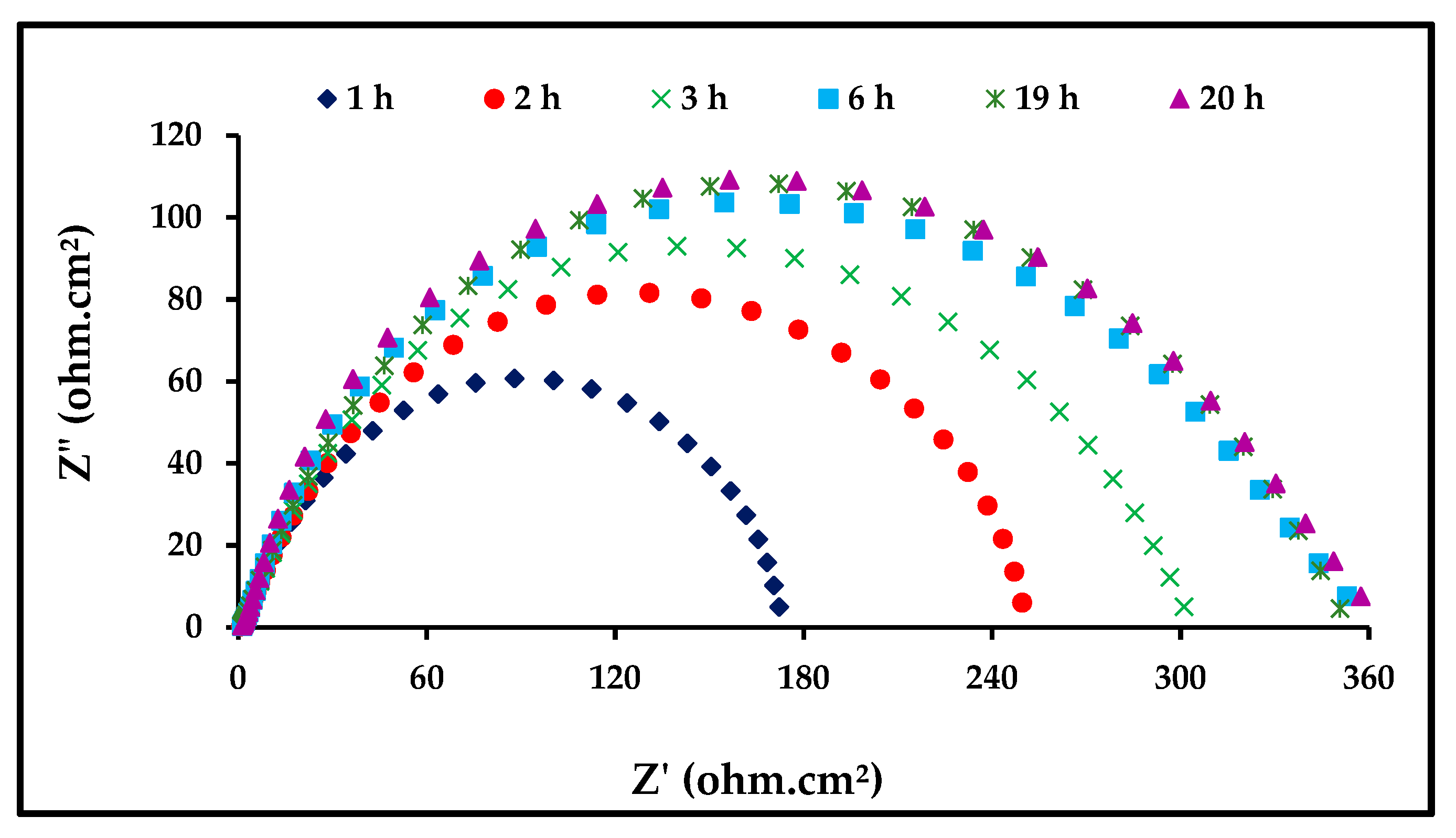
3.3.3. Effect of Immersion Time
3.4. Adsorption Isotherm Models and Thermodynamic
3.5. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-Ray Spectroscopy (EDS)
3.6. Mechanism of Corrosion Inhibition
- The inhibitor molecules are adsorbed onto the carbon steel surface by electrostatic interaction between the electrons adsorbed on the carbon steel surface (physical adsorption).
- The presence of heteroatoms having free electron pair enhances the chemical adsorption.
- The carbon steel surface becomes more negative for the accumulation of electrons on it.
