Effect of Olive-Pine Bottom Ash on Properties of Geopolymers Based on Metakaolin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Raw Materials Characterization
2.3. Preparation of MK-OPBA Geopolymers
2.4. Characterization of MK-OPBA Geopolymers
3. Results and Discussion
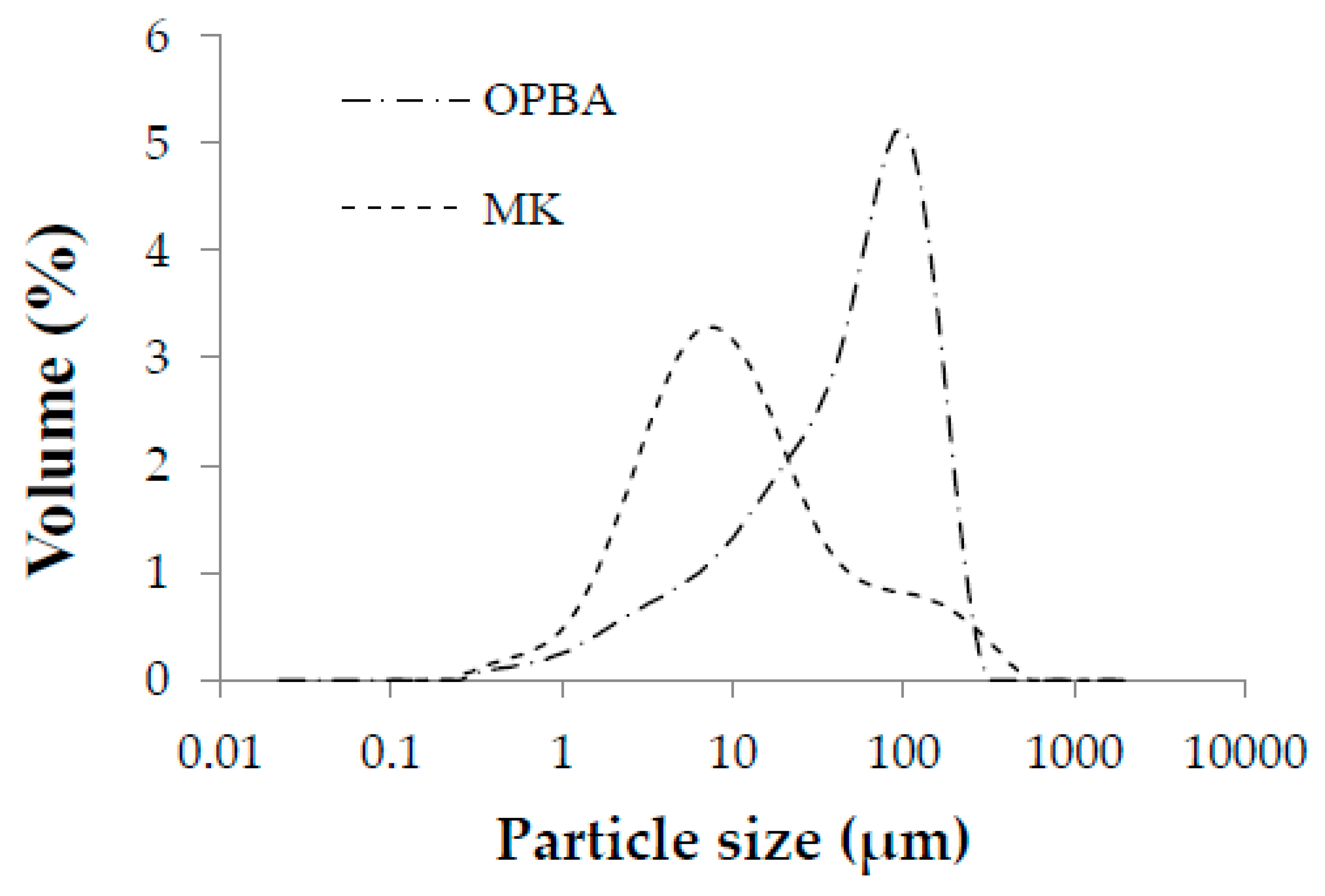
3.1. Raw Materials Characterization Results
3.2. MK-OPBA Geopolymers Characterization Results
3.2.1. FTIR of MK-OPBA Geopolymers
3.2.2. XRD of MK-OPBA Geopolymer
3.2.3. Apparent Porosity, Water Absorption and Bulk Density of MK-OPBA Geopolymers
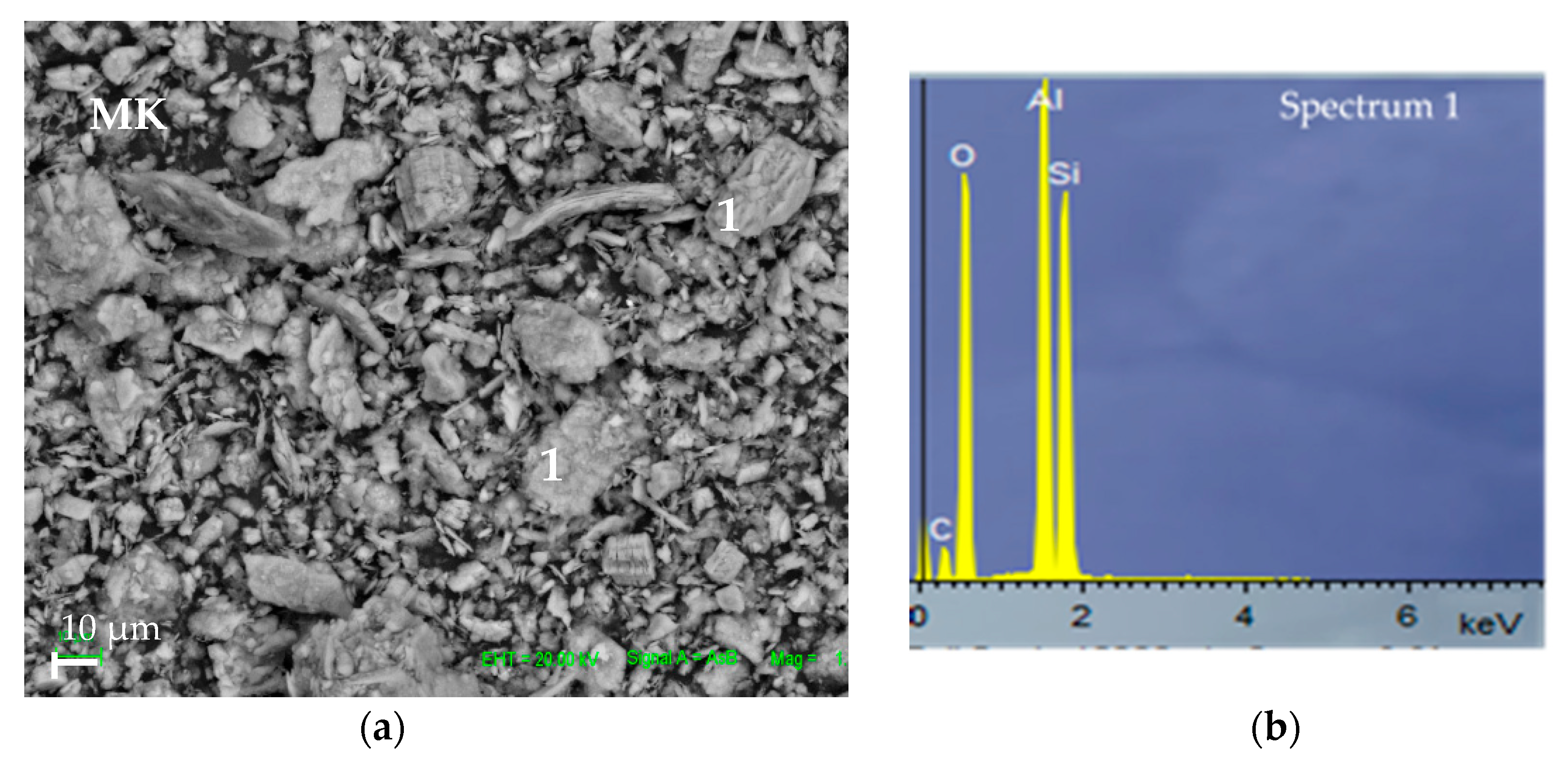
3.2.4. SEM Study of MK-OPBA Geopolymers
3.2.5. Compressive strength of MK-OPBA Geopolymers
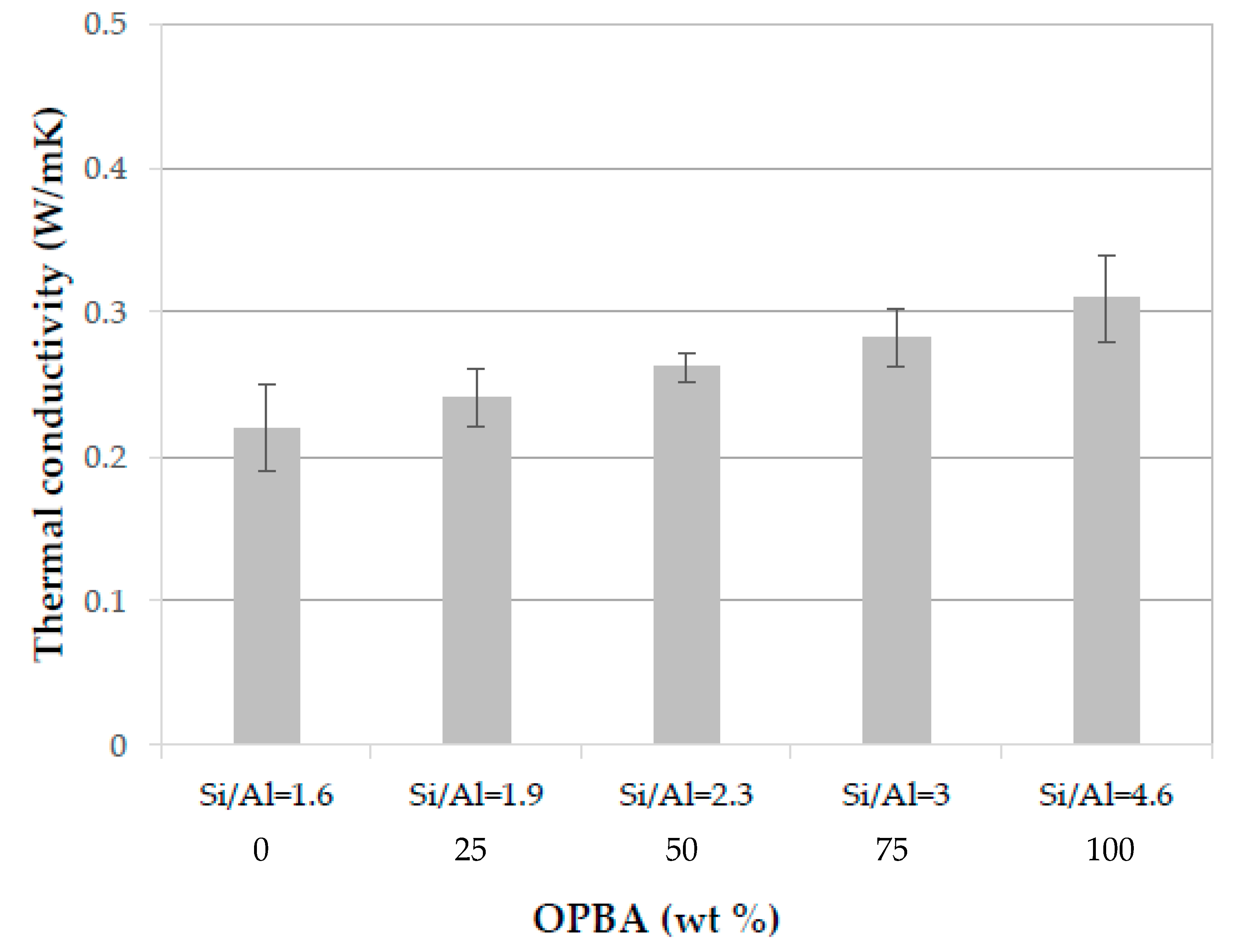
3.2.6. Thermal Conductivity of MK-OPBA Geopolymers
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bosoaga, A.; Masek, O.; Oakey, J.E. CO2 Capture Technologies for Cement Industry. Energy Procedia 2009, 1, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Goñi, S.; Guerrero, A. Hydraulic activity of belite cement from class C coal fly ash. Effect of curing and admixtures. Mater. Construcc. 2006, 56, 61–77. [Google Scholar]
- Popescu, C.D.; Muntean, M.; Shar, J.H. Industrial trial production of low energy belite cement. Cem. Concr. Compos. 2003, 25, 689–693. [Google Scholar] [CrossRef]
- de la Torre, A.G.; Aranda, M.A.G.; de Aza, A.H.; Pena, P.; de Aza, S. Belite Portland Cements. Synthesis and Mineralogical analysis. Boletín de la Sociedad Española de Cerámica y 2005, 44, 185–191. [Google Scholar] [CrossRef]
- Habert, G.; d’Espinose de Lacaillerie, J.B.; Roussel, N. An Environmental evaluation of geopolymer based concrete production: Reviewing current research trends. J. Clean. Prod. 2011, 19, 1229–1238. [Google Scholar] [CrossRef]
