Effect of Relative Humidity on Mechanical Degradation of Medium Mn Steels
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Exposing Test in Humidity Condition
2.3. Constant Load Test and Slow Strain Rate Tensile Test
3. Results Discussion
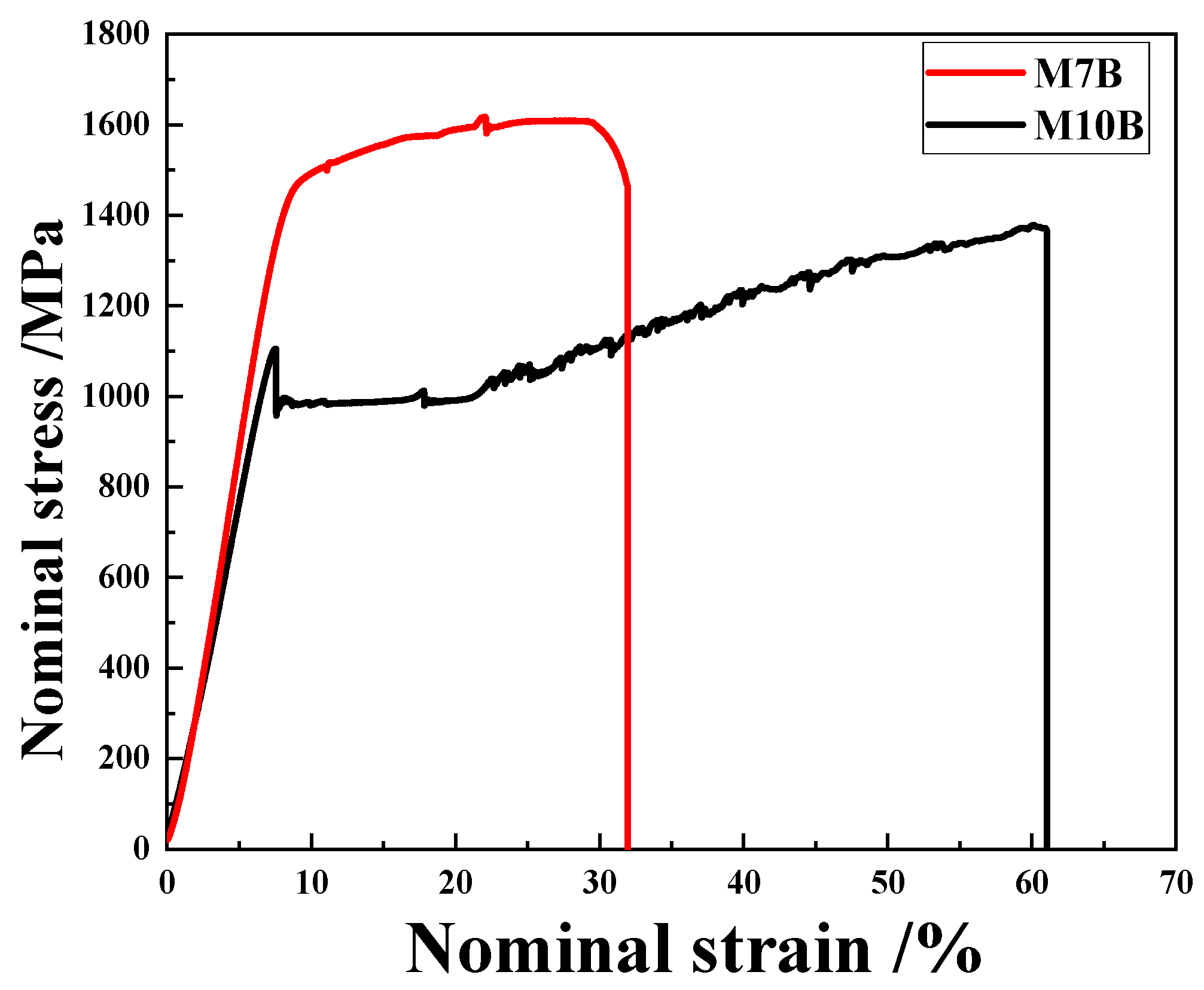
3.1. Mechanical Properties
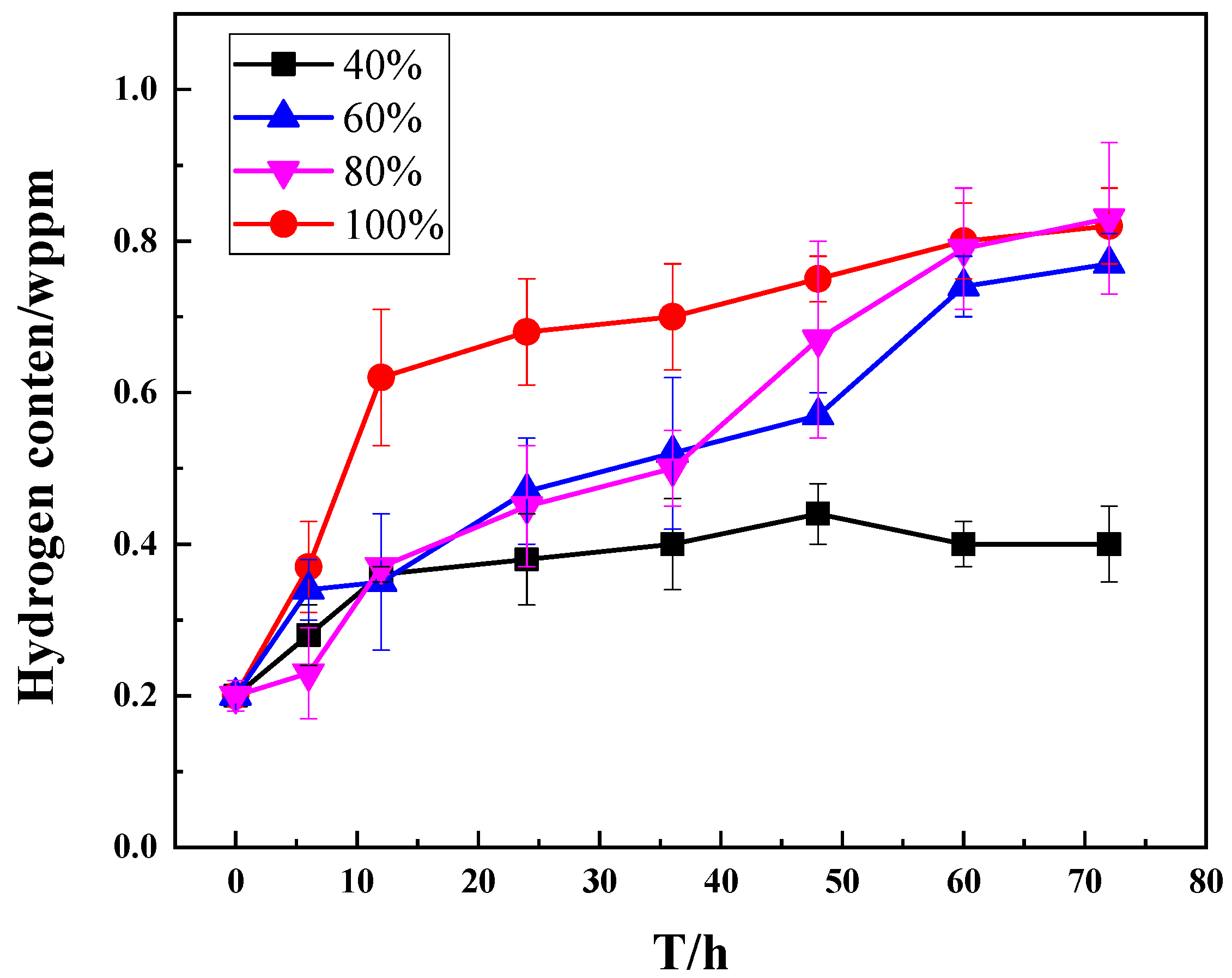
3.2. Exposure Test Results
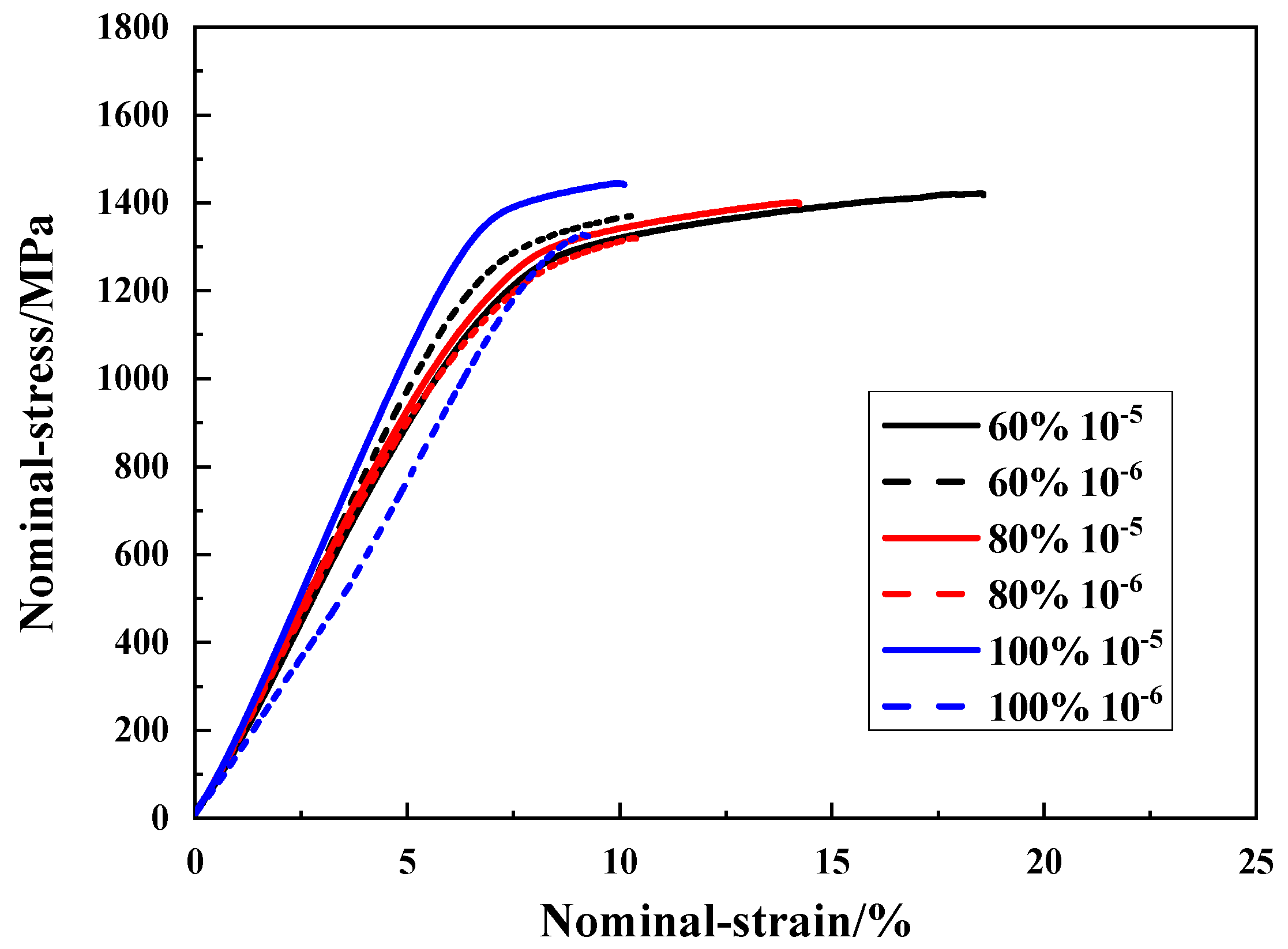
