Hybrid TiO2–Polyaniline Photocatalysts and their Application in Building Gypsum Plasters
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Photocatalysts
2.3. Preparation of Photocatalytic Gypsum Plaster
2.4. Characterization Techniques
2.5. Photocatalytic Activity
3. Results
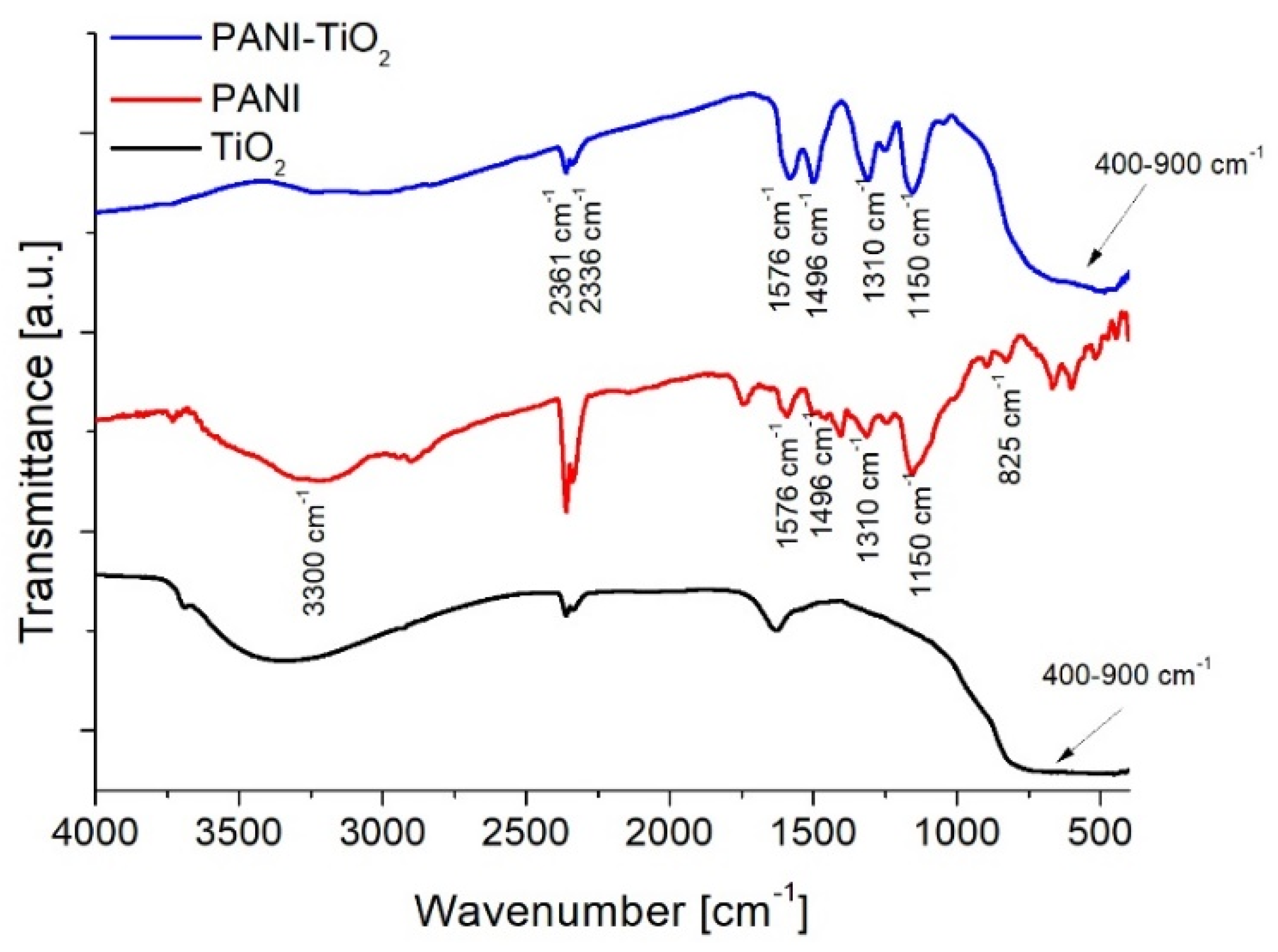
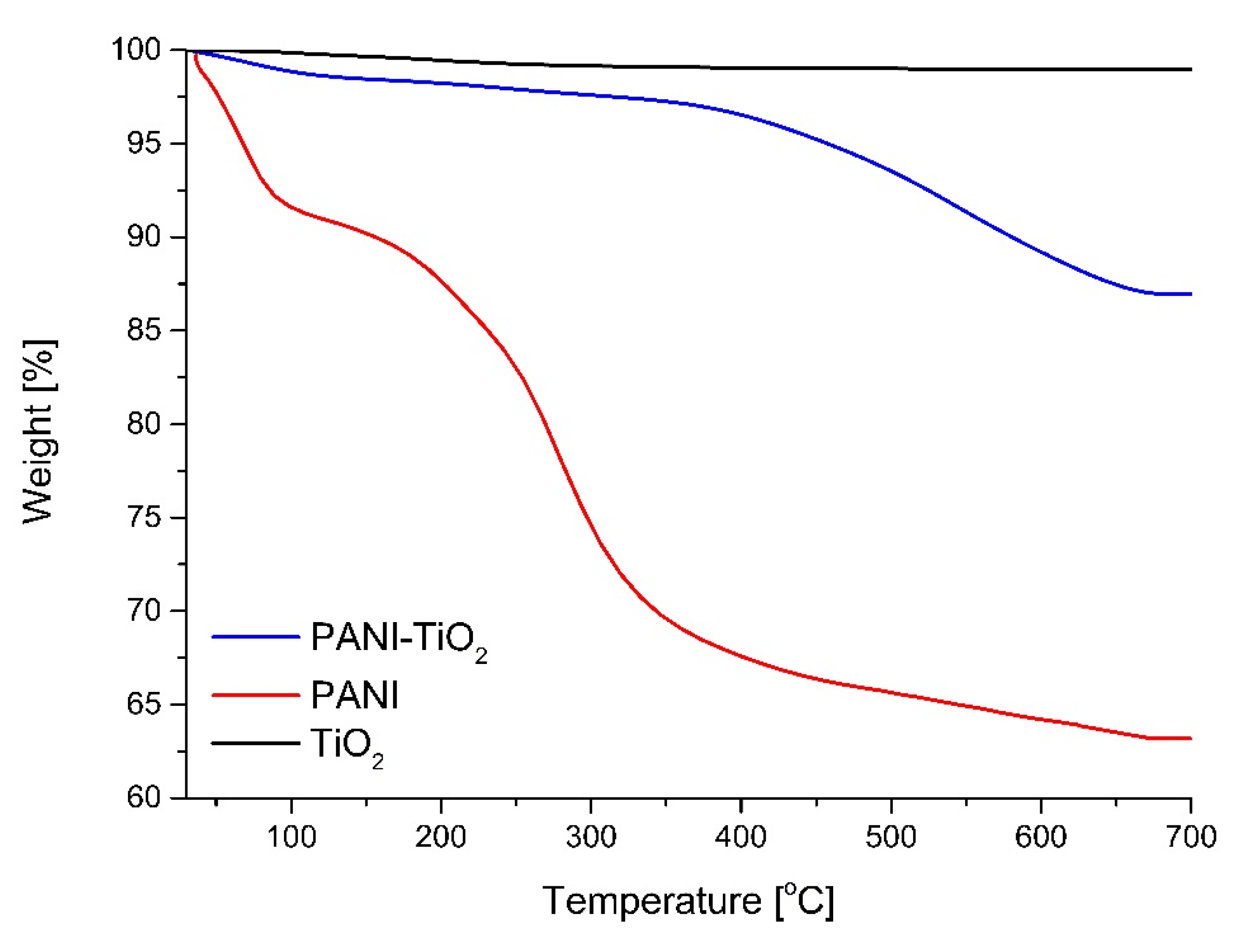
3.1. Characterization of Photocatalysts
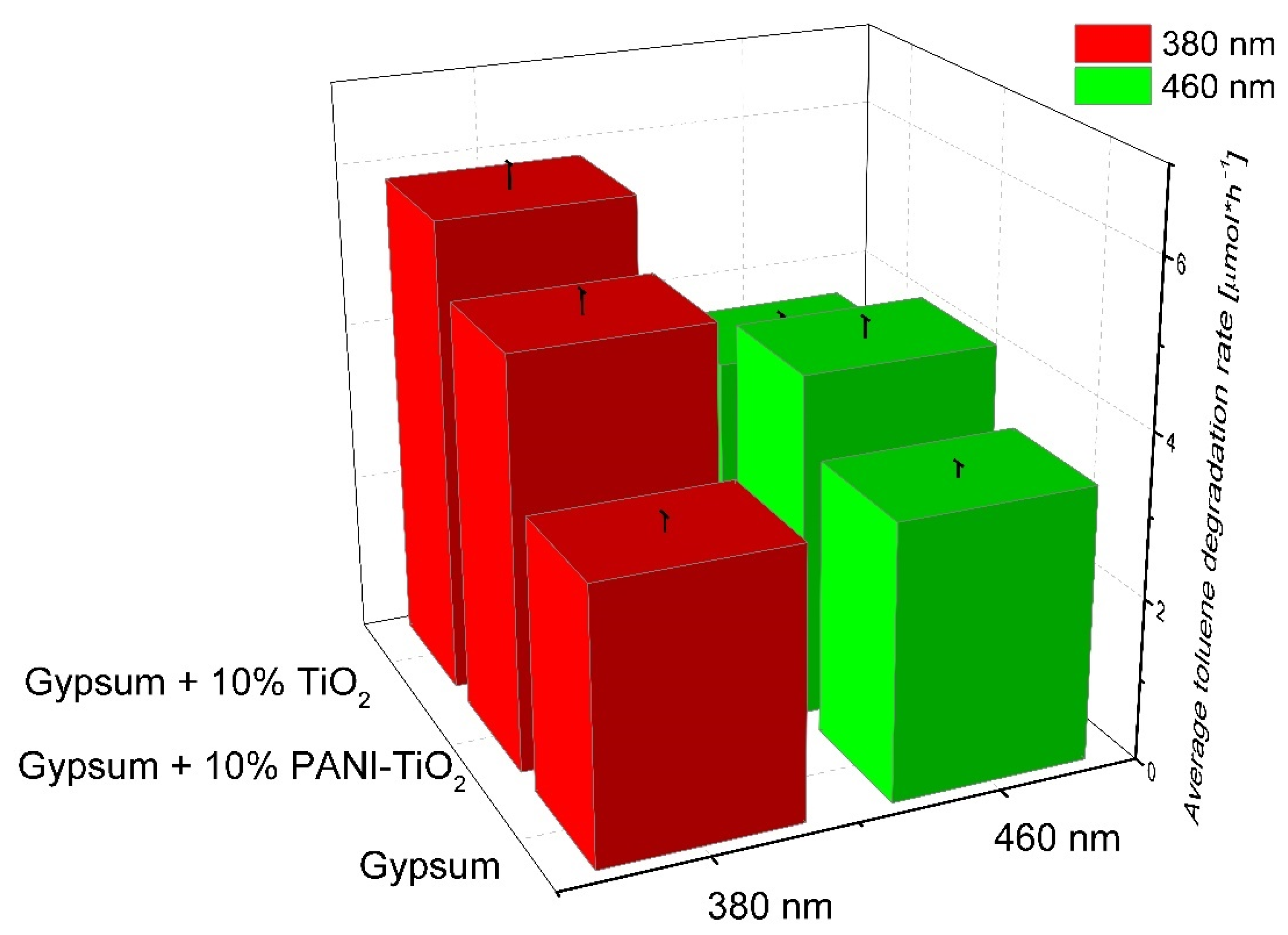
3.2. Photocatalytic Activity
4. Photocatalytic Gypsum Plasters
5. Discussion and Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced Oxidation Processes (AOP) for Water Purification and Recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Guo, N.; Liang, Y.; Lan, S.; Liu, L.; Zhang, J.; Ji, G.; Gan, S. Microscale Hierarchical Three-Dimensional Flowerlike TiO 2 /PANI Composite: Synthesis, Characterization, and Its Remarkable Photocatalytic Activity on Organic Dyes under UV-Light and Sunlight Irradiation. J. Phys. Chem. C 2014, 118, 18343–18355. [Google Scholar] [CrossRef]
- Veres, Á.; Rica, T.; Janovák, L.; Dömök, M.; Buzás, N.; Zöllmer, V.; Seemann, T.; Richardt, A.; Dékány, I. Silver and Gold Modified Plasmonic TiO2 Hybrid Films for Photocatalytic Decomposition of Ethanol under Visible Light. Catal. Today 2012, 181, 156–162. [Google Scholar] [CrossRef]
- Fan, J.W.; Liu, X.H.; Zhang, J. The Synthesis of TiO2 and TiO2-Pt and Their Application in the Removal of Cr (VI). Environ. Technol. 2011, 32, 427–437. [Google Scholar] [CrossRef]
- Espino-Estévez, M.R.; Fernández-Rodríguez, C.; González-Díaz, O.M.; Araña, J.; Espinós, J.P.; Ortega-Méndez, J.A.; Doña-Rodríguez, J.M. Effect of TiO2-Pd and TiO2-Ag on the Photocatalytic Oxidation of Diclofenac, Isoproturon and Phenol. Chem. Eng. J. 2016, 298, 82–95. [Google Scholar] [CrossRef]
- Gilson, R.C.; Black, K.C.L.; Lane, D.D.; Achilefu, S. Hybrid TiO2 –Ruthenium Nano-Photosensitizer Synergistically Produces Reactive Oxygen Species in Both Hypoxic and Normoxic Conditions. Angew. Chemie-Int. Ed. 2017, 56, 10717–10720. [Google Scholar] [CrossRef]