- Van den Heede, P.; De Belie, N. Environmental impact and life cycle assessment (LCA) of traditional and “Green” concretes: Literature review and theoretical calculations. Cement Concrete Comp. 2012, 34, 431–442. [Google Scholar] [CrossRef]
- Bhutta, A.; Farooq, M.; Zanotti, C.; Banthia, N. Pull-out behavior of different fibers in geopolymer mortars: Effect of alkaline solution concentration and curing. Mater. Struct. 2017, 50, 1–13. [Google Scholar] [CrossRef]
- Font, A.; Soriano, L.; Moraes-Pinheiro, S.M.; Tashima, M.M.; Monzó, J.; Borrachero, M.V.; Payá, J. Design and properties of 100% waste-based ternary alkali-activated mortars: Blast furnace slag, olive-stone biomass ash and rice husk ash. J. Clean. Prod. 2020, 243, 118568. [Google Scholar] [CrossRef]
- Sata, V.; Sathonsaowaphak, A.; Chindaprasirt, P. Resistance of lignite bottom ash geopolymer mortar to sulfate and sulfuric acid attack. Cem. Concr. Compos. 2012, 34, 700–708. [Google Scholar] [CrossRef]
- Mobili, A.; Belli, A.; Giosuè, C.; Telesca, A.; Marroccoli, M.; Tittarelli, F. Calcium sulfoaluminate, geopolymeric, and cementitious mortars for structural applications. Environments 2017, 4, 64. [Google Scholar] [CrossRef] [Green Version]
- Giosuè, C.; Mobili, A.; Di Perna, C.; Tittarelli, F. Performance of lightweight cement-based and alkali-activated mortars exposed to high-temperature. Const. Build Mater. 2019, 220, 565–576. [Google Scholar] [CrossRef]
- Xu, H.; van Deventer, J.S.J. Effect of Source Materials on geopolymerization. Ind. Eng. Chem. Res. 2003, 42, 1698–1706. [Google Scholar] [CrossRef]
- Rostovskaya, G.; Illyin, V.; Brodko, O. The investigation of Service Properties of the Slag Alkaline Concretes. In Proceedings of the International Conference on Non-Traditional Cement and Concrete, Brno, Czech Republic, 11–13 June 2002; pp. 510–523. [Google Scholar]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the composition and application of biomass ash. Part 1. Phase-mineral and chemical composition and classification. Fuel 2013, 105, 40–76. [Google Scholar] [CrossRef]
