Influence of Firing Temperature on the Physical, Thermal and Microstructural Properties of Kankara Kaolin Clay: A Preliminary Investigation
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Chemical Composition
3.2. X-ray Diffraction of Raw and Fired Clay
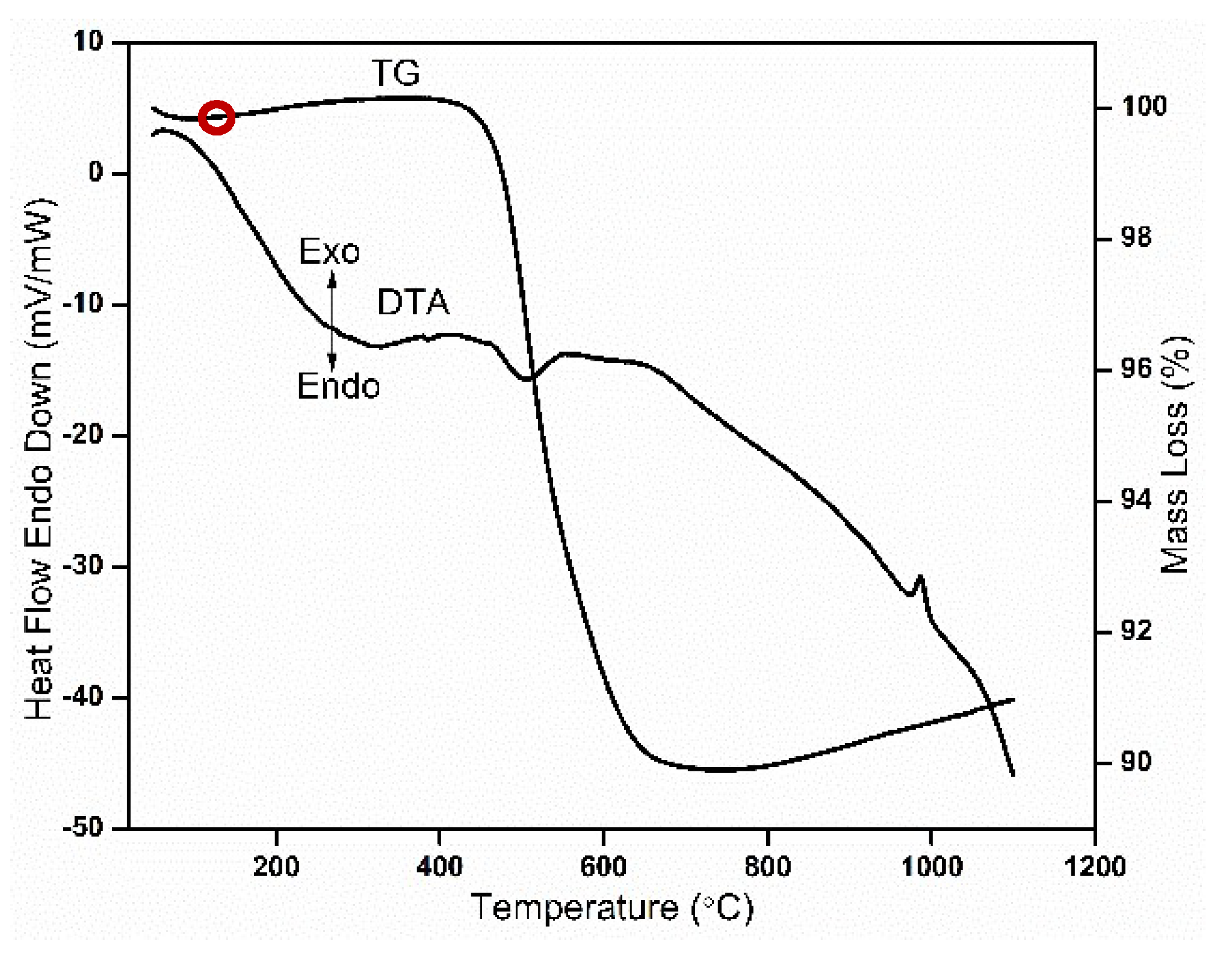
3.3. Thermogravimetric and Differential Thermal Analysis
