Separation and Recovery of Refined Si from Al–Si Melt by Modified Czochralski Method
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
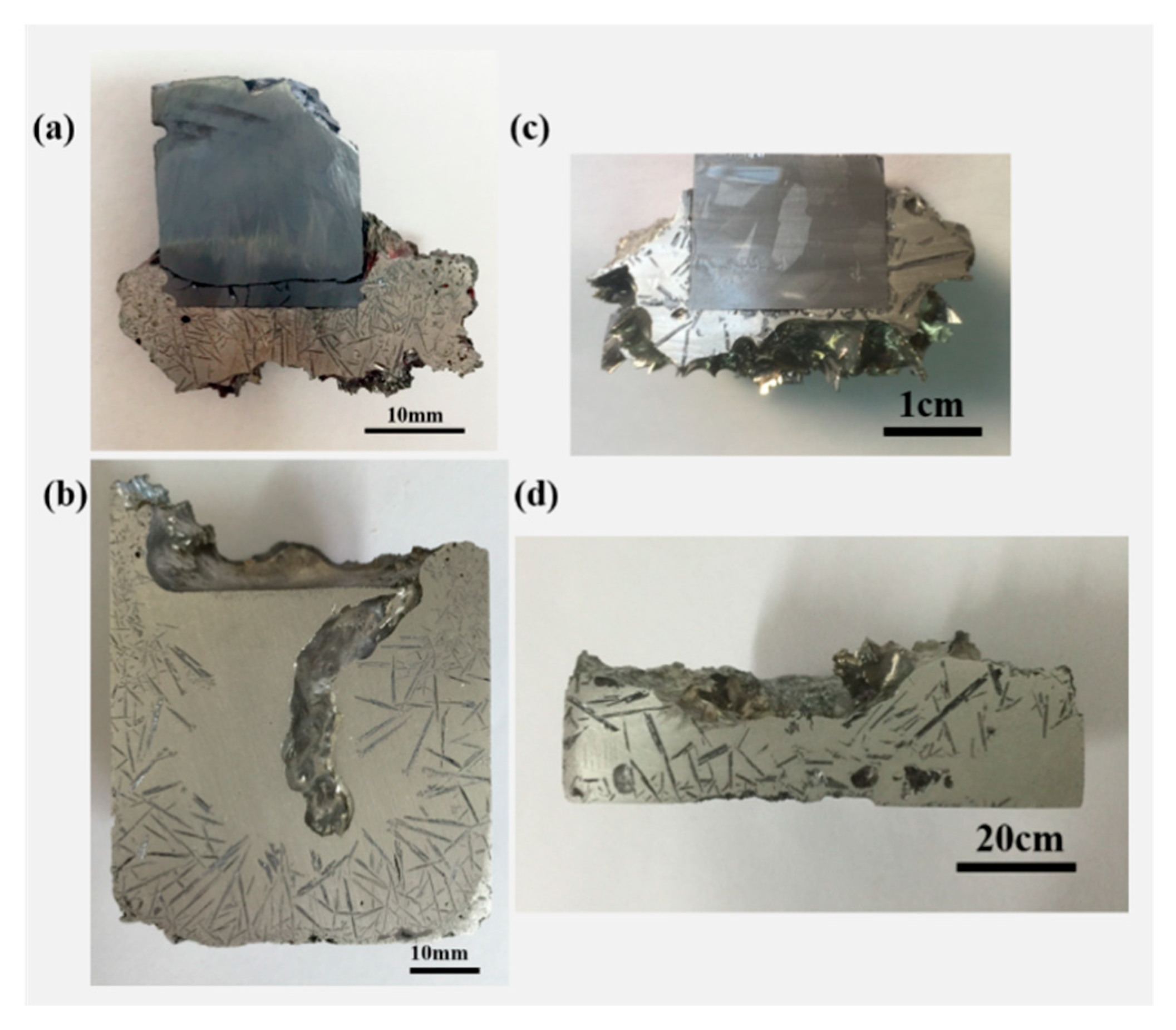
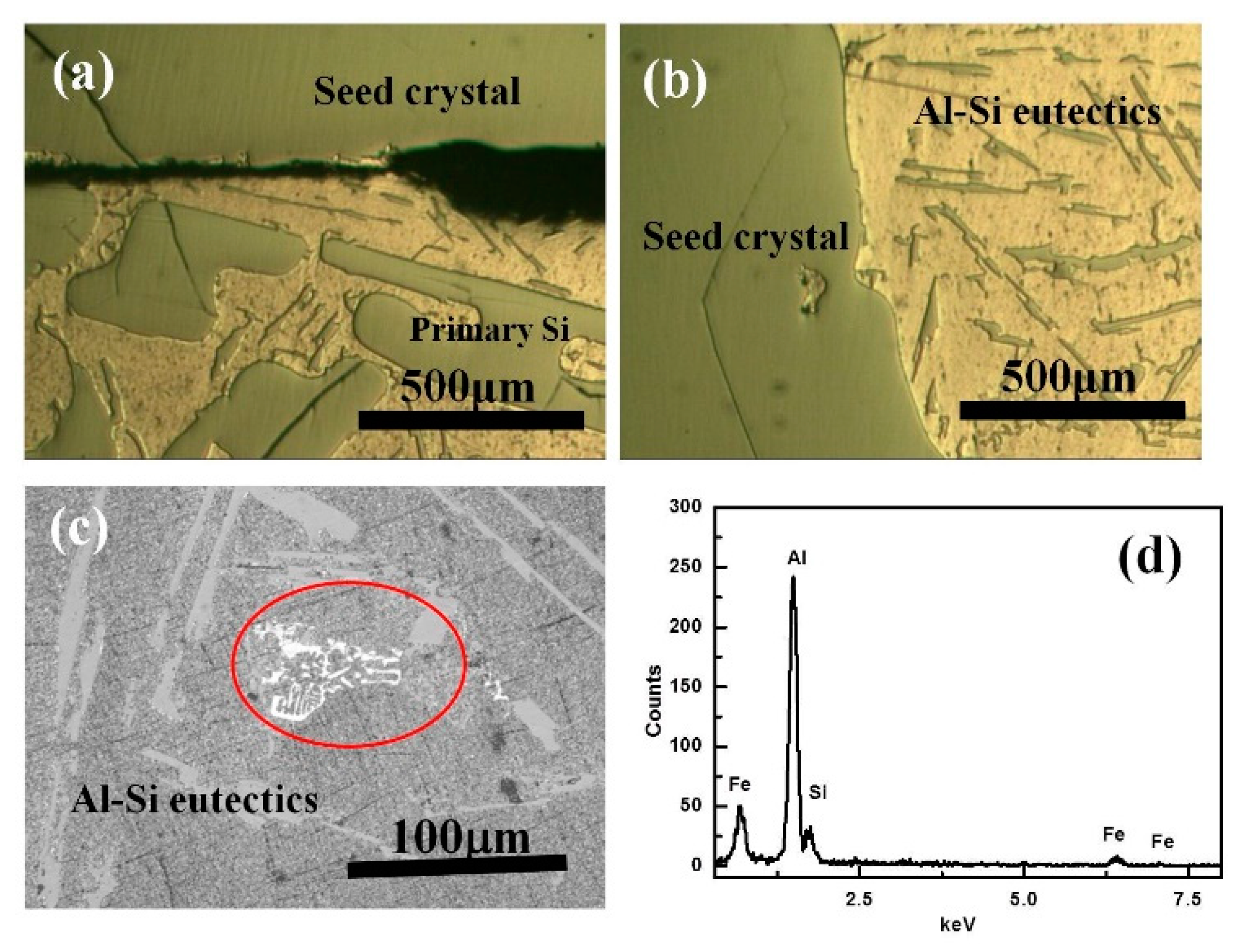
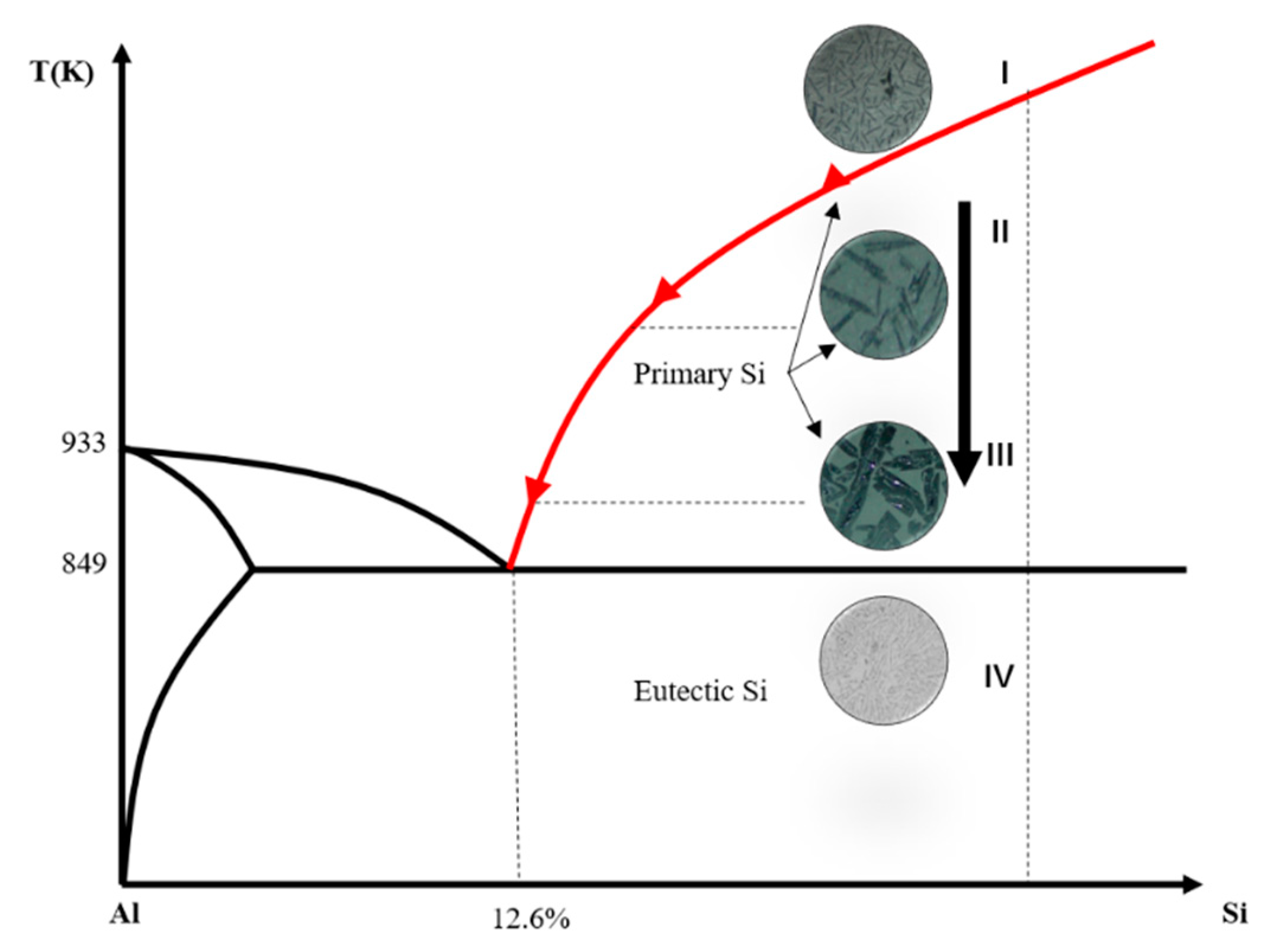
3.1. Morphologies and Distribution of the Refined Si
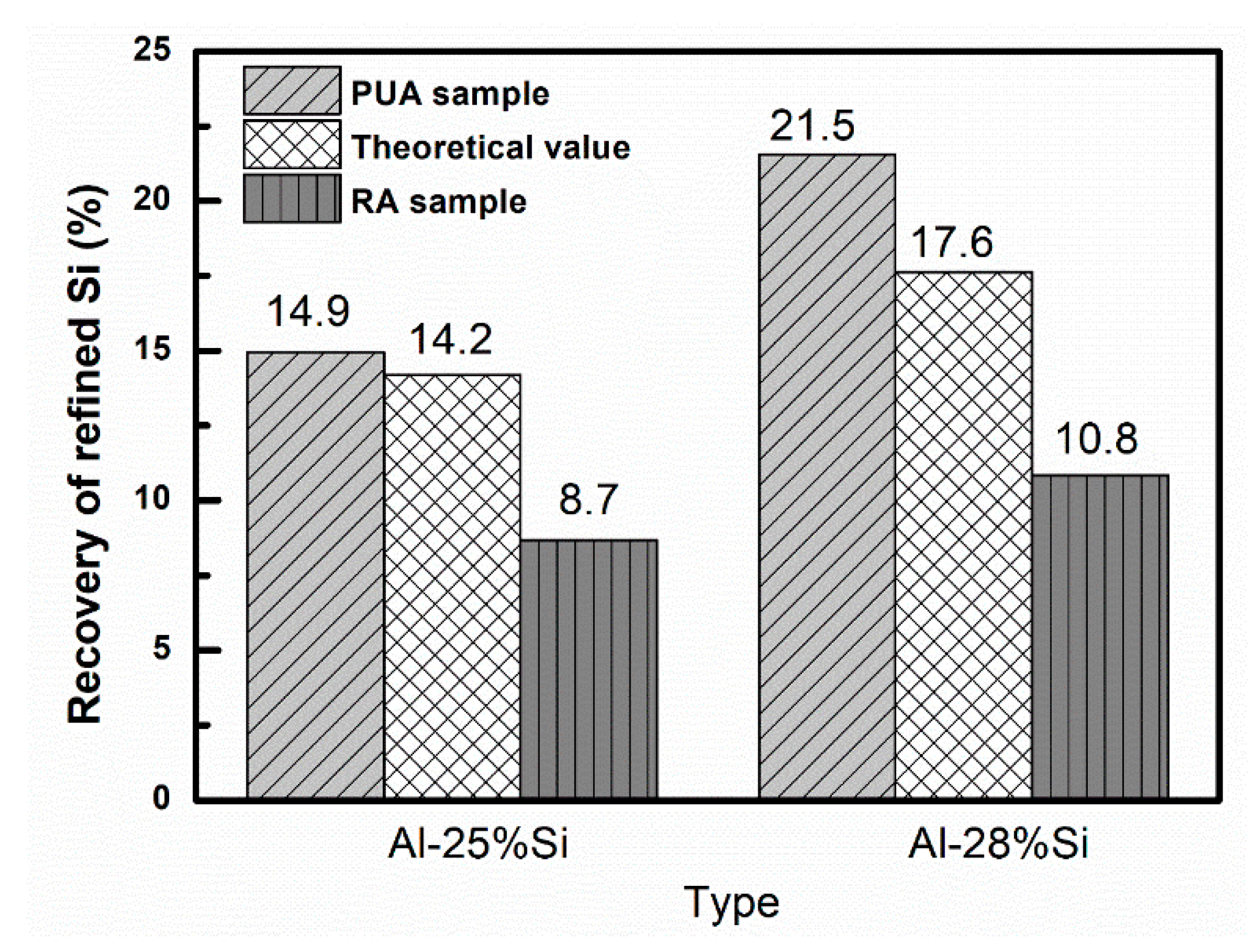
3.2. Recovery of Refined Si by Modified Czochralski Method
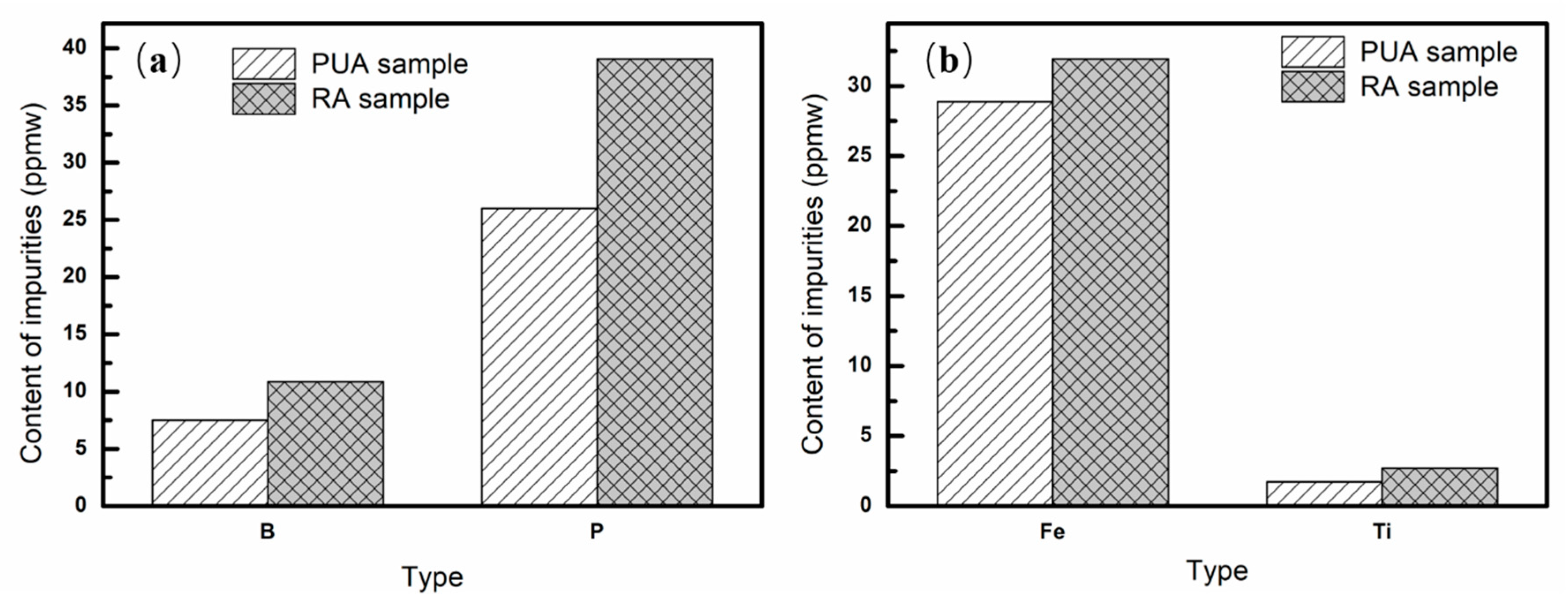
3.3. Removal Fraction of Impurities at Different Locations
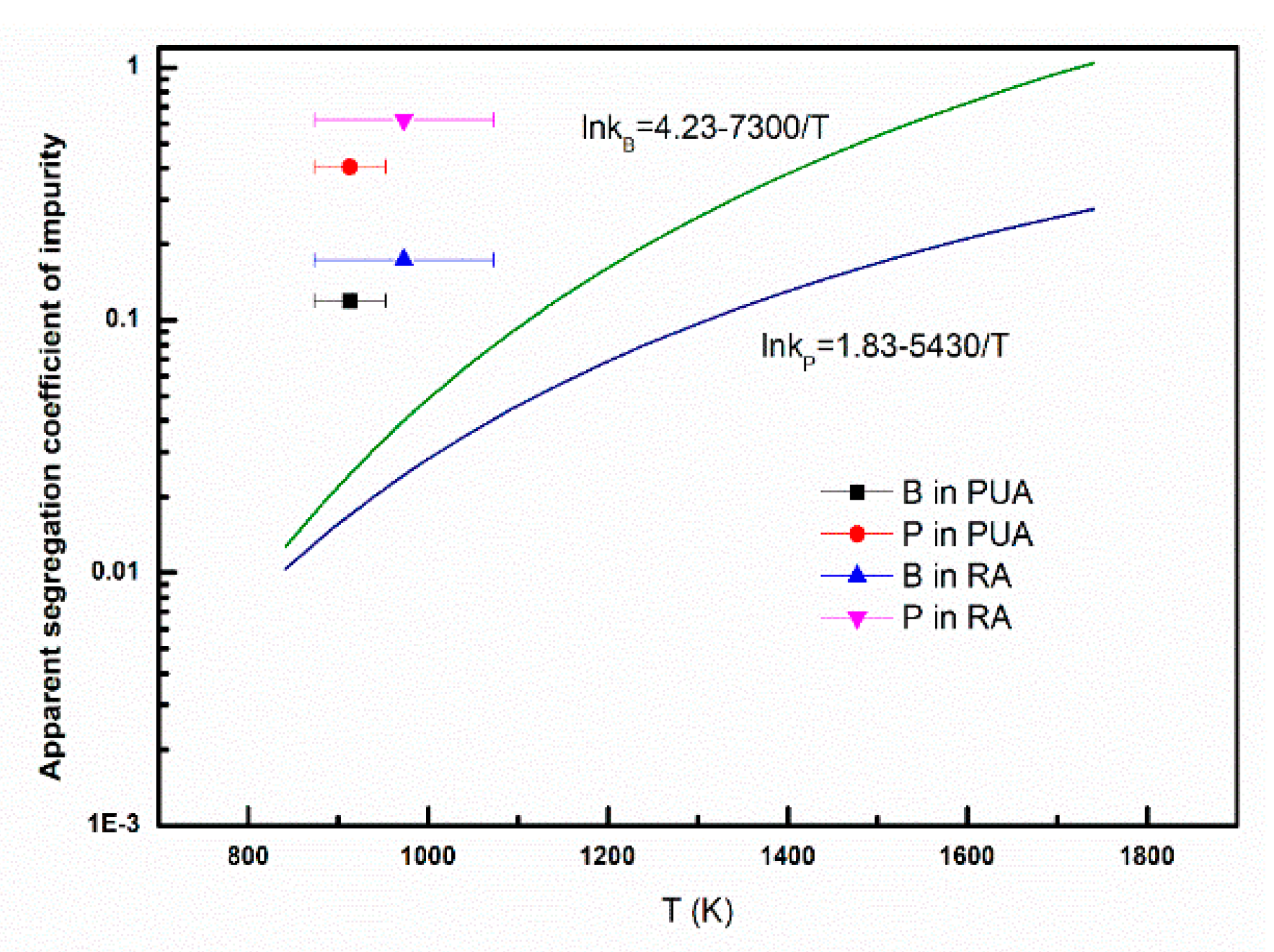
3.4. Apparent Segregation Coefficients of Impurities
3.5. Distribution Mechanism of Refined Si
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chigondo, F. From Metallurgical-Grade to Solar-Grade Silicon: An Overview. Silicon 2017, 10, 789–798. [Google Scholar] [CrossRef]
- Bye, G.; Ceccaroli, B. Solar grade silicon: Technology status and industrial trends. Sol. Energy Mater. Sol. Cells 2014, 130, 634–646. [Google Scholar] [CrossRef]