3.3. Slow Strain Rate Tensile Test
3.3.1. Slow Strain Rate Tensile Test Results under Different Humidity Conditions for M7B
3.3.2. SSRTT Result under Different Humidity for M10B
3.4. Constant Load Test (CLT)
Constant Load Test Result under Different Humidity for M7B and M10B
4. Conclusions
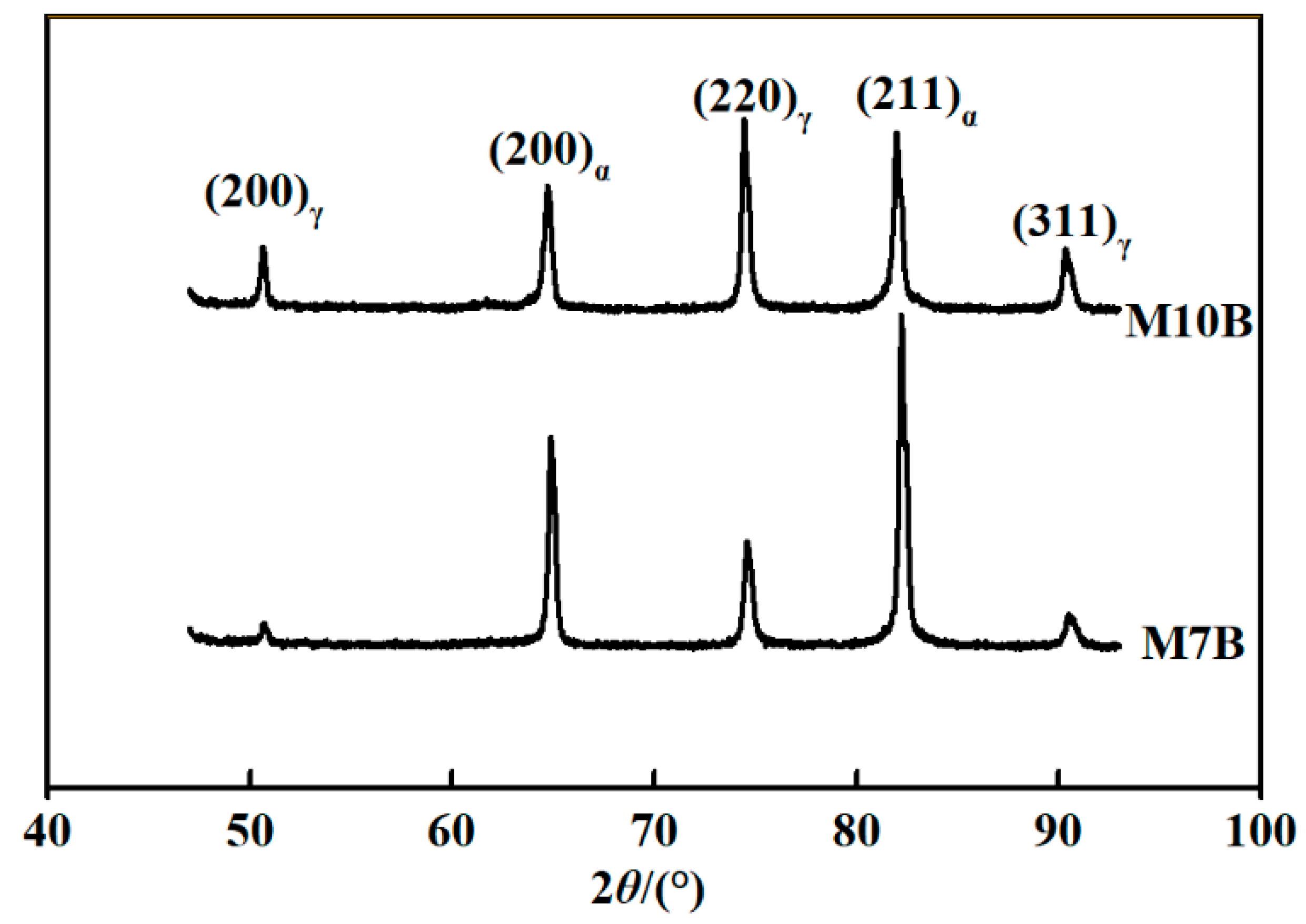
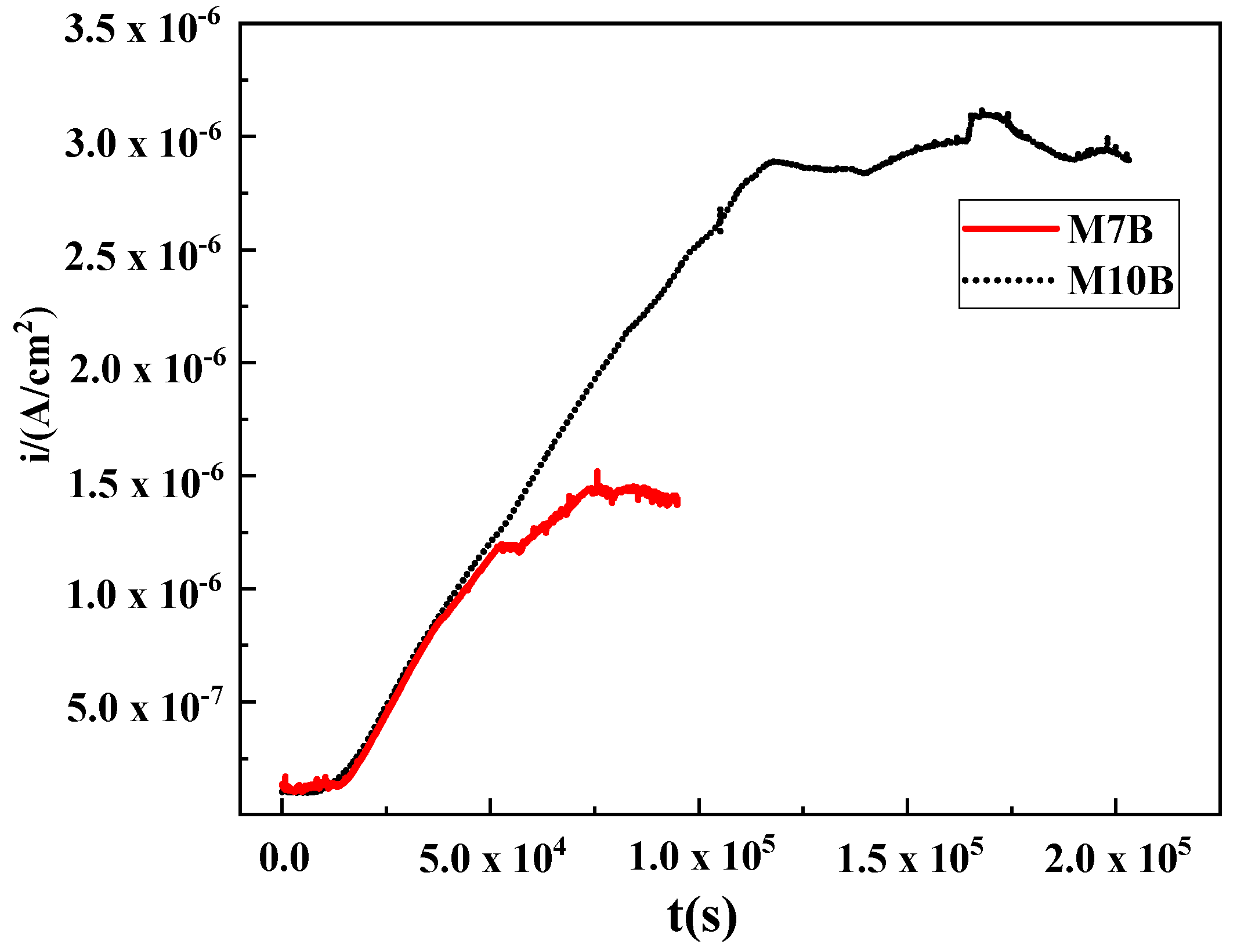
- Different Mn contents will cause changes in the volume fraction of austenite. The content of austenite in M10B steel is much higher than that in M7B. The elongation of M10B is higher than M7B, because more austenite tends to deform during the tensile test. The effective hydrogen diffusion coefficients of M7B and M10B steel are 1.08 × 10−7 cm2/s and 4.41 × 10−9 cm2/s, respectively. The behavior of hydrogen diffusion is also influenced by different content of austenite, which hinders the diffusion of hydrogen.