- Jaworek, A.; Czech, T.; Sobczyk, A.T.; Krupa, A. Properties of biomass vs. coal fly ashes deposited in electrostatic precipitator. J. Electrostat. 2013, 71, 165–175. [Google Scholar] [CrossRef]
- Toniolo, N.; Boccaccini, A.R. Fly ash-based geopolymer containing added silicate waste. A review. Ceram. Int. 2017, 43, 14545–14551. [Google Scholar] [CrossRef]
- Pérez-Villarejo, L.; Eliche-Quesada, D.; Martín-Pascual, J.; Martín-Morales, M.; Zamorano, M. Comparative study of the use of different biomass from olive grove in the manufacture of sustainable ceramic lightweight bricks. Constr. Build. Mater. 2020, 231, 117103. [Google Scholar] [CrossRef]
- Balo, A.M.; Rahier, H.; Mobili, A.; Katsiki, A.; Fagel, N.; Chinje, U.M.; Njopwouo, D. Metakaolin-based inorganic polymer synthesis using cotton shell ash as sole alkaline activator. Const. Build. Mater. 2018, 191, 1011–1022. [Google Scholar] [CrossRef]
- Alonso, M.M.; Gascó, C.; Martín-Morales, M.; Suárez-Navarro, J.A.; Zamorano, M.; Puertas, F. Olive biomass ash as an alternative activator in geopolymer formation: A study of Strength, radiology and leaching behaviour. Cement Concrete Comp. 2019, 104, 103384. [Google Scholar] [CrossRef]
- Eliche-Quesada, D.; Felipe-Sesé, M.; Moreno-Molina, A.; Franco, F.; Infantes-Molina, A. Investigation of using bottom or fly pine-olive pruning ash to produce Environmental friendly ceramic Materials. Appl. Clay Sci. 2017, 135, 333–346. [Google Scholar] [CrossRef]
- Beltrán, M.; Barbudo, A.; Agrela, F.; Jiménez, J.; de Brito, J. Mechanical performance of bedding mortars made with olive biomass bottom ash. Constr. Build. Mater. 2016, 112, 669–707. [Google Scholar] [CrossRef]