- Gu, X.; Yu, X.; Yang, D. Low-cost solar grade silicon purification process with Al–Si system using a powder metallurgy technique. Sep. Purifi. Technol. 2011, 77, 33–39. [Google Scholar] [CrossRef]
- Mostafa, A.; Medraj, M. Binary Phase Diagrams and Thermodynamic Properties of Silicon and Essential Doping Elements (Al, As, B, Bi, Ga, In, N, P, Sb and Tl). Materials 2017, 10, 676. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Chen, J.; Danaei, A.; Thomas, S.; Huang, L.; Luo, X.; Barati, M. Effect of Ti addition to Cu-Si alloy on the boron distribution in various phases. J. Alloy Compd. 2018, 734, 235–242. [Google Scholar] [CrossRef]
- Li, J.; Bai, X.; Ban, B.; He, Q.; Chen, J. Mechanism of boron removal from Si–Al melt by Ar–H2 gas mixtures. Trans. Nonferrous Met. Soc. China 2016, 26, 3046–3051. [Google Scholar] [CrossRef]
- Jiang, D.; Ren, S.; Shi, S.; Dong, W.; Qiu, J.; Tan, Y.; Li, J. Phosphorus Removal from Silicon by Vacuum Refining and Directional Solidification. J. Electron. Mater. 2013, 43, 314–319. [Google Scholar] [CrossRef]