- Suwannaruang, T.; Kamonsuangkasem, K.; Kidkhunthod, P.; Chirawatkul, P.; Saiyasombat, C.; Chanlek, N.; Wantala, K. Influence of Nitrogen Content Levels on Structural Properties and Photocatalytic Activities of Nanorice-like N-Doped TiO2 with Various Calcination Temperatures. Mater. Res. Bull. 2018, 105, 265–276. [Google Scholar] [CrossRef]
- Irie, H.; Watanabe, Y.; Hashimoto, K. Carbon-Doped Anatase TiO2 Powders as a Visible-Light Sensitive Photocatalyst. Chem. Lett. 2003, 32, 772–773. [Google Scholar] [CrossRef]
- Rockafellow, E.M.; Stewart, L.K.; Jenks, W.S. Is Sulfur-Doped TiO2 an Effective Visible Light Photocatalyst for Remediation? Appl. Catal. B Environ. 2009, 91, 554–562. [Google Scholar] [CrossRef]
- Chowdhury, P.; Moreira, J.; Gomaa, H.; Ray, A.K. Visible-Solar-Light-Driven Photocatalytic Degradation of Phenol with Dye-Sensitized TiO2: Parametric and Kinetic Study. Ind. Eng. Chem. Res. 2012, 51, 4523–4532. [Google Scholar] [CrossRef]
- Shang, J.; Zhao, F.; Zhu, T.; Li, J. Photocatalytic Degradation of Rhodamine B by Dye-Sensitized TiO2 under Visible-Light Irradiation. Sci. China Chem. 2011, 54, 167–172. [Google Scholar] [CrossRef]
- Yang, C.; Dong, W.; Cui, G.; Zhao, Y.; Shi, X.; Xia, X.; Tang, B.; Wang, W. Enhanced Photocatalytic Activity of PANI/TiO2 Due to Their Photosensitization-Synergetic Effect. Electrochim. Acta 2017, 247, 486–495. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, Y.; Yang, H.; Xu, S.; Jiang, L.; Dan, Y. A New Carboxyl-Functionalized P3HT/TiO2 Composite Photocatalyst: Preparation, Structure and Prompted Activity through Interfacial Engineering. Proc. Nat. Res. Soc. 2017, 1. [Google Scholar] [CrossRef][Green Version]
- Faisal, M.; Harraz, F.A.; Ismail, A.A.; El-Toni, A.M.; Al-Sayari, S.A.; Al-Hajry, A.; Al-Assiri, M.S. Polythiophene/Mesoporous SrTiO3 Nanocomposites with Enhanced Photocatalytic Activity under Visible Light. Sep. Purif. Technol. 2018, 190, 33–44. [Google Scholar] [CrossRef]
- Xu, J.; Hu, Y.; Zeng, C.; Zhang, Y.; Huang, H. Polypyrrole Decorated BiOI Nanosheets: Efficient Photocatalytic Activity for Treating Diverse Contaminants and the Critical Role of Bifunctional Polypyrrole. J. Colloid Interface Sci. 2017, 505, 719–727. [Google Scholar] [CrossRef]