- When exposed to air, the hydrogen concentration in the M7B sample is 0.4 ppm. When M7B is exposed to 60% or higher RH, the hydrogen concentration increased to 0.8 ppm. For M7B, an increase of RH and a decrease of strain rate accelerate the hydrogen degradation with an increase of hydrogen concentration of the material, thus reducing its service performance.
- Similar hydrogen degradation was also found in M10B, as fracture strength and elongation decrease gradually under strain rate of 10−4 s−1 with the increase of relative humidity and hydrogen concentration, resulting in final failure. Elongation decreases gradually under a strain rate of 10−6 s−1, with higher relative humidity increasing hydrogen content in M10B.
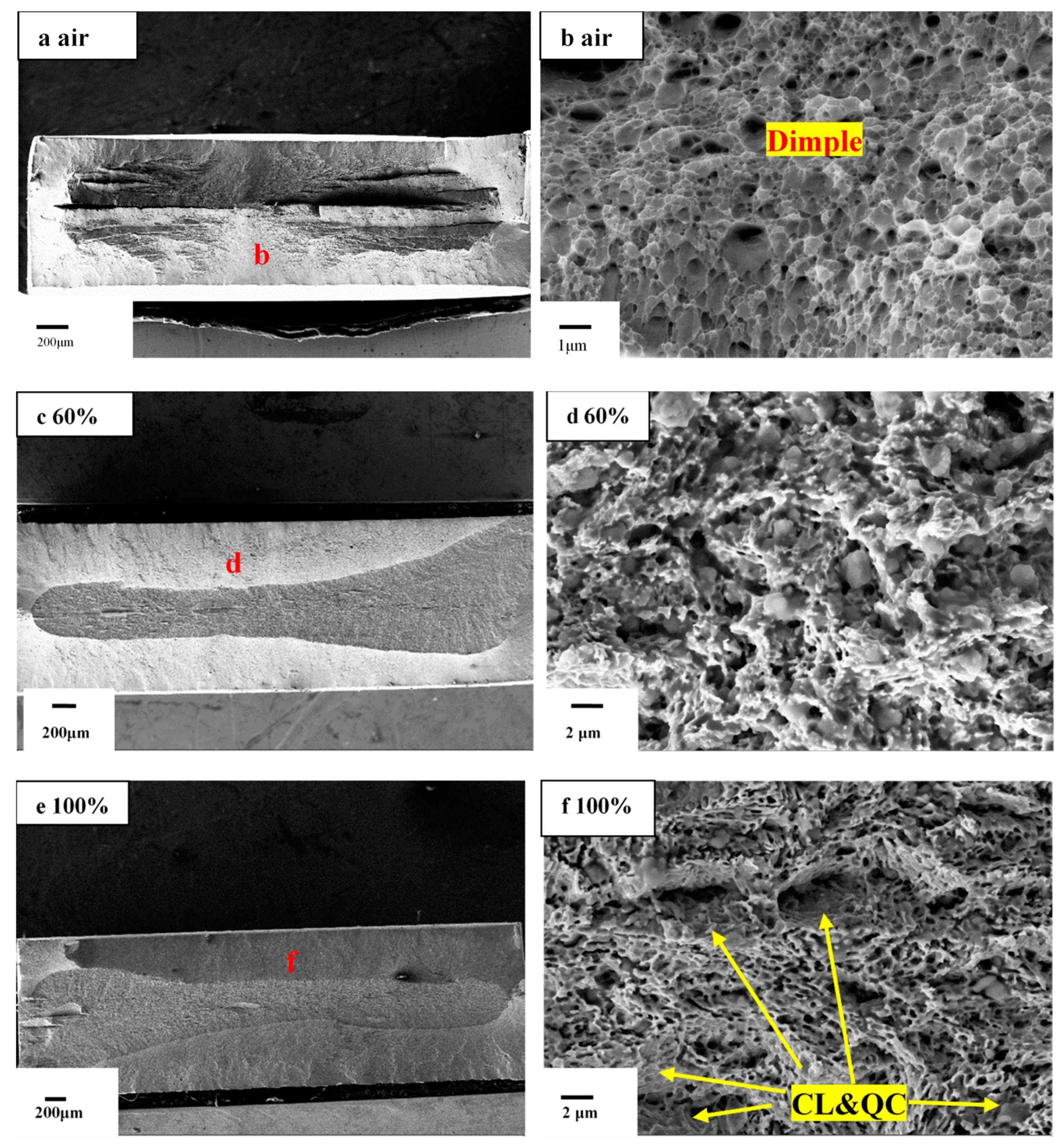
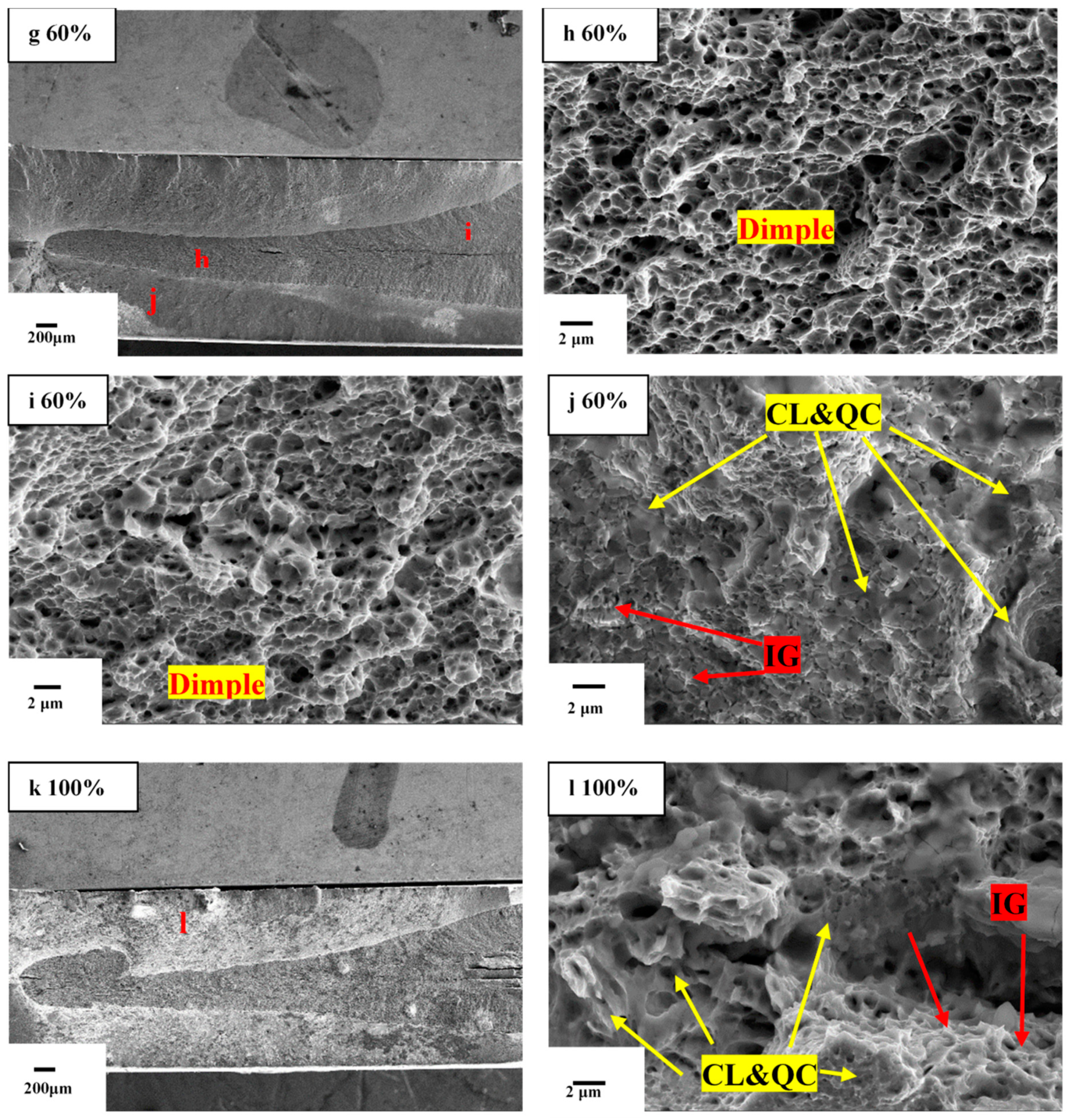
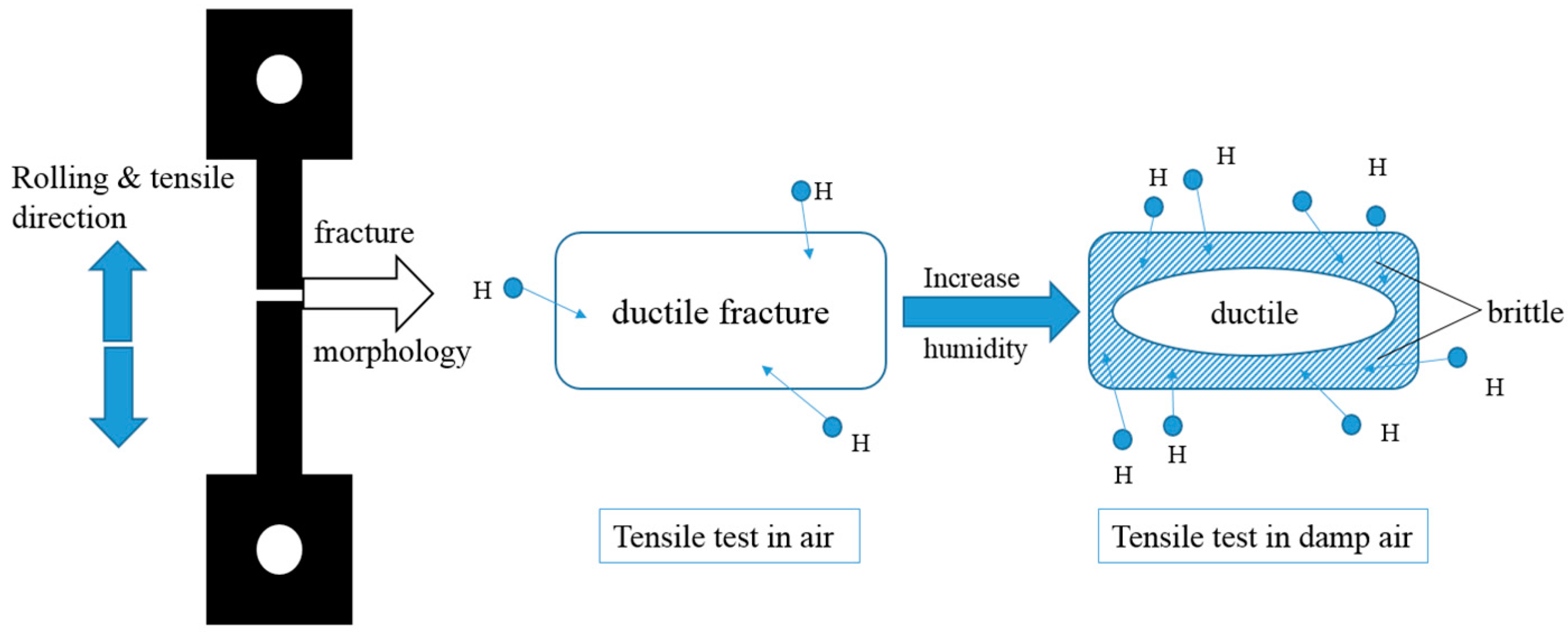
- Due to the entry of hydrogen into samples in environments with high relative humidity, fracture morphology changes from ductile mode with obvious dimples to brittle mode characterization with intergranular fracture.