- Martorano, M.A.; Neto, J.B.F.; Oliveira, T.S.; Tsubaki, T.O. Refining of metallurgical silicon by directional solidification. Mater. Sci. Eng. B 2011, 176, 217–226. [Google Scholar] [CrossRef]
- Jiang, W.; Yu, W.; Li, J.; You, Z.; Li, C.; Lv, X. Segregation and Morphological Evolution of Si Phase during Electromagnetic Directional Solidification of Hypereutectic Al-Si Alloys. Materials 2018, 12, 10. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Wu, J.; Ma, W.; Xu, M.; Lei, Y.; Yang, B. Removal of impurities from metallurgical grade silicon by addition of ZnO to calcium silicate slag. Sep. Purif. Technol. 2016, 170, 248–255. [Google Scholar] [CrossRef]
- Huang, L.; Chen, J.; Fang, M.; Thomas, S.; Danaei, A.; Luo, X.; Barati, M. Clean enhancing elimination of boron from silicon kerf using Na2O-SiO2 slag treatment. J. Clean. Prod. 2018, 186, 718–725. [Google Scholar] [CrossRef]
- Jiang, D.; Shi, S.; Tan, Y. Progress in Research and Development of Solar-grade Silicon Preparation by Electron Beam Melting. J. Inorg. Mater. 2015, 30, 785. [Google Scholar]