- Nguyen, H.Q.; Rainbolt, E.A.; Sista, P.; Stefan, M.C. Synthesis and Polymerization of Fused-Ring Thienodipyrrole Monomers. Macromol. Chem. Phys. 2012, 213, 425–430. [Google Scholar] [CrossRef]
- Kang, E.T.; Neoh, K.G.; Tan, K.L. Polyaniline: A Polymer with Many Interesting Intrinsic Redox States. Prog. Polym. Sci. 1998, 23, 277–324. [Google Scholar] [CrossRef]
- Asadollahi, A.; Sohrabnezhad, S.; Ansari, R.; Zanjanchi, M.A. P-n Heterojuction in Organic (Polyaniline)-Inorganic (Ag2CO3) Polymer-Based Heterojuction Photocatalyst. Mater. Sci. Semicond. Process. 2018, 87, 119–125. [Google Scholar] [CrossRef]
- Bu, Y.; Chen, Z. Role of Polyaniline on the Photocatalytic Degradation and Stability Performance of the Polyaniline/Silver/Silver Phosphate Composite under Visible Light. ACS Appl. Mater. Interfaces 2014, 6, 17589–17598. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, Y.; Ji, X.; Huang, S.; Xia, J.; Xie, M.; Yan, J.; Xu, H.; Li, H. Conjugated Conducting Polymers PANI Decorated Bi12O17Cl2 Photocatalyst with Extended Light Response Range and Enhanced Photoactivity. Appl. Surf. Sci. 2019, 464, 552–561. [Google Scholar] [CrossRef]
- Wang, Q.; Hui, J.; Li, J.; Cai, Y.; Yin, S.; Wang, F.; Su, B. Photodegradation of Methyl Orange with PANI-Modified BiOCl Photocatalyst under Visible Light Irradiation. Appl. Surf. Sci. 2013, 283, 577–583. [Google Scholar] [CrossRef]
- Yu, W.J.; Cheng, Y.; Zou, T.; Liu, Y.; Wu, K.; Peng, N. Preparation of BiPO4-Polyaniline Hybrid and Its Enhanced Photocatalytic Performance. Nano 2018, 13, 1850009. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, Z.; Wang, W.; Ju, T.; She, H.; Wang, Q. Synthesis and Characterization of Polyaniline-Modified BiOI: A Visible-Light-Response Photocatalyst. J. Mater. Sci. Mater. Electron. 2018, 29, 18343–18351. [Google Scholar] [CrossRef]
- Hao, X.; Gong, J.; Ren, L.; Zhang, D.; Xiao, X.; Jiang, Y.; Zhang, F.; Tong, Z. Preparation of Polyaniline Modified BiOBr with Enhanced Photocatalytic Activities. Funct. Mater. Lett. 2017, 10, 1750040. [Google Scholar] [CrossRef]