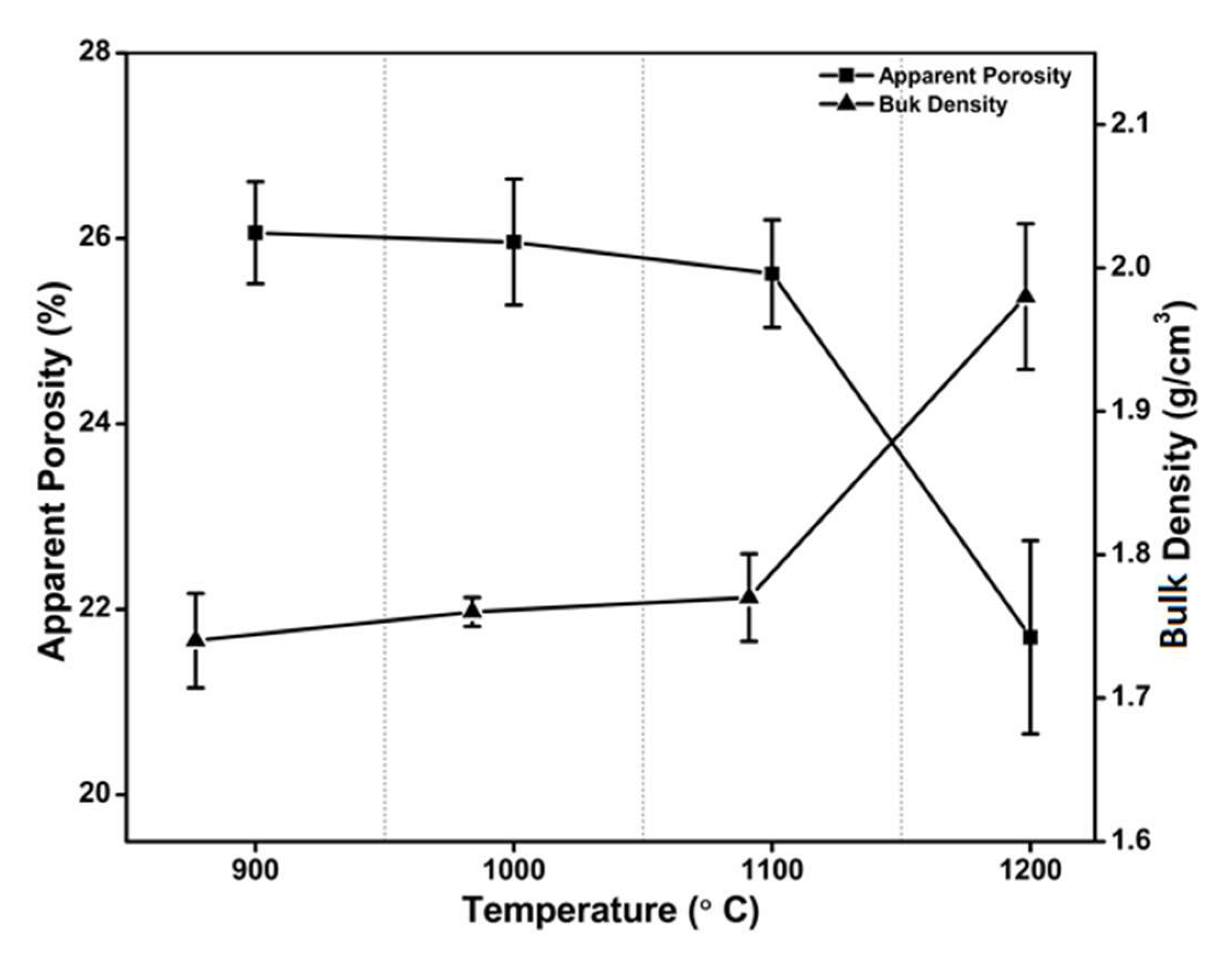
3.4. Bulk Density and Apparent Porosity
4. Conclusions
- The XRD analysis of the sintered material shows the presence of the mullite phase and the disappearance of other phases. This result revealed the superiority in terms of thermal properties of the fired clay.