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Tang, L.; Li, X.; Lau, S.P.; Li, L.; Mu, G.; Liu, G. A Kinetic Model to Study the Corrosion Inhibition of 500μM PAR for Steel Corrosion in 0.5-3.0 M Hydrochloric Acid. Recent Patents Corros. Sci. 2011, 1, 56–62. [Google Scholar] [CrossRef]
- Fouda, A.E.A.E.S.; Megahed, H.E.; Fouad, N.; Elbahrawi, N.M. Corrosion Inhibition of Carbon Steel in 1 M Hydrochloric Acid Solution by Aqueous Extract of Thevetia peruviana. J. Bio- Tribo-Corrosion 2016, 2, 16. [Google Scholar] [CrossRef] [Green Version]
- Saeed, M.; Saleem, M.; Usmani, S.; Malik, I.A.; Al-Shammari, F.A.; Deen, K.M. Corrosion inhibition of mild steel in 1 M HCl by sweet melon peel extract. J. King Saud Univ. Sci. 2019, 31, 1344–1351. [Google Scholar] [CrossRef]
- Sliem, M.H.; Afifi, M.; Radwan, A.B.; Fayyad, E.M.; Shibl, M.; Heakal, F.E.-T.; Abdullah, A.M. AEO7 Surfactant as an Eco-Friendly Corrosion Inhibitor for Carbon Steel in HCl solution. Sci. Rep. 2019, 9, 2319. [Google Scholar] [CrossRef] [Green Version]
- Bouanis, M.; Tourabi, M.; Nyassi, A.; Zarrouk, A.; Jama, C.; Bentiss, F. Corrosion inhibition performance of 2,5-bis(4-dimethylaminophenyl)-1,3,4-oxadiazole for carbon steel in HCl solution: Gravimetric, electrochemical and XPS studies. Appl. Surf. Sci. 2016, 389, 952–966. [Google Scholar] [CrossRef]
- Ijuo, G.; Chahul, H.; Eneji, I. Kinetic and Thermodynamic Studies of Corrosion Inhibition of Mild Steel using Bridelia ferruginea Extract in Acidic Environment Kinetic and Thermodynamic Studies of Corrosion Inhibition of Mild Steel using Bridelia ferruginea Extract in Acidic Environment. J. Adv. Electrochem. 2016, 2, 107–112. [Google Scholar]
- Abeng, F.; Idim, V.; Nna, P. Kinetics and Thermodynamic Studies of Corrosion Inhibition of Mild Steel Using Methanolic Extract of Erigeron floribundus (Kunth) in 2 M HCl Solution. World News of Nat. Sci. 2017, 10, 26–38. [Google Scholar]
- Iroha, N.; Hamilton-Amachree, A. Inhibition and adsorption of oil extract of Balanites aegyptiaca seeds on the corrosion of mild steel in hydrochloric acid environment. World Sci. News 2019, 126, 183–197. [Google Scholar]
- Al-Senani, G. Department of Chemistry, College of Science, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia Study the Corrosion Inhibition of Carbon Steel in 1 M HCl Using Extracts of Date Palm Waste. Int. J. Electrochem. Sci. 2018, 13, 3777–3788. [Google Scholar] [CrossRef]
- AL-Senani, G.; AL-Saeedi, S.; AL-Mufarij, R. Coriandrum sativum leaves extract (CSL) as an eco-friendly green inhibitor for corrosion of carbon steel in acidic media. J. Mater. Environ. Sci. 2016, 7, 2240–2251. [Google Scholar]
- Odewunmi, N.; A Umoren, S.; Gasem, Z. Utilization of watermelon rind extract as a green corrosion inhibitor for mild steel in acidic media. J. Ind. Eng. Chem. 2015, 21, 239–247. [Google Scholar] [CrossRef]
- Nnanna, L.; Uchendu, K.; Nwosu, F.; Ihekoronye, U.; Eti, E. Gmelina Arborea Bark Extracts as a Corrosion Inhibitor for Mild Steel in an Acidic Environment. Int. J. Mater. Chem. 2014, 4, 34–39. [Google Scholar] [CrossRef]