- Shang, M.; Wang, W.; Sun, S.; Ren, J.; Zhou, L.; Zhang, L. Efficient Visible Light-Induced Photocatalytic Degradation of Contaminant by Spindle-like PANI/BiVO4. J. Phys. Chem. C 2009, 113, 20228–20233. [Google Scholar] [CrossRef]
- Xiong, P.; Chen, Q.; He, M.; Sun, X.; Wang, X. Cobalt Ferrite-Polyaniline Heteroarchitecture: A Magnetically Recyclable Photocatalyst with Highly Enhanced Performances. J. Mater. Chem. 2012, 22, 17485–17493. [Google Scholar] [CrossRef]
- Feizpoor, S.; Habibi-Yangjeh, A.; Yubuta, K.; Vadivel, S. Fabrication of TiO2/CoMoO4/PANI Nanocomposites with Enhanced Photocatalytic Performances for Removal of Organic and Inorganic Pollutants under Visible Light. Mater. Chem. Phys. 2019, 224, 10–21. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, J.; Meng, G.; Guo, X.; Liu, C.; Liu, Z. One-Step Synthesis of Novel PANI-Fe3O4@ZnO Core-Shell Microspheres: An Efficient Photocatalyst under Visible Light Irradiation. Appl. Surf. Sci. 2016, 366, 486–493. [Google Scholar] [CrossRef]
- Jing, L.; Xu, Y.; Xie, M.; Liu, J.; Deng, J.; Huang, L.; Xu, H.; Li, H. Three Dimensional Polyaniline/MgIn2S4 Nanoflower Photocatalysts Accelerated Interfacial Charge Transfer for the Photoreduction of Cr(VI), Photodegradation of Organic Pollution and Photocatalytic H2 Production. Chem. Eng. J. 2019, 360, 1601–1612. [Google Scholar] [CrossRef]
- Chen, Q.; He, Q.; Lv, M.; Liu, X.; Wang, J.; Lv, J. The Vital Role of PANI for the Enhanced Photocatalytic Activity of Magnetically Recyclable N-K2Ti4O9/MnFe2O4/PANI Composites. Appl. Surf. Sci. 2014, 311, 230–238. [Google Scholar] [CrossRef]
- Arshadnia, I.; Movahedi, M.; Rasouli, N. SnFe2O4/SnO2/PANI Magnetically Separable Photocatalyst for Decolorization of Two Dye Mixture in Aqueous Solution. Surfaces and Interfaces 2017, 8, 91–96. [Google Scholar] [CrossRef]
- Niu, B.; Xu, Z. A Stable Ta3N5@PANI Core-Shell Photocatalyst: Shell Thickness Effect, High-Efficient Photocatalytic Performance and Enhanced Mechanism. J. Catal. 2019, 371, 175–184. [Google Scholar] [CrossRef]