- For M7B, the threshold stress does not significantly decrease with an increase in RH, but shows a better delayed cracking resistance than M10B. Eighty percent RH can be accepted as the critical humidity for M10B.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hui, W.; Zhang, H.; Zhang, Y.; Zhao, X.; Shao, C. Effect of nickel on hydrogen embrittlement behavior of medium-carbon high strength steels. Mater. Sci. Eng. A 2016, 674, 615–625. [Google Scholar] [CrossRef]
- Miller, R.L. Ultrafine-grained microstructures and mechanical properties of alloy steels. Metall. Mater. Trans. B 1972, 3, 905–912. [Google Scholar] [CrossRef]
- Han, J.; Lee, S.-J.; Lee, Y.-K.; Jung, J.-G. The effects of the initial martensite microstructure on the microstructure and tensile properties of intercritically annealed Fe–9Mn–0.05C steel. Acta Mater. 2014, 78, 369–377. [Google Scholar] [CrossRef]
- Gibbs, P.J.; De Moor, E.; Merwin, M.J.; Clausen, B.; Speer, J.G.; Matlock, D.K. Austenite Stability Effects on Tensile Behavior of Manganese-Enriched-Austenite Transformation-Induced Plasticity Steel. Metall. Mater. Trans. A 2011, 42, 3691–3702. [Google Scholar] [CrossRef]
- Lee, S.; De Cooman, B.C. On the Selection of the Optimal Intercritical Annealing Temperature for Medium Mn TRIP Steel. Metall. Mater. Trans. A 2013, 44, 5018–5024. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Lee, S.-J.; Santhosh Kumar, S.; Lee, K.; Cooman, B.C.D. Localized Deformation in Multiphase, Ultra-Fine-Grained 6 Pct Mn Transformation-Induced Plasticity Steel. Metall. Mater. Trans. A 2011, 42, 3638–3651. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Shi, J.; Wang, C.; Cao, W.; Sun, X.; Dong, H. Experimental and numerical analysis on formation of stable austenite during the intercritical annealing of 5Mn steel. Acta Mater. 2011, 59, 4002–4014. [Google Scholar] [CrossRef]
- Lee, S.; Lee, S.-J.; De Cooman, B.C. Austenite stability of ultrafine-grained transformation-induced plasticity steel with Mn partitioning. Scr. Mater. 2011, 65, 225–228. [Google Scholar] [CrossRef]
- Cao, W.Q.; Wang, M.Q.; Wang, C.; Shi, J.; Hui, W.J.; Dong, H. Microstructure and mechanical properties of Fe–0.2C–5Mn steel processed by ART-annealing. Mater. Sci. Eng. A 2011, 528, 6661–6666. [Google Scholar] [CrossRef]
- Jang, J.-M.; Kim, S.-J.; Kang, N.H.; Cho, K.-M.; Suh, D.-W. Effects of annealing conditions on microstructure and mechanical properties of low carbon, manganese transformation-induced plasticity steel. Met. Mater. Int. 2009, 15, 909–916. [Google Scholar] [CrossRef]
- Arlazarov, A.; Gouné, M.; Bouaziz, O.; Hazotte, A.; Petitgand, G.; Barges, P. Evolution of microstructure and mechanical properties of medium Mn steels during double annealing. Mater. Sci. Eng. A 2012, 542, 31–39. [Google Scholar] [CrossRef]
- Han, J.; Lee, Y.-K. The effects of the heating rate on the reverse transformation mechanism and the phase stability of reverted austenite in medium Mn steels. Acta Mater. 2014, 67, 354–361. [Google Scholar] [CrossRef]
- Furukawa, T.; Huang, H.; Matsumura, O. Effects of carbon content on mechanical properties of 5% Mn steels exhibiting transformation induced plasticity. Mater. Sci. Technol. 1994, 10, 964–970. [Google Scholar] [CrossRef]
- Suh, D.-W.; Park, S.-J.; Lee, T.-H.; Oh, C.-S.; Kim, S.-J. Influence of Al on the Microstructural Evolution and Mechanical Behavior of Low-Carbon, Manganese Transformation-Induced-Plasticity Steel. Metall. Mater. Trans. A 2009, 41, 397. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Estrin, Y.; De Cooman, B.C. Constitutive Modeling of the Mechanical Properties of V-added Medium Manganese TRIP Steel. Metall. Mater. Trans. A 2013, 44, 3136–3146. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, T. Dependence of strength–ductility characteristics on thermal history in lowcarbon, 5 wt-% Mn steels. Mater. Sci. Technol. 1989, 5, 465–470. [Google Scholar] [CrossRef]