- Zheng, X.; Gong, M.; Li, Q.; Guo, L. Corrosion inhibition of mild steel in sulfuric acid solution by loquat (Eriobotrya japonica Lindl.) leaves extract. Sci. Rep. 2018, 8, 9140. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Potential of Borage flower aqueous extract as an environmentally sustainable corrosion inhibitor for acid corrosion of mild steel: Electrochemical and theoretical studies. J. Mol. Liq. 2019, 277, 895–911. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B. A detailed electrochemical/theoretical exploration of the aqueous Chinese gooseberry fruit shell extract as a green and cheap corrosion inhibitor for mild steel in acidic solution. J. Mol. Liq. 2019, 282, 366–384. [Google Scholar] [CrossRef]
- Al-Dahiri, R.H. The Application of Zinc Oxide Nanoparticles as An Eco- Friendly Inhibitor for Steel in Acidic Solution. Int. J. Electrochem. Sci. 2020, 15, 442–457. [Google Scholar] [CrossRef]
- Khamis, E.A.; Hamdy, A.; Morsi, R.E. Magnetite nanoparticles/polyvinyl pyrrolidone stabilized system for corrosion inhibition of carbon steel. Egypt. J. Pet. 2018, 27, 919–926. [Google Scholar] [CrossRef]
- Quadri, T.; Olasunkanmi, L.O.; Fayemi, O.; Solomon, M.M.; Ebenso, E.E. Zinc Oxide Nanocomposites of Selected Polymers: Synthesis, Characterization, and Corrosion Inhibition Studies on Mild Steel in HCl Solution. ACS Omega 2017, 2, 8421–8437. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Fu, L.; Han, F.; Wang, A.; Cai, W.; Yu, J.; Yang, J.; Peng, F. Green biosynthesis and characterization of zinc oxide nanoparticles using Corymbia citriodora leaf extract and their photocatalytic activity. Green Chem. Lett. Rev. 2015, 8, 59–63. [Google Scholar] [CrossRef]
- Rao, M.D.; Gautam, P. Synthesis and characterization of ZnO nanoflowers usingChlamydomonas reinhardtii: A green approach. Environ. Prog. Sustain. Energy 2016, 35, 1020–1026. [Google Scholar] [CrossRef]
- Soto-Robles, C.; Nava, O.; Vilchis-Nestor, A.; Castro-Beltran, A.; Gomez-Gutierrez, C.; Lugo-Medina, E.; Olivas, A.; Luque, P. Biosynthesized zinc oxide using Lycopersicon esculentum peel extract for methylene blue degradation. J. Mater. Sci. Mater. Electron. 2018, 29, 3722–3729. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. The chemical constituents and pharmacological effects of Convolvulus arvensis and Convolvulus scammonia—A review. IOSR J. Pharm. 2016, 6, 64–75. [Google Scholar]
- Convolvulus. Available online: https://en.wikipedia.org/wiki/Convolvulus (accessed on 17 February 2020).
- Convolvulus. The Jepson eFlora. Available online: https://ucjeps.berkeley.edu/eflora/eflora_display.php?tid=11474 (accessed on 17 February 2020).
- Rachitha, P.; Krupashree, K.; Jayashree, G.; Kandikattu, H.K.; Amruta, N.; Gopalan, N.; Rao, M.; Khanum, F. Chemical composition, antioxidant potential, macromolecule damage and neuroprotective activity of Convolvulus pluricaulis. J. Tradit. Complement. Med. 2018, 8, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Ngoepe, N.; Mbita, Z.; Mathipa, M.; Mketo, N.; Ntsendwana, B.; Hintsho-Mbita, N. Biogenic synthesis of ZnO nanoparticles using Monsonia burkeana for use in photocatalytic, antibacterial and anticancer applications. Ceram. Int. 2018, 44, 16999–17006. [Google Scholar] [CrossRef]
- Ahmed, S.K.; Ali, W.B.; Khadom, A.A. Synthesis and investigations of heterocyclic compounds as corrosion inhibitors for mild steel in hydrochloric acid. Int. J. Ind. Chem. 2019, 10, 159–173. [Google Scholar] [CrossRef] [Green Version]
- Fiori-Bimbi, M.V.; Alvarez, P.E.; Vaca, H.; Gervasi, C.A. Corrosion inhibition of mild steel in HCL solution by pectin. Corros. Sci. 2015, 92, 192–199. [Google Scholar] [CrossRef]