- (Physical properties): The sharp increase in bulk density occurring above 1100 °C sintering temperature and the continuous reduction in apparent porosity observed over the 900–1200 °C range can be explained in view of the consolidation of the clay.
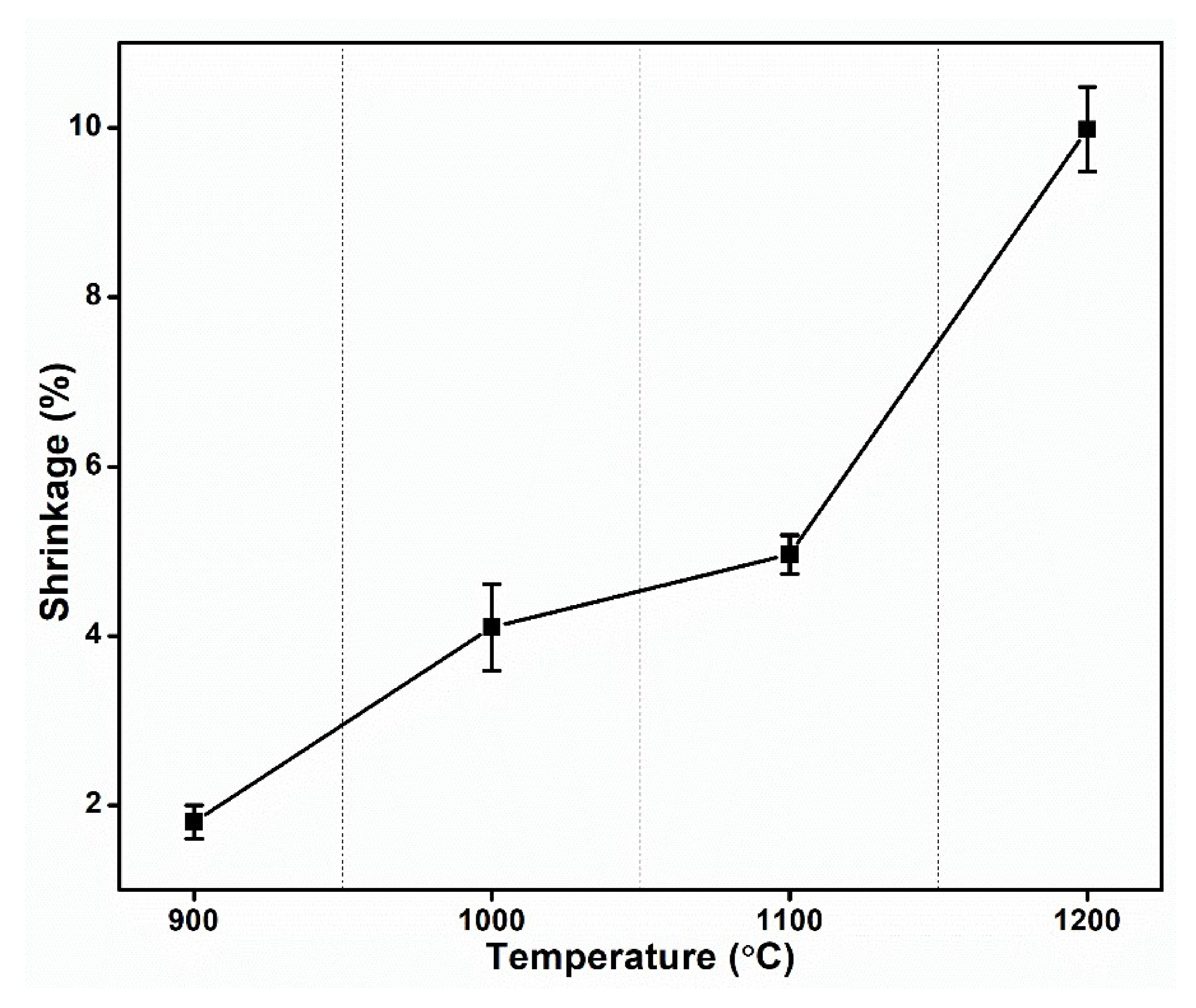
- The linear shrinkage of the material increases by 500% as sintering temperature rises from 900 °C to 1200 °C. Therefore, the densification of the clay increased.