- Baek, S.-H.; Lee, H.; Min, D.; Choi, S.; Moon, B.-M.; Jung, H.-D. Novel Recycling Method for Boron Removal from Silicon by Thermal Plasma Treatment Coupled with Steam and Hydrogen Gases. Metals 2017, 7, 401. [Google Scholar] [CrossRef] [Green Version]
- Ban, B.; Zhang, T.; Li, J.; Bai, X.; Pan, X.; Chen, J.; Hadi Tabaian, S. Solidification refining of MG-Si by Al-Si alloy under rotating electromagnetic field with varying frequencies. Sep. Purif. Technol. 2018, 202, 266–274. [Google Scholar] [CrossRef]
- Lei, Y.; Ma, W.; Sun, L.; Wu, J.; Dai, Y.; Morita, K. Removal o f B from Si by Hf addition during Al-Si solvent refining process. Sci. Technol. Adv. Mater. 2016, 17, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Bian, X.; Liu, X. Effect of strontium addition on hydrogen content and porosity shape of Al-Si alloys. Int. J. Cast Met. Res. 2016, 14, 31–35. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Morita, K. Refining of silicon during its solidification from a Si–Al melt. J. Cryst. Growth. 2009, 311, 776–779. [Google Scholar] [CrossRef]
- Schmitz, J.; Hallstedt, B.; Brillo, J.; Egry, I.; Schick, M. Density and thermal expansion of liquid Al–Si alloys. J. Mater. Sci. 2012, 47, 3706–3712. [Google Scholar] [CrossRef]
- He, Y.; Ma, W.; Lv, G.; Zhang, Y.; Lei, Y.; Yang, X. An efficient method to separate silicon from high-silicon aluminum alloy melts by electromagnetic directional solidification. J. Clean. Prod. 2018, 185, 389–398. [Google Scholar] [CrossRef]
- Li, J.; Guo, Z.; Tang, H.; Wang, Z.; Sun, S. Si purification by solidification of Al–Si melt with super gravity. Trans. Nonferrous Met. Soc. China 2012, 22, 958–963. [Google Scholar] [CrossRef]
- Yu, W.; Ma, W.; Lv, G.; Ren, Y.; Dai, Y.; Morita, K. Low-Cost Process for Silicon Purification with Bubble Adsorption in Al-Si Melt. Metall. Mater. Trans. B 2014, 45, 1573–1578. [Google Scholar] [CrossRef]
- Zou, Q.C.; Jie, J.C.; Liu, S.C.; Wang, T.M.; Yin, G.M.; Li, T.J. Effect of traveling magnetic field on separation and purification of Si from Al–Si melt during solidification. J. Cryst. Growth. 2015, 429, 68–73. [Google Scholar] [CrossRef]
