Preparation and Characterization of Novel Mixed Periodic Mesoporous Organosilica Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Remarks
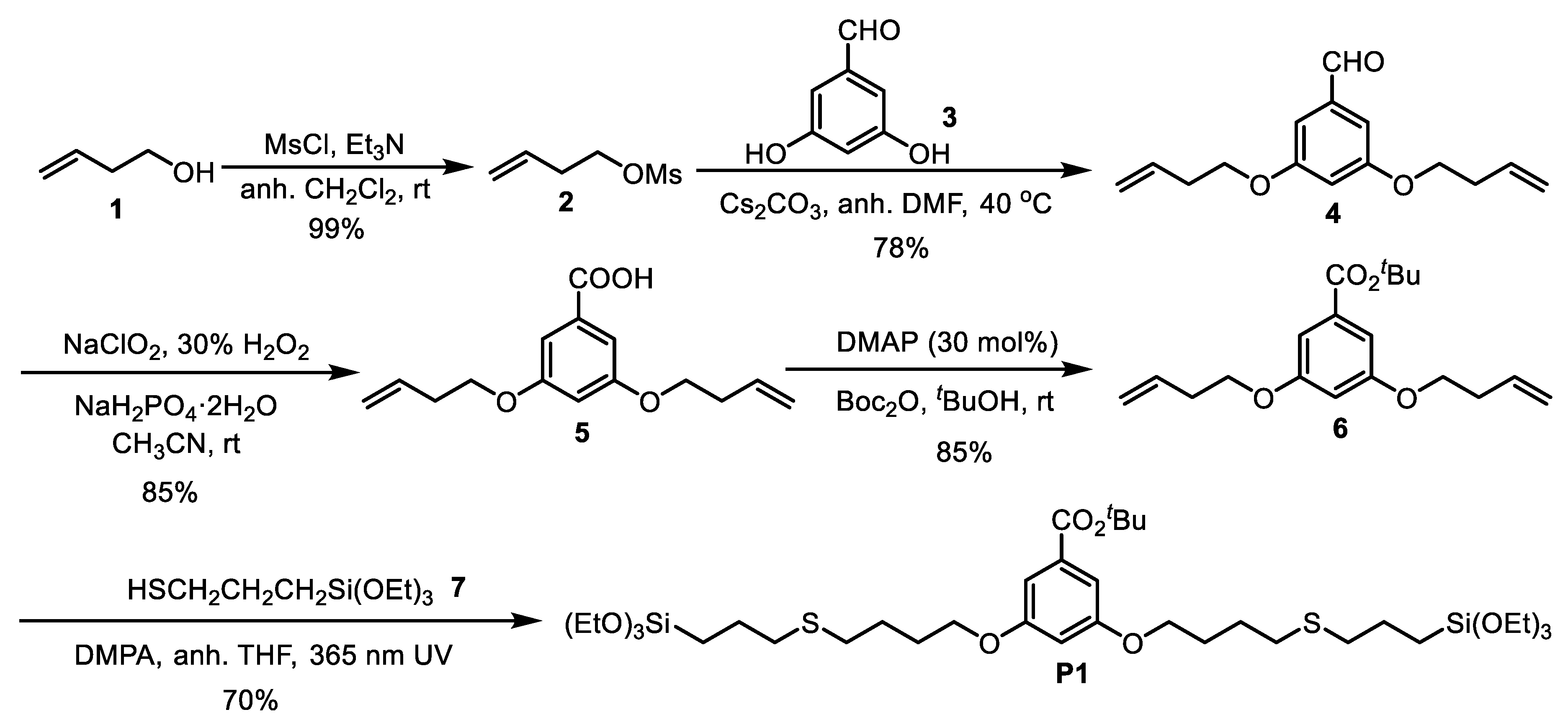
2.2. Synthesis of 2
2.3. Synthesis of 4
2.4. Synthesis of 5
2.5. Synthesis of 6