- UNE-EN 772-13. Test Methods for Masonry Pieces. Determination of the Absolute dry Density and Dry Bulk Density of Parts for Masonry Factory (Except Natural Stone); Asociación Española de Normalización (AENOR): Madrid, Spain, 2001. [Google Scholar]
- ASTM C373—Test. Method for Water Absorption, Bulk Density, Apparent Density, Apparent Porosity and Apparent Specific Gravity of Fired Whiteware Products; American Society for testing and Materials: West Conshohocken, PA, USA, 1994. [Google Scholar]
- UNE-EN 772-1:2011. Methods of Test for Masonry Units—Part. 1: Determination of Compressive Strength; Asociación Española de Normalización (AENOR): Madrid, Spain, 2011. [Google Scholar]
- ISO 8302: 1991. Thermal Insulation-Determination of Steady-State Thermal Resistance and Related Properties-Guarded Hot Plate Apparatus; International Standards Organization: Geneva, Switzerland, 1991. [Google Scholar]
- García-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; Mcphee, D.E. Compatibility studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram Na2O–CaO–Al2O3–SiO2–H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Provis, J.L.; van Deventer, J.S.J. Time-resolved and spatially-resolved infrared spectroscopic observation of seeded nucleation controlling geopolymer gel formation. J. Colloid Interf. Sci. 2011, 357, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.K.W.; van Deventer, J.S.J. The effects of inorganic salt contamination on the strength and durability of geopolymers. Colloids Surf. A. 2002, 211, 115–126. [Google Scholar] [CrossRef]
- Rodríguez, E.; Mejía de Gutiérrez, R.; Bernal, S.; Gordillo, M. Effect of the SiO2/Al2O3 and Na2O/SiO2 ratios on the properties of geopolymers based on MK. Rev. Fac. Ing. Univ. Antioq. 2009, 49, 30–40. [Google Scholar]