- Li, W.; Tian, Y.; Zhao, C.; Zhang, Q.; Geng, W. Synthesis of Magnetically Separable Fe3O4 at PANI/TiO2 Photocatalyst with Fast Charge Migration for Photodegradation of EDTA under Visible-Light Irradiation. Chem. Eng. J. 2016, 303, 282–291. [Google Scholar] [CrossRef]
- Kundu, S.; Satpati, B.; Kar, T.; Pradhan, S.K. Microstructure Characterization of Hydrothermally Synthesized PANI/V2O5·nH2O Heterojunction Photocatalyst for Visible Light Induced Photodegradation of Organic Pollutants and Non-Absorbing Colorless Molecules. J. Hazard. Mater. 2017, 339, 161–173. [Google Scholar] [CrossRef]
- Zou, T.; Wang, C.; Tan, R.; Song, W.; Cheng, Y. Preparation of Pompon-like ZnO-PANI Heterostructure and Its Applications for the Treatment of Typical Water Pollutants under Visible Light. J. Hazard. Mater. 2017, 338, 276–286. [Google Scholar] [CrossRef]
- Carević, M.V.; Abazović, N.D.; Mitrić, M.N.; Ćirić-Marjanović, G.; Mojović, M.D.; Ahrenkiel, S.P.; Čomor, M.I. Properties of Zirconia/Polyaniline Hybrid Nanocomposites and Their Application as Photocatalysts for Degradation of Model Pollutants. Mater. Chem. Phys. 2018, 205, 130–137. [Google Scholar] [CrossRef]
- Ahmad, R.; Mondal, P.K. Adsorption and Photodegradation of Methylene Blue by Using PAni/TiO2 Nanocomposite. J. Dispers. Sci. Technol. 2012, 33, 380–386. [Google Scholar] [CrossRef]
- Pan, H.; Liao, W.; Sun, N.; Murugananthan, M.; Zhang, Y. Highly Efficient and Visible Light Responsive Heterojunction Composites as Dual Photoelectrodes for Photocatalytic Fuel Cell. Catalysts 2018, 8, 30. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Q.; Liu, Y.; Xiong, R.; Pan, C.; Shi, J. Synthesis and Photocatalytic Activity of Polyaniline-TiO2 Composites with Bionic Nanopapilla Structure. J. Nanoparticle Res. 2011, 13, 3157–3165. [Google Scholar] [CrossRef]
- Yu, Q.L.; Brouwers, H.J.H. Design of a Novel Photocatalytic Gypsum Plaster: With the Indoor Air Purification Property. Adv. Mater. Res. 2013, 651, 751–756. [Google Scholar] [CrossRef]
- Zajac, K.; Janus, M.; Morawski, A.W. Improved Self-Cleaning Properties of Photocatalytic Gypsum Plaster Enriched with Glass Fiber. Materials (Basel) 2019, 12, 357. [Google Scholar] [CrossRef] [PubMed]