- Meng, Y.; Ning, W.; Xu, B.; Yang, W.; Zhang, K.; Chen, Y.; Li, L.; Liu, X.; Zheng, J.; Zhang, Y. Inhibition of mild steel corrosion in hydrochloric acid using two novel pyridine Schiff base derivatives: A comparative study of experimental and theoretical results. RSC Adv. 2017, 7, 43014–43029. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Zhang, S.; Guo, L.; Feng, L.; Tan, B. Experimental and Theoretical Studies on the Corrosion Inhibition of Carbon Steel by Two Indazole Derivatives in HCl Medium. Materials. 2019, 12, 1339. [Google Scholar] [CrossRef] [Green Version]
- Ahamad, I.; Prasad, R.; Quraishi, M.A. Thermodynamic, electrochemical and quantum chemical investigation of some Schiff bases as corrosion inhibitors for mild steel in hydrochloric acid solutions. Corros. Sci. 2010, 52, 933–942. [Google Scholar] [CrossRef]
- Ahamad, I.; Prasad, R.; Quraishi, M.A. Adsorption and inhibitive properties of some new Mannich bases of Isatin derivatives on corrosion of mild steel in acidic media. Corros. Sci. 2010, 52, 1472–1481. [Google Scholar] [CrossRef]
- Ahmed, R.; Farghali, R.; Fekry, A. Study for the Stability and Corrosion Inhibition of Electrophoretic Deposited Chitosan on Mild Steel Alloy in Acidic Medium. Int. J. Electrochem. Sci. 2012, 7, 7270–7282. [Google Scholar]
- Izadi, M.; Shahrabi, T.; Ramezanzadeh, B. Active corrosion protection performance of an epoxy coating applied on the mild steel modified with an eco-friendly sol-gel film impregnated with green corrosion inhibitor loaded nanocontainers. Appl. Surf. Sci. 2018, 440, 491–505. [Google Scholar] [CrossRef]
- Usman, B.J.; A Umoren, S.; Gasem, Z.M. Inhibition of API 5L X60 steel corrosion in CO2-saturated 3.5% NaCl solution by tannic acid and synergistic effect of KI additive. J. Mol. Liq. 2017, 237, 146–156. [Google Scholar] [CrossRef]
- Ituen, E.; Akaranta, O.; James, A. Evaluation of Performance of Corrosion Inhibitors Using Adsorption Isotherm Models: An Overview. Chem. Sci. Int. J. 2017, 18, 1–34. [Google Scholar] [CrossRef]
- Sophie, P.; Anthony, N. Kinetic, Thermodynamic and Adsorption Studies for Corrosion Inhibition of Carbon Steel by Asparagaeus Setaceus L. + Mn2+ in Neutral Media. Int. J. Res. Appl. Sci. Eng. Technol. 2018, 6, 3125–3133. [Google Scholar] [CrossRef]
- Santos, É.D.C.D.; Cordeiro, R.; Dos Santos, M.; Rodrigues, P.R.P.; Singh, A.; D’Elia, E. Barley Agro-industrial Residues as Corrosion Inhibitor for Mild Steel in 1mol L-1HCl Solution. Mater. Res. 2019, 22. [Google Scholar] [CrossRef]
- Saha, S.K.; Dutta, A.; Sukul, D.; Ghosh, P.; Banerjee, P. Adsorption and corrosion inhibition effect of Schiff base molecules on the mild steel surface in 1 M HCl medium: A combined experimental and theoretical approach. Phys. Chem. Chem. Phys. 2015, 17, 5679–5690. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.A. Adsorption behavior of 8,9-bis(4 (dimethyl amino)phenyl)benzo[4,5]imidazo[1,2-a]pyridine-6,7-dicarbonitrile on mild steel surface in 1 M HCl. J. Assoc. Arab. Univ. Basic Appl. Sci. 2017, 22, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Raja, A.S.; Rajendran, S.; Satyabama, P. Inhibition of Corrosion of Carbon Steel in Well Water by DL-Phenylalanine-Zn2+ System. J. Chem. 2012, 2013, 720965. [Google Scholar] [CrossRef]
- Essien, E.A.; Kavaz, D.; Ituen, E.; A Umoren, S. Synthesis, characterization and anticorrosion property of olive leaves extract-titanium nanoparticles composite. J. Adhes. Sci. Technol. 2018, 32, 1773–1794. [Google Scholar] [CrossRef]