- Xue, H.; Lv, G.; Ma, W.; Chen, D.; Yu, J. Separation Mechanism of Primary Silicon from Hypereutectic Al-Si Melts Under Alternating Electromagnetic Fields. Metall. Mater. Trans. A 2015, 46, 2922–2932. [Google Scholar] [CrossRef]
- Li, J.; Guo, Z.; Li, J.; Yu, L. Super Gravity Separation of Purified Si from Solvent Refining with the Al-Si Alloy System for Solar Grade Silicon. Silicon 2014, 7, 239–246. [Google Scholar] [CrossRef]
- Czochralski, J. A new method for the measurement of the crystallization rate of metals. Zeitschrift für Chemie 1918, 92, 219–221. [Google Scholar]
- Miller, F.P.; Vandome, A.F.; John, M. Czochralski Process; VDM Publishing Group: Saarbrücken, Gernamy, 2010; p. 104. [Google Scholar]
- Arivanandhan, M.; Gotoh, R.; Watahiki, T.; Fujiwara, K.; Hayakawa, Y.; Uda, S.; Konagai, M. The impact of Ge codoping on the enhancement of photovoltaic characteristics of B-doped Czochralski grown Si crystal. J. Appl. Phys. 2012, 111, 043707. [Google Scholar] [CrossRef]
- Gao, C.; Ma, X.; Zhao, J.; Yang, D. Effect of tin on point defects and oxygen precipitation in Czochralski silicon: Experimental and theoretical studies. J. Appl. Phys. 2013, 113, 093511. [Google Scholar] [CrossRef]