- Sánchez de Rojas, M.I.; Frías, M. The pozzolanic activity of different materials, its influence on the hydration heat in mortars. Cem. Concr. Res. 1996, 26, 203–213. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Fernández-Jiménez, A.; Palomo, A.; Macphee, D. Effect of calcium additions on N-A-S-H cementitious gels. J. Am. Ceram. Soc. 2010, 93, 1934–1940. [Google Scholar] [CrossRef]
- Bernal, S.A.; Rodríguez, E.D.; Mejía de Gutiérrez, R.; Provis, J.L.; Delvasto, S. Activation of metakaolin/slag blends using alkaline solutions based on chemically modified silica fume and rice husk ash. Waste Biomass Valori. 2011, 3, 99–108. [Google Scholar] [CrossRef]
- Kamhangrittirong, P.; Suwanvitaya, P.; Witayakul, W.; Suwanvitaya, P.; Chindaprasirt, P. Factors influence on shrinkage of high calcium fly ash geopolymer paste. Adv. Mater. Res. 2013, 610–613, 2275–2281. [Google Scholar] [CrossRef]
- Lloyd, R.; Provis, J.; Van Deventer, J. Microscopy and microanalysis of inorganic polymer cements. 2: The gel binder. J. Mater. Sci. 2009, 44, 620–631. [Google Scholar] [CrossRef]
- Yip, C.; Van Deventer, J. Microanalysis of calcium silicate hydrate gel formed within a geopolymeric binder. J. Mater. Sci. 2003, 38, 3851–3860. [Google Scholar] [CrossRef]
- Palomo, A.; Fernández-Jiménez, A.; Kovalchuk, G.; Ordoñez, L.M.; Naranjo, M.C. OPC-fly ash cementitious systems: Study of gel binders produced during alkaline hydration. J. Mater. Sci. 2007, 42, 2958–2966. [Google Scholar] [CrossRef]
- Rowles, M.; Connor, B.O. Chemical optimization of the compressive strength of aluminosilicate geopolymers synthesized by sodium silicate activation of metakaolinite. J. Mater. Chem. 2003, 13, 1161–1165. [Google Scholar] [CrossRef]