- Dutton, R.; Nuttall, K.; Puls, M.P.; Simpson, L.A. Mechanisms of hydrogen induced delayed cracking in hydride forming materials. Metall. Trans. A 1977, 8, 1553–1562. [Google Scholar] [CrossRef]
- Steigerwald, E.A.; Schaller, F.W.; Troiano, A.R. The role of stress in hydrogen induced delayed failure. Trans. Metall. Soc. AIME 1960, 218, 832–841. [Google Scholar]
- Chu, W.-Y.; Hsiao, C.-M.; Ju, S.-Y.; Wang, C. Hydrogen induced delayed cracking of type III steel specimens. Corrosion 1982, 38, 446–452. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, S.; Dong, J.; Ke, W. Evolution of atmospheric corrosion of MnCuP weathering steel in a simulated coastal-industrial atmosphere. Corros. Sci. 2012, 59, 270–276. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Wu, L.; Han, R.; Sun, Y. Study of the corrosion behavior of weathering steels in atmospheric environments. Corros. Sci. 2013, 67, 1–10. [Google Scholar] [CrossRef]
- Cai, Y.; Zhao, Y.; Ma, X.; Zhou, K.; Chen, Y. Influence of environmental factors on atmospheric corrosion in dynamic environment. Corros. Sci. 2018, 137, 163–175. [Google Scholar] [CrossRef]
- Li, S.; Akiyama, E.; Yuuji, K.; Tsuzaki, K.; Uno, N.; Zhang, B. Hydrogen embrittlement property of a 1700-MPa-class ultrahigh-strength tempered martensitic steel. Sci. Technol. Adv. Mater. 2010, 11, 025005. [Google Scholar] [CrossRef] [PubMed]
- Omura, T. Hydrogen Entry and its Effect on Delayed Fracture Susceptibility of High Strength Steel Bolts under Atmospheric Corrosion. ISIJ Int. 2012, 52, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhang, Z.; Akiyama, E.; Tsuzaki, K.; Zhang, B. Evaluation of susceptibility of high strength steels to delayed fracture by using cyclic corrosion test and slow strain rate test. Corros. Sci. 2010, 52, 1660–1667. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, S.; Shen, L.; Zhou, Q.; Li, J.; Su, Y.; Qiao, L.; Yan, Y. The effect of hydrogen concentration on the fracture surface of medium Mn steels. Eng. Fail. Anal. 2020, 108, 104263. [Google Scholar] [CrossRef]
- Turnbull, A.; Saenz de Santa Maria, M.; Thomas, N.D. The effect of H2S concentration and pH on hydrogen permeation in AISI 410 stainless steel in 5% NaCl. Corros. Sci. 1989, 29, 89–104. [Google Scholar] [CrossRef]
- Kan, B.; Yang, Z.X.; Wang, Z.; Li, J.X.; Zhou, Q.J.; Su, Y.J.; Qiao, L.J.; Volinsky, A.A. Hydrogen redistribution under stress-induced diffusion and corresponding fracture behaviour of a structural steel. Mater. Sci. Technol. 2017, 33, 1539–1547. [Google Scholar] [CrossRef]
- Marsh, K.N. Recommended Reference Materials for the Realization of Physicochemical Properties; Blackwell Scientific Publication: Oxford, UK, 1987; pp. 152–162. [Google Scholar]
- Wei, G.; Wang, G.; Xu, C.; Ju, X.; Xing, L.; Du, X.; Yang, Y. Selection principles and thermophysical properties of high temperature phase change materials for thermal energy storage: A review. Renew. Sustain. Energy Rev. 2018, 81, 1771–1786. [Google Scholar] [CrossRef]
- Herington, E.F.G.; Durst, R.A.; Cali, J.P. Recommended Reference Materials for Realization of Physicochemical Properties. Pure Appl. Chem. 1974, 40, 391–472. [Google Scholar]
- Fu, H.; Wang, W.; Zhao, H.; Jin, F.; Li, J. Study of hydrogen-induced delayed fracture in high-Mn TWIP/TRIP steels during in situ electrochemical hydrogen-charging: Role of microstructure and strain rate in crack initiation and propagation. Corros. Sci. 2020, 162, 108191. [Google Scholar] [CrossRef]
- Tsong-Pyng, P.; Altstetter, C.J. Effects of deformation on hydrogen permeation in austenitic stainless steels. Acta Metall. 1986, 34, 1771–1781. [Google Scholar] [CrossRef]
- Jebaraj, J.J.M.; Morrison, D.J.; Suni, I.I. Hydrogen diffusion coefficients through Inconel 718 in different metallurgical conditions. Corros. Sci. 2014, 80, 517–522. [Google Scholar] [CrossRef]