- (Thermal properties): The thermal analysis of the clay revealed the presence of an endothermic peak within 70–100 °C due to the removal of physical combined water present in the clay. An exothermic peak at 560 °C revealed that the complete transformation of kaolin to metakaolin is due to dehydroxylation (DHX) process.
- (Microstructural properties): SEM analysis of fractured surfaces showed poor consolidation of the fractured surface sintered at 900 °C. The consolidation becomes more marked as sintering temperature increases from 900 ° to 1200 °C and the fractured surface shows a more consolidated shape and the presence of well-defined cracks for the firing temperature of 1200 °C. This can be explained by the formation of the glassy phase.
Author Contributions
Funding
Conflicts of Interest
References
- Murray, H.H. Traditional and new applications for kaolin, smectite, and palygorskite: A general overview. Appl. Clay Sci. 2000, 17, 207–221. [Google Scholar] [CrossRef]
- Ngun, B.K.; Mohamad, H.; Sulaiman, S.K.; Okada, K.; Ahmad, Z.A. Some ceramic properties of clays from central Cambodia. Appl. Clay Sci. 2011, 53, 33–41. [Google Scholar] [CrossRef]
- Ekosse, G.-I.E. Kaolin deposits and occurrences in Africa: Geology, mineralogy and utilization. Appl. Clay Sci. 2010, 50, 212–236. [Google Scholar] [CrossRef]
- Ekosse, G.-I.E. The Makoro kaolin deposit, Southeastern Botswana: Its genesis and possible industrial applications. Appl. Clay Sci. 2000, 16, 301–320. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Ramaswamy, S. Influence of mineral impurities on the properties of kaolin and its thermally treated products. Appl. Clay Sci. 2002, 21, 133–142. [Google Scholar] [CrossRef]
- Castelein, O.; Soulestin, B.; Bonnet, J.P.; Blanchart, P. The influence of heating rate on the thermal behaviour and mullite formation from a kaolin raw material. Ceram. Int. 2001, 27, 517–522. [Google Scholar] [CrossRef]
- Abubakar, M.; Fawziah, S.; Ahmad, N. Effect of milling time on the performance of ceramic membrane from ball clay for the treatment of nickel plating wastewater. J. Aust. Ceram. Soc. 2019, 55, 667–679. [Google Scholar] [CrossRef]
- ASTM. Standard Test Method for Water Absorption, Bulk Density, Apparent Porosity, and Apparent Specific Gravity of Fired Whiteware Products; C 373-88; ASTM International: West Conshohocken, PA, USA, 1999; pp. 1–2. [Google Scholar]
- ASTM. Standard Test Methods for Density of Compacted or Sintered Powder Metallurgy (PM) Products Using Archimedes’ Principle; ASTM B962-14; ASTM International: West Conshohocken, PA, USA, 2014; pp. 1–7. [Google Scholar]
- ASTM. Standard Test Method for Drying and Firing Shrinkages of Ceramic Whiteware Clays; ASTM C326-09 1, 1; ASTM International: West Conshohocken, PA, USA, 2014; pp. 1–2. [Google Scholar]
- Khemakhem, S.; Larbot, A.; Ben Amar, R. New ceramic microfiltration membranes from Tunisian natural materials: Application for the cuttlefish effluents treatment. Ceram. Int. 2009, 35, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Majouli, A.; Tahiri, S.; Alami Younssi, S.; Loukili, H.; Albizane, A. Elaboration of new tubular ceramic membrane from local Moroccan Perlite for microfiltration process. Application to treatment of industrial wastewaters. Ceram. Int. 2012, 38, 4295–4303. [Google Scholar] [CrossRef]
- Harabi, A.; Guechi, A.; Condom, S. Production of supports and filtration membranes from Algerian kaolin and limestone. Proced. Eng. 2012, 33, 220–224. [Google Scholar] [CrossRef] [Green Version]