- Steveson, M.; Sagoe Crentsil, K. Relationships between composition, structure and strength of inorganic polymers Part 2 fly ash-derived inorganic polymers. J. Mater. Sci. 2005, 40, 4247–4259. [Google Scholar] [CrossRef]
- Williams, R.P.; van Riessen, A. Development of alkali activated borosilicate inorganic polymers. J. Eur. Ceram. Soc. 2011, 31, 1513–1516. [Google Scholar] [CrossRef]
- Kamseu, E.; Ceron, B.; Tobias, H.; Leonelli, E.; Bignozzi, M.C.; Muscio, A.; Libbra, A. Insulating behavior of metakaolin-based geopolymer materials assess with heat flux meter and laser flash techniques. J. Therm. Anal. Calorim. 2011, 108, 1189–1199. [Google Scholar] [CrossRef]
- Villaquirán-Caicedo. M.A.; Mejía de Gutiérrez, R.; Sulekar, S.; Davis, C.; Nino, J.C. Thermal properties of novel binary geopolymers based on metakaolin and alternative silica sources. Appl. Clay Sci. 2015, 118, 276–282. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.C.; van Deventer, J.S.J. Thermal Conductivity of Metakaolin Geopolymers Used as a First Approximation for Determining Gel Interconnectivity. Ind. Eng. Chem. Res. 2006, 45, 7781–7788. [Google Scholar] [CrossRef]
- Kamseu, E.; Nait-Ali, B.; Bignozzi, M.C.; Leonelli, C.; Rossignol, S.; Smith, D.S. Bulk composition and microstructure dependence of effective thermal conductivity of porous inorganic polymer cements. J. Eur. Ceram. Soc. 2012, 32, 1593–1603. [Google Scholar] [CrossRef]
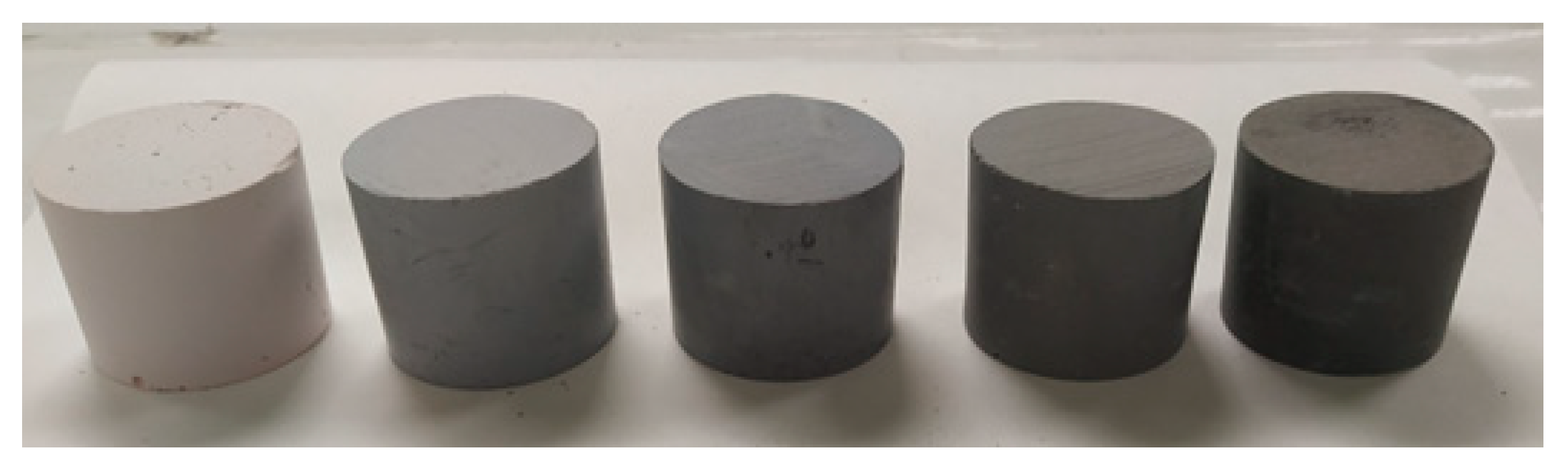










| Sample | Si/Al Molar Ratio | Si/Na Molar Ratio | MK (g) | OPBA (g) | NaOH (g) | Water (g) | Na2SiO3 (g) | Liquid/Solid Ratio | Degree of Reaction (%) |
|---|---|---|---|---|---|---|---|---|---|
| 100MK-0OPBBA | 1.63 | 0.42 | 450.0 | 0 | 80 | 195 | 300 | 0.85 | 54 |
| 75MK-25OPBA | 1.91 | 0.44 | 337.5 | 112.5 | 80 | 195 | 300 | 0.85 | 50 |