- Janus, M.; Zatorska, J.; Zając, K.; Kusiak-Nejman, E.; Czyżewski, A.; Morawski, A.W. The Mechanical and Photocatalytic Properties of Modified Gypsum Materials. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2018, 236–237, 1–9. [Google Scholar] [CrossRef]
- Gnayem, H.; Uvarov, V.; Lahad, O.; Sasson, Y. Hybrid Bismuth Oxyhalides@gypsum as Self-Cleaning Composites: Novel Aspects of a Sustainable Photocatalytic Technology for Solar Environmental Cleanup. RSC Adv. 2015, 5, 66650–66656. [Google Scholar] [CrossRef]
- Singh, V.P.; Mishra, D.; Kabachkov, E.N.; Shul, Y.M.; Vaish, R. The Characteristics of BiOCl/Plaster of Paris Composites and Their Photocatalytic Performance under Visible Light Illumination for Self- Cleaning. Mater. Sci. Energy Technol. 2020, 3, 299–307. [Google Scholar] [CrossRef]
- Xia, Y.; Wiesinger, J.M.; MacDiarmid, A.G.; Epstein, A.J. Camphorsulfonic Acid Fully Doped Polyaniline Emeraldine Salt: Conformations in Different Solvents Studied by an Ultraviolet/Visible/Near-Infrared Spectroscopic Method. Chem. Mater. 1995, 7, 443–445. [Google Scholar] [CrossRef]
- Nair, R.V.; Gayathri, P.K.; Gummaluri, V.S.; Nambissan, P.M.G.; Vijayan, C. Large Bandgap Narrowing in Rutile TiO2 Aimed towards Visible Light Applications and Its Correlation with Vacancy-Type Defects History and Transformation. J. Phys. D. Appl. Phys. 2018, 51. [Google Scholar] [CrossRef]
- Rohom, A.B.; Londhe, P.U.; Mahapatra, S.K.; Kulkarni, S.K.; Chaure, N.B. Electropolymerization of Polyaniline Thin Films. High Perform. Polym. 2014, 26, 641–646. [Google Scholar] [CrossRef]
- Banerjee, S.; Sarmah, S.; Kumar, A. Photoluminescence Studies in HCl-Doped Polyaniline Nanofibers. J. Opt. 2009, 38, 124–130. [Google Scholar] [CrossRef]
- Asha; Goyal, S.L.; Kumar, D.; Kumar, S.; Kishore, N. Synthesis and Characterization of Polyaniline/TiO2 Composites. Indian J. Pure Appl. Phys. 2014, 52, 341–347. [Google Scholar]
- Qi, Y.N.; Xu, F.; Sun, L.X.; Zeng, J.L.; Liu, Y.Y. Thermal Stability and Glass Transition Behavior of PANI/α-Al 2O3 Composites. J. Therm. Anal. Calorim. 2008, 94, 553–557. [Google Scholar] [CrossRef]