- Gupta, S.P. Intermetallic compound formation in Fe–Al–Si ternary system: Part I. Mater. Charact. 2002, 49, 269–291. [Google Scholar] [CrossRef]
- Raghavan, V. Al-Fe-Si (Aluminum-Iron-Silicon). J. Phase Equilib Diff. 2010, 32, 140–142. [Google Scholar] [CrossRef]
- Novak, P.; Nova, K. Oxidation Behavior of Fe-Al, Fe-Si and Fe-Al-Si Intermetallics. Materials 2019, 12, 1748. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Ban, B.; Li, J.; Zhang, T.; Bai, X.; Chen, J.; Dai, S. Effect of Cooling Rate on Phosphorus Removal During Al-Si Solvent Refining. Metall. Mater. Trans. B 2015, 46, 542–544. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Morita, K. Removal of phosphorus by the solidification refining with Si-Al melts. Sci. Technol. Adv. Mat. 2004, 4, 531–537. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, T.; Morita, K. Removal of B from Si by Solidification Refining with Si-Al Melts. Metall. Mater. Trans. B 2005, 36, 731–736. [Google Scholar] [CrossRef]
- Murray, J.L.; McAIister, A.J. The AI-Si (Aluminum-Silicon) System. Bull. Alloy Phase Diagr. 1984, 5, 74–84. [Google Scholar] [CrossRef]


| B | P | Fe | Ti | |
|---|---|---|---|---|
| Si-A | 28 | 46 | 2818 | 437 |
| Si-B | 207 | 217 | 1696 | 263 |
| High purity Al | - | - | 7.00 | 1.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, J.; Lin, Y.; Shi, J.; Ban, B.; Liu, G.; Yang, W.; Chen, J. Separation and Recovery of Refined Si from Al–Si Melt by Modified Czochralski Method. Materials 2020, 13, 996. https://doi.org/10.3390/ma13040996
Li J, Li J, Lin Y, Shi J, Ban B, Liu G, Yang W, Chen J. Separation and Recovery of Refined Si from Al–Si Melt by Modified Czochralski Method. Materials. 2020; 13(4):996. https://doi.org/10.3390/ma13040996
Chicago/Turabian StyleLi, Jingwei, Juncheng Li, Yinhe Lin, Jian Shi, Boyuan Ban, Guicheng Liu, Woochul Yang, and Jian Chen. 2020. "Separation and Recovery of Refined Si from Al–Si Melt by Modified Czochralski Method" Materials 13, no. 4: 996. https://doi.org/10.3390/ma13040996