- McNabb, A.; Foster, P.K. A new analysis of the diffusion of hydrogen in iron and ferritic steels. Trans. Metall. Soc. AIME 1963, 227, 618–627. [Google Scholar]
- Chen, J.H.; Cao, R. Chapter 1—Introduction. In Micromechanism of Cleavage Fracture of Metals; Chen, J.H., Cao, R., Eds.; Butterworth-Heinemann: Boston, MA, USA, 2015; pp. 1–54. [Google Scholar]
- Beachem, C.D. Orientation of cleavage facets in tempered martensite (quasi-cleavage) by single surface trace analysis. Metall. Trans. 1973, 4, 1999–2000. [Google Scholar] [CrossRef]
- Lynch, S.P. 2—Hydrogen embrittlement (HE) phenomena and mechanisms. In Stress Corrosion Cracking; Raja, V.S., Shoji, T., Eds.; Woodhead Publishing: Cambridge, UK, 2011; pp. 90–130. [Google Scholar]
- Birbilis, N.; Hinton, B. 19—Corrosion and corrosion protection of aluminium. In Fundamentals of Aluminium Metallurgy; Lumley, R., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 574–604. [Google Scholar]
- Beachem, C.D. A new model for hydrogen-assisted cracking (hydrogen “embrittlement”). Metall. Mater. Trans. B 1972, 3, 441–455. [Google Scholar] [CrossRef]
- Akiyama, E.; Matsukado, K.; Wang, M.; Tsuzaki, K. Evaluation of hydrogen entry into high strength steel under atmospheric corrosion. Corros. Sci. 2010, 52, 2758–2765. [Google Scholar] [CrossRef]
- Tao, J.; Hu, S.; Yan, F.; Zhang, Y.; Langley, M. A study of the mechanism of delamination fracture in bainitic magnetic yoke steel. Mater. Des. 2016, 108, 429–439. [Google Scholar] [CrossRef]


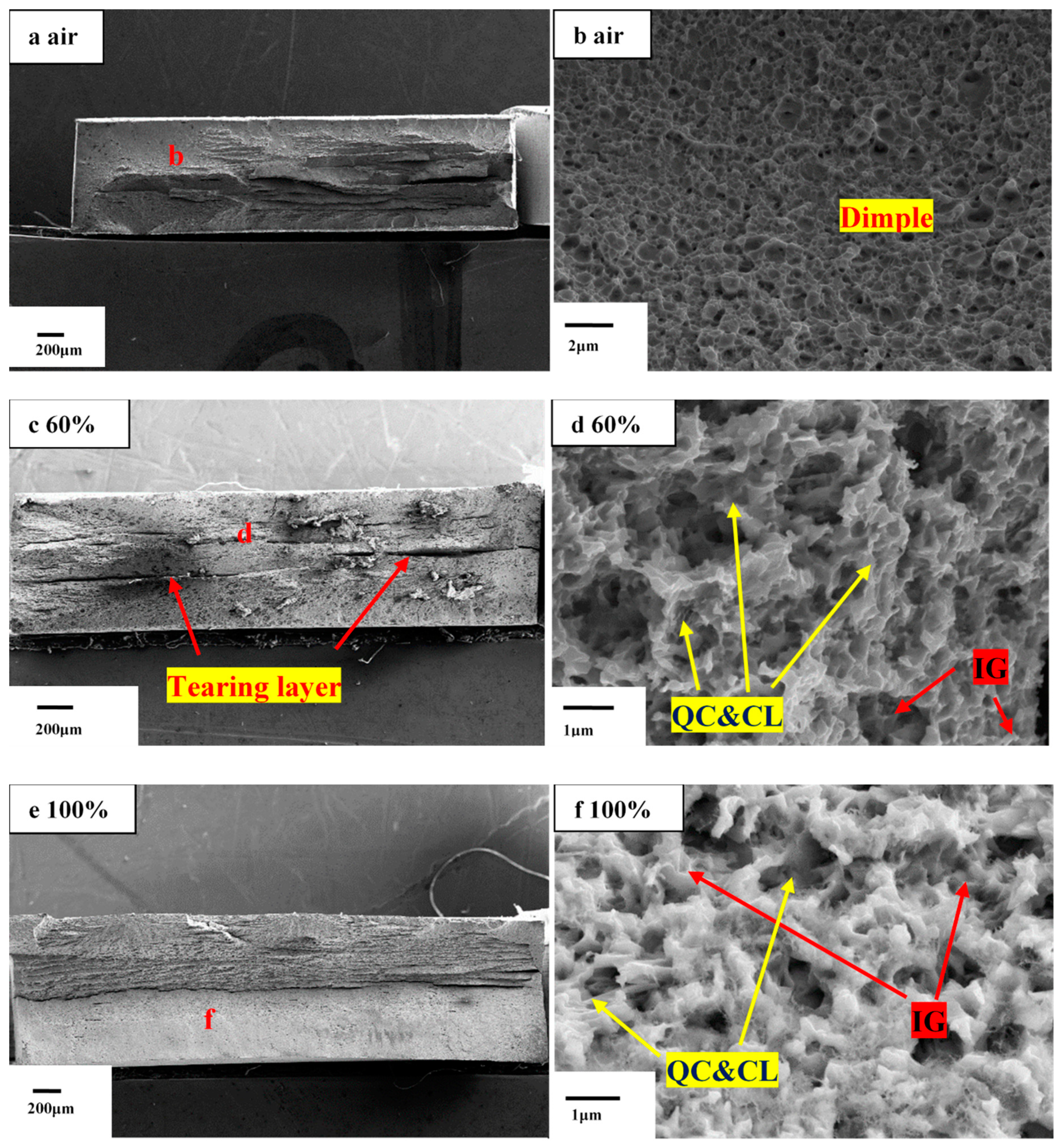
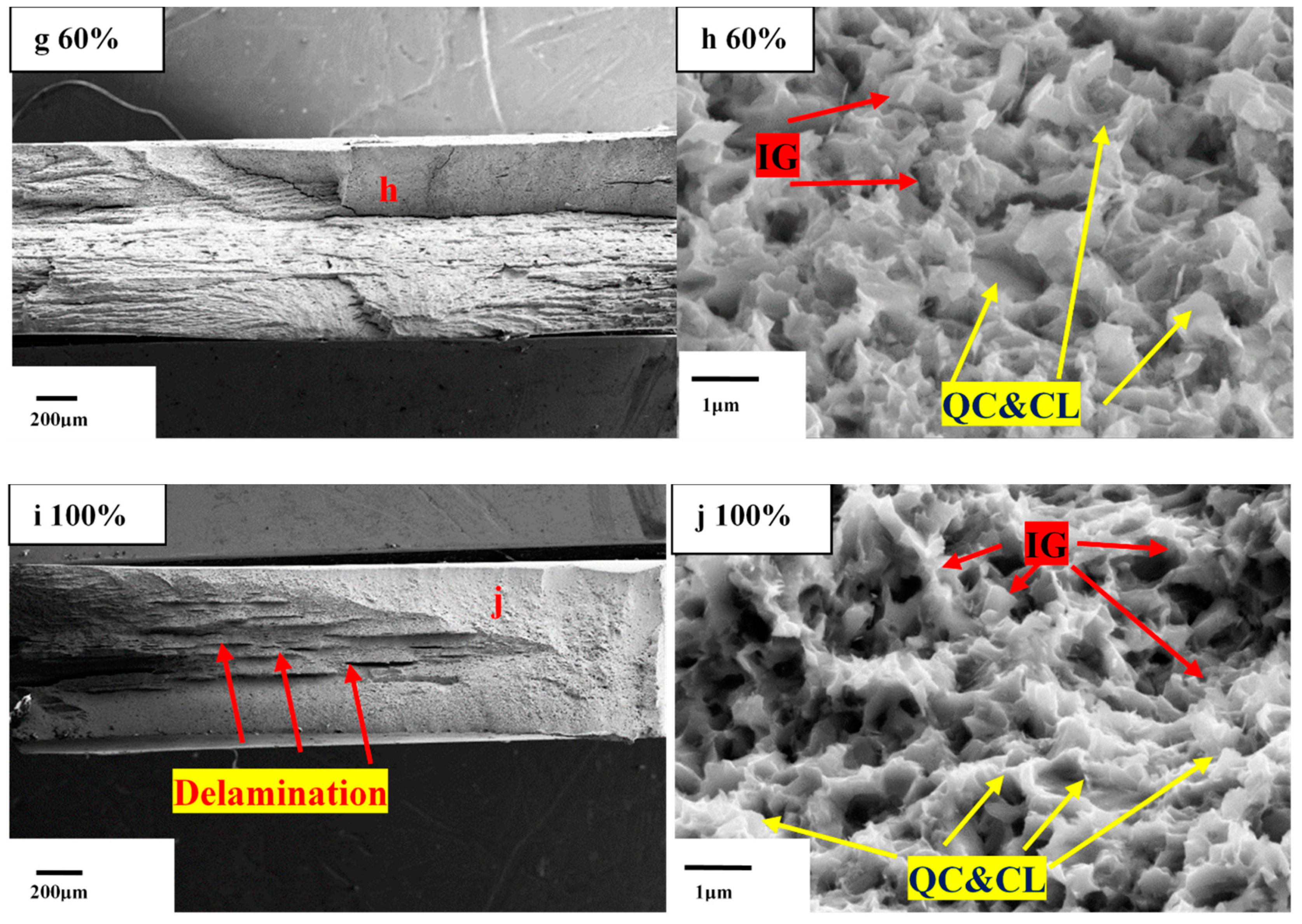

| Material/Element | C | Si | Mn | S | Al | V | Fe | Heat Treatment | YS/TS, MPa | Elongation, % | PSE, GPa•% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| M7B | 0.20 | 0.24 | 7.51 | 0.014 | 0.035 | 0.11 | Bal | batch annealing 12 h | 1450/1614 | 22.0 | 35.5 |
| M10B | 0.15 | 0.17 | 10.4 | 0.013 | 1.49 | <0.01 | Bal | batch annealing 12 h | 980/1390 | 30.3 | 42.1 |
| Strain Rate | Humidity | Fracture Strength/MPa | Elongation /% | PSE/ GPa•% | Sensitivity of HE /% | CH/wppm |
|---|---|---|---|---|---|---|
| 10−4 s−1 | Air (about 40% RH) | 1594 | 22 | 35.07 | 0 | 0.35 ± 0.03 |
| 10−5 s−1 | 60% RH | 1381 | 15 | 20.7 | 31.8 | 1.02 ± 0.08 |
| 80% RH | 1395 | 13.5 | 18.8 | 38.7 | 1.63 ± 0.05 | |
| 100% RH | 1455 | 7.5 | 10.9 | 65.9 | 1.81 ± 0.12 | |
| 10−6 s−1 | 60% RH | 1406 | 9 | 12.7 | 59.1 | 1.68 ± 0.04 |
| 80% RH | 1380 | 8 | 11.0 | 63.6 | 1.92 ± 0.03 | |
| 100% RH | 1415 | 6 | 8.5 | 72.7 | 2.32 ± 0.15 |
| Strain Rate | Humidity | Fracture Strength/MPa | Elongation /% | PSE/GPa•% | Sensitivity of HE /% | CH/wppm |
|---|---|---|---|---|---|---|
| 10−4 s−1 | Air (about 40% RH) | 1380 | 30.3 | 41.81 | 0 | 0.43 ± 0.04 |
| 10−4 s−1 | 60% RH | 1330 | 29.2 | 38.84 | 3.6 | 0.48 ± 0.05 |
| 80% RH | 1260 | 22.5 | 28.35 | 25.7 | 0.91 ± 0.14 | |
| 100% RH | 1200 | 20.87 | 25.04 | 31.1 | 1.35 ± 0.08 | |
| 10−6 s−1 | 60% RH | 1050 | 10.1 | 10.61 | 67.0 | 2.13 ± 0.21 |
| 80% RH | 1030 | 7.3 | 7.52 | 75.9 | 2.31 ± 0.15 | |
| 100% RH | 1010 | 6.6 | 6.67 | 78.2 | 2.23 ± 0.27 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Xu, J.; Shen, L.; Zhou, Q.; Su, Y.; Qiao, L.; Yan, Y. Effect of Relative Humidity on Mechanical Degradation of Medium Mn Steels. Materials 2020, 13, 1304. https://doi.org/10.3390/ma13061304
Liu Q, Xu J, Shen L, Zhou Q, Su Y, Qiao L, Yan Y. Effect of Relative Humidity on Mechanical Degradation of Medium Mn Steels. Materials. 2020; 13(6):1304. https://doi.org/10.3390/ma13061304
Chicago/Turabian StyleLiu, Qingyang, Juanping Xu, Liancheng Shen, Qingjun Zhou, Yanjing Su, Lijie Qiao, and Yu Yan. 2020. "Effect of Relative Humidity on Mechanical Degradation of Medium Mn Steels" Materials 13, no. 6: 1304. https://doi.org/10.3390/ma13061304