| 50MK-50OPBA | 2.32 | 0.46 | 225.0 | 225.0 | 80 | 195 | 300 | 0.85 | 48 |
| 25MK-75OPBA | 3.05 | 0.49 | 112.5 | 337.5 | 80 | 195 | 300 | 0.85 | 42 |
| 0MK-100OPBA | 4.62 | 0.52 | 0 | 450.0 | 80 | 195 | 300 | 0.85 | 33 |
| Oxide Content (%) | OPBA | MK |
|---|---|---|
| SiO2 | 46.10 | 58.03 |
| Al2O3 | 12.04 | 40.29 |
| Fe2O3 | 4.78 | 0.42 |
| CaO | 19.65 | 0.09 |
| MgO | 3.71 | 0.11 |
| MnO | 0.09 | 0.01 |
| Na2O | 0.78 | 0.02 |
| K2O | 4.59 | 0.39 |
| TiO2 | 0.83 | 0.15 |
| P2O5 | 1.12 | 0.07 |
| SO3 | 0.41 | 0.01 |
| LOI | 5.58 | 0.36 |
| Sample | Si | Al | Na | Ca | Si/Al | Si/Na | Si/Ca |
|---|---|---|---|---|---|---|---|
| 100MK-0OPBA Gel NASH | 27.92 | 15.40 | 8.08 | 0 | 1.81 | 3.46 | - |
| 50MK-50OPBA Gel NASH | 23.63 | 12.45 | 12.50 | 4.56 | 1.90 | 1.89 | 5.18 |
| 50MK-50OPBA Gel CASH | 17.77 | 7.86 | 4.25 | 23.70 | 2.26 | 4.18 | 0.75 |
| 0MK-100OPBA Gel NASH | 25.40 | 12.55 | 12.70 | 2.40 | 2.03 | 2.0 | 10.60 |
| 0MK-100OPBA Gel CASH | 16.38 | 6.14 | 2.80 | 25.25 | 2.66 | 5.84 | 0.64 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonet-Martínez, E.; García-Cobo, P.; Pérez-Villarejo, L.; Castro, E.; Eliche-Quesada, D. Effect of Olive-Pine Bottom Ash on Properties of Geopolymers Based on Metakaolin. Materials 2020, 13, 901. https://doi.org/10.3390/ma13040901
Bonet-Martínez E, García-Cobo P, Pérez-Villarejo L, Castro E, Eliche-Quesada D. Effect of Olive-Pine Bottom Ash on Properties of Geopolymers Based on Metakaolin. Materials. 2020; 13(4):901. https://doi.org/10.3390/ma13040901
Chicago/Turabian StyleBonet-Martínez, Eduardo, Pedro García-Cobo, Luis Pérez-Villarejo, Eulogio Castro, and Dolores Eliche-Quesada. 2020. "Effect of Olive-Pine Bottom Ash on Properties of Geopolymers Based on Metakaolin" Materials 13, no. 4: 901. https://doi.org/10.3390/ma13040901