- Mohsen, Q.; El-Maghraby, A. Characterization and assessment of Saudi clays raw material at different area. Arab. J. Chem. 2010, 3, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Ptacek, P. Isothermal kinetic analysis of the thermal decomposition of kaolinite: The thermogravimetric study. Thermochim. Acta 2010, 501, 24–29. [Google Scholar] [CrossRef]
- Panda, A.K.; Mishra, B.G.; Mishra, D.K.; Singh, R.K. Effect of sulphuric acid treatment on the physico-chemical characteristics of kaolin clay. Colloids Surf. A Physicochem. Eng. Asp. 2010, 363, 98–104. [Google Scholar] [CrossRef]
- Gougazeh, M.; Buhl, J.C. Synthesis and characterization of zeolite A by hydrothermal transformation of natural Jordanian kaolin. J. Assoc. Arab Univ. Basic Appl. Sci. 2014, 15, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Soto, P.J.; Eliche-Quesada, D.; Martínez-Martínez, S.; Garzon-Garzon, E.; Perez-Villarejo, L.; Ma Rincón, J. The effect of vitreous phase on mullite and mullite-based ceramic composites from kaolin wastes as by-products of mining, sericite clays and kaolinite. Mater. Lett. 2018, 223, 154–158. [Google Scholar] [CrossRef]
- Iglesia, P.G.; García-Moreno, O.; Menendez, J.L.; De Aza, A.H.; Álvarez-Clemares, I.; Torrecillas, R. Microstructural development and mechanical performance of mullitealumina and hibonite-alumina ceramics with controlled addition of a glass phase. Ceram. Int. 2017, 44, 2292–2299. [Google Scholar] [CrossRef] [Green Version]
- Lecomte-Nana, G.L.; Bonnet, J.P.; Blanchart, P. Investigation of the firing mechanisms of kaolin–muscovite. Appl. Clay Sci. 2011, 51, 445–451. [Google Scholar] [CrossRef]
- Furlani, E.; Tonello, G.; Aneggi, E.; Maschio, S. Preparation and characterization of sintered ceramics made with spent foundry olivine sand and clay. Ceram. Int. 2012, 38, 2619–2625. [Google Scholar] [CrossRef]
- Sahnoun, R.D.; Baklouti, S. Characterization of flat ceramic membrane supports prepared with kaolin-phosphoric acid-starch. Appl. Clay Sci. 2013, 83–84, 399–404. [Google Scholar] [CrossRef]
- Chen, C.Y.; Lan, G.S.; Tuan, W.H. Preparation of mullite by the reaction sintering of kaolinite and alumina. J. Eur. Ceram. Soc. 2000, 20, 2519–2525. [Google Scholar] [CrossRef]



| Compound | SiO2 | Al2O3 | K2O | Fe2O3 | CaO | TiO2 | MgO | MnO | P2O5 |
|---|---|---|---|---|---|---|---|---|---|
| Concentration (%) | 55.40 | 42.90 | 1.30 | 0.31 | 0.07 | 0.05 | 0.04 | 0.01 | 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abubakar, M.; Muthuraja, A.; Rajak, D.K.; Ahmad, N.; Pruncu, C.I.; Lamberti, L.; Kumar, A. Influence of Firing Temperature on the Physical, Thermal and Microstructural Properties of Kankara Kaolin Clay: A Preliminary Investigation. Materials 2020, 13, 1872. https://doi.org/10.3390/ma13081872
Abubakar M, Muthuraja A, Rajak DK, Ahmad N, Pruncu CI, Lamberti L, Kumar A. Influence of Firing Temperature on the Physical, Thermal and Microstructural Properties of Kankara Kaolin Clay: A Preliminary Investigation. Materials. 2020; 13(8):1872. https://doi.org/10.3390/ma13081872
Chicago/Turabian StyleAbubakar, Muazu, Ayyankalai Muthuraja, Dipen Kumar Rajak, Norhayati Ahmad, Catalin I. Pruncu, Luciano Lamberti, and Ashwini Kumar. 2020. "Influence of Firing Temperature on the Physical, Thermal and Microstructural Properties of Kankara Kaolin Clay: A Preliminary Investigation" Materials 13, no. 8: 1872. https://doi.org/10.3390/ma13081872