- Singh, P.; Chauhan, D.; Srivastava, K.; Srivastava, V.; Quraishi, M. Expired atorvastatin drug as corrosion inhibitor for mild steel in hydrochloric acid solution. Int. J. Ind. Chem. 2017, 8, 363–372. [Google Scholar] [CrossRef]





| Temperature | 298 K | 333 K | ||||
|---|---|---|---|---|---|---|
| Concentration (mg/mL) | Crate (mg/cm2∙h) | θ | Einh (%) | Crate (mg/cm2∙h) | θ | Einh% |
| Blank | 1.14 | - | - | 5.72 | - | - |
| 0.006 | 0.52 | 0.55 | 54.55 | 2.73 | 0.52 | 52.27 |
| 0.03 | 0.21 | 0.82 | 81.82 | 1.43 | 0.75 | 75.00 |
| 0.06 | 0.16 | 0.86 | 86.36 | 1.07 | 0.81 | 81.36 |
| 0.09 | 0.10 | 0.91 | 90.91 | 0.78 | 0.86 | 86.36 |
| 0.12 | 0.09 | 0.92 | 91.82 | 0.62 | 0.89 | 89.09 |
| Concentration (mg/mL) | 298 K | 333 K | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ecorr (mV) | βa (mV/dec) | βc (mV/dec) | icorr (mA/cm2) | Einh (%) | Ecorr (mV) | βa (mV/dec) | βc (mV/dec) | icorr (mA/cm2) | Einh (%) | |
| Blank | −441.01 | 76.90 | 123.28 | 5.15 | 0 | −451.83 | 109.87 | 150.38 | 9.93 | 0 |
| 0.006 | −464.22 | 70.78 | 133.75 | 2.24 | 56.60 | −468.61 | 91.34 | 147.17 | 4.29 | 58.35 |
| 0.03 | −466.08 | 69.55 | 144.68 | 1.02 | 80.17 | −480.03 | 71.25 | 145.87 | 2.37 | 77.02 |
| 0.06 | −464.29 | 67.06 | 145.97 | 0.67 | 87.07 | −486.48 | 67.38 | 143.65 | 1.78 | 82.76 |
| 0.09 | −473.69 | 65.83 | 147.10 | 0.50 | 90.39 | −491.62 | 66.73 | 137.29 | 1.39 | 86.55 |
| 0.12 | −484.89 | 68.11 | 136.95 | 0.45 | 91.26 | −511.89 | 64.75 | 132.25 | 1.09 | 89.47 |
| Concentration (mg/mL) | 298 K | 333 K | ||||
|---|---|---|---|---|---|---|
| Rct (ohms∙cm2) | Cdl (mF) | Einh (%) | Rct (ohms∙cm2) | Cdl (mF) | Einh (%) | |
| Blank | 21.55 | 0.56 | - | 5.73 | 7.33 | - |
| 0.006 | 55.48 | 0.47 | 61.16 | 12.58 | 1.00 | 54.45 |
| 0.03 | 127.00 | 0.43 | 81.26 | 27.86 | 0.85 | 79.43 |
| 0.06 | 191.30 | 0.36 | 88.73 | 35.61 | 0.63 | 83.91 |
| 0.09 | 220.10 | 0.36 | 90.21 | 45.34 | 0.54 | 87.36 |
| 0.12 | 226.70 | 0.30 | 90.49 | 59.48 | 0.52 | 90.37 |
| Time (h) | Rct (ohms∙cm2) | Cdl (mF) |
|---|---|---|
| 1 | 200.0 | 0.53 |
| 2 | 276.4 | 0.49 |
| 3 | 302.0 | 0.32 |
| 6 | 343.4 | 0.30 |
| 19 | 353.0 | 0.29 |
| 20 | 359.3 | 0.28 |
| Isotherm Models | Temperature (K) | Kads | R2 | n | a | ΔG°ads (kJ/mol) | ΔH°ads (kJ/mol) | ΔS°ads (J/mol K) |
|---|---|---|---|---|---|---|---|---|
| Langmuir | 298 | 200 | 0.9998 | - | - | −23.0778 | −2.66 | −68.51 |
| 333 | 178.57 | 0.9989 | - | - | −25.4746 | |||
| Freundlich | 298 | 1.35 | 0.9604 | 0.164 | - | −10.6899 | −2.15 | −28.65 |
| 333 | 1.23 | 0.9862 | 0.143 | - | −11.6902 | |||
| Temkin | 298 | 22,324.87 | 0.9766 | - | 4.205 | −34.7599 | 19.22 | −181.15 |
| 333 | 50,633.28 | 0.9955 | - | −4.845 | −41.1097 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Senani, G.M. Synthesis of ZnO-NPs Using a Convolvulus arvensis Leaf Extract and Proving Its Efficiency as an Inhibitor of Carbon Steel Corrosion. Materials 2020, 13, 890. https://doi.org/10.3390/ma13040890
Al-Senani GM. Synthesis of ZnO-NPs Using a Convolvulus arvensis Leaf Extract and Proving Its Efficiency as an Inhibitor of Carbon Steel Corrosion. Materials. 2020; 13(4):890. https://doi.org/10.3390/ma13040890
Chicago/Turabian StyleAl-Senani, Ghadah M. 2020. "Synthesis of ZnO-NPs Using a Convolvulus arvensis Leaf Extract and Proving Its Efficiency as an Inhibitor of Carbon Steel Corrosion" Materials 13, no. 4: 890. https://doi.org/10.3390/ma13040890