- Schnitzler, D.C.; Meruvia, M.S.; Hümmelgen, I.A.; Zarbin, A.J.G. Preparation and Characterization of Novel Hybrid Materials Formed from (Ti,Sn)O2 Nanoparticles and Polyaniline. Chem. Mater. 2003, 15, 4658–4665. [Google Scholar] [CrossRef]
- Brédas, J.L.; Silbey, R.; Boudreaux, D.S.; Chance, R.R. Chain-Length Dependence of Electronic and Electrochemical Properties of Conjugated Systems: Polyacetylene, Polyphenylene, Polythiophene, and Polypyrrole. J. Am. Chem. Soc. 1983, 105, 6555–6559. [Google Scholar] [CrossRef]
- Wysocka, I.; Markowska-Szczupak, A.; Szweda, P.; Ryl, J.; Endo-Kimura, M.; Kowalska, E.; Nowaczyk, G.; Zielińska-Jurek, A. Gas-phase Removal of Indoor Volatile Organic Compounds and Airborne Microorganisms over Mono- and Bimetal-modified (Pt, Cu, Ag) Titanium(IV) Oxide Nanocomposites. Indoor Air 2019, 29, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Wysocka, I.; Kowalska, E.; Ryl, J.; Nowaczyk, G.; Zieli, A. Morphology, Photocatalytic and Antimicrobial Properties of TiO 2 Modified with Mono- and Bimetallic Copper, Platinum and Silver Nanoparticles. Nanomaterials 2019, 9, 1129. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A Review on the Visible Light Active Titanium Dioxide Photocatalysts for Environmental Applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Wysocka, I.; Kowalska, E.; Trzciński, K.; Łapiński, M.; Nowaczyk, G.; Zielińska-Jurek, A. UV-Vis-Induced Degradation of Phenol over Magnetic Photocatalysts Modified with Pt, Pd, Cu and Au Nanoparticles. Nanomaterials 2018, 8, 28. [Google Scholar] [CrossRef]
- Heeger, A.J. Nobel Lecture: Semiconducting and Metallic Polymers: The Fourth Generation of Polymeric Materials. Rev. Mod. Phys. 2001, 73, 681–700. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-Doped Titanium Dioxide as Visible-Light-Sensitive Photocatalyst: Designs, Developments, and Prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Puga, A.V. Photocatalytic Production of Hydrogen from Biomass-Derived Feedstocks. Coord. Chem. Rev. 2016, 315, 1–66. [Google Scholar] [CrossRef]












| Sample Label | BET Surface Area (m2·g−1) | Average Crystallite Size (nm) | |||
|---|---|---|---|---|---|
| Anatase | Rutile | ||||
| Size (nm) | Phase Content (%) | Size (nm) | Phase Content (%) | ||
| PANI | 11 | - | - | - | - |
| TiO2 | 55 | 18.2 ± 0.9 | 86.3 ± 0.3 | 25.4 ± 0.7 | 13.7 ± 0.2 |
| PANI–TiO2 | 55 | 19.4 ± 0.9 | 81.3 ± 0.9 | 28.1 ± 0.7 | 12.9 ± 0.2 |
| Sample Label | Average Phenol Degradation Rate (µmol·h−1) | ||||||
|---|---|---|---|---|---|---|---|
| UV-Vis | Vis λ > 400 nm | Vis λ > 420 nm | Scavenger (UV-Vis) | ||||
| BQ | t-BuOH | AgNO3 | AO | ||||
| PANI | 0.27 ± 0.01 | 0.18 ± 0.01 | 0.12 ± 0.01 | 0.14 ± 0.01 | 0.14 ± 0.01 | 0.13 ± 0.01 | 0.54 ± 0.03 |
| TiO2 | 4.63 ± 0.23 | 0.40 ± 0.02 | 0.12 ± 0.01 | 2.96 ± 0.15 | 1.16± 0.06 | 3.36 ± 0.17 | 4.92 ± 0.25 |
| PANI–TiO2 | 2.01 ± 0.10 | 0.07 ± 0.01 | 0.26 ± 0.01 | 2.33 ± 0.12 | 2.02 ± 0.10 | 2.30 ± 0.11 | 1.40 ± 0.07 |
| Sample Label | Contact Angle (°) | 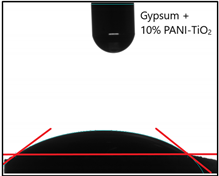 |
| Gypsum | 42.1 ± 1.9 | |
| Gypsum + 10 wt % TiO2 | 20.6 ± 2.7 | |
| Gypsum + 10 wt % PANI–TiO2 | 28.9 ± 0.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulowska, A.; Wysocka, I.; Pelczarski, D.; Karczewski, J.; Zielińska-Jurek, A. Hybrid TiO2–Polyaniline Photocatalysts and their Application in Building Gypsum Plasters. Materials 2020, 13, 1516. https://doi.org/10.3390/ma13071516
Sulowska A, Wysocka I, Pelczarski D, Karczewski J, Zielińska-Jurek A. Hybrid TiO2–Polyaniline Photocatalysts and their Application in Building Gypsum Plasters. Materials. 2020; 13(7):1516. https://doi.org/10.3390/ma13071516
Chicago/Turabian StyleSulowska, Agnieszka, Izabela Wysocka, Daniel Pelczarski, Jakub Karczewski, and Anna Zielińska-Jurek. 2020. "Hybrid TiO2–Polyaniline Photocatalysts and their Application in Building Gypsum Plasters" Materials 13, no. 7: 1516. https://doi.org/10.3390/ma13071516
APA StyleSulowska, A., Wysocka, I., Pelczarski, D., Karczewski, J., & Zielińska-Jurek, A. (2020). Hybrid TiO2–Polyaniline Photocatalysts and their Application in Building Gypsum Plasters. Materials, 13(7), 1516. https://doi.org/10.3390/ma13071516