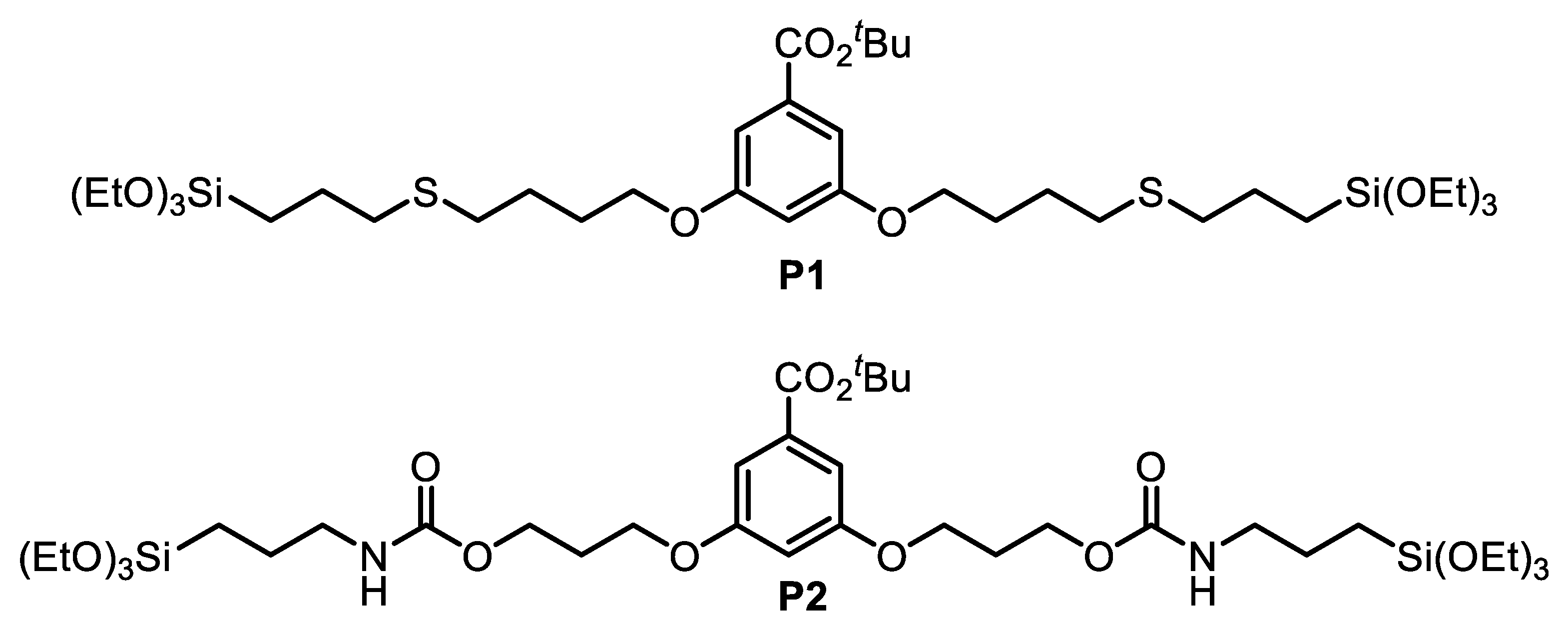
2.6. Synthesis of P1
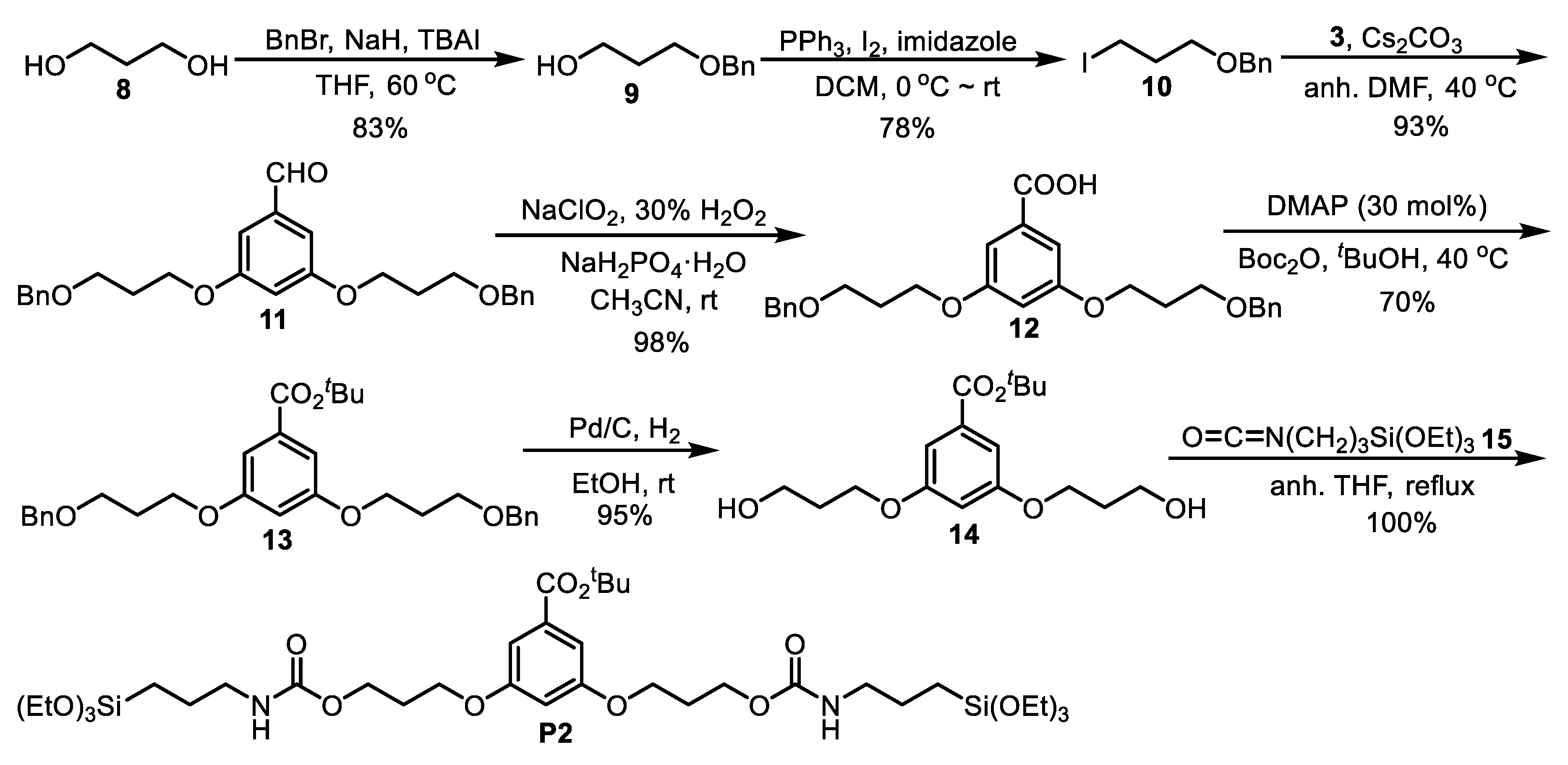
2.7. Synthesis of 9
2.8. Synthesis of 10
2.9. Synthesis of 11
2.10. Synthesis of 12
2.11. Synthesis of 13
2.12. Synthesis of 14
2.13. Synthesis of P2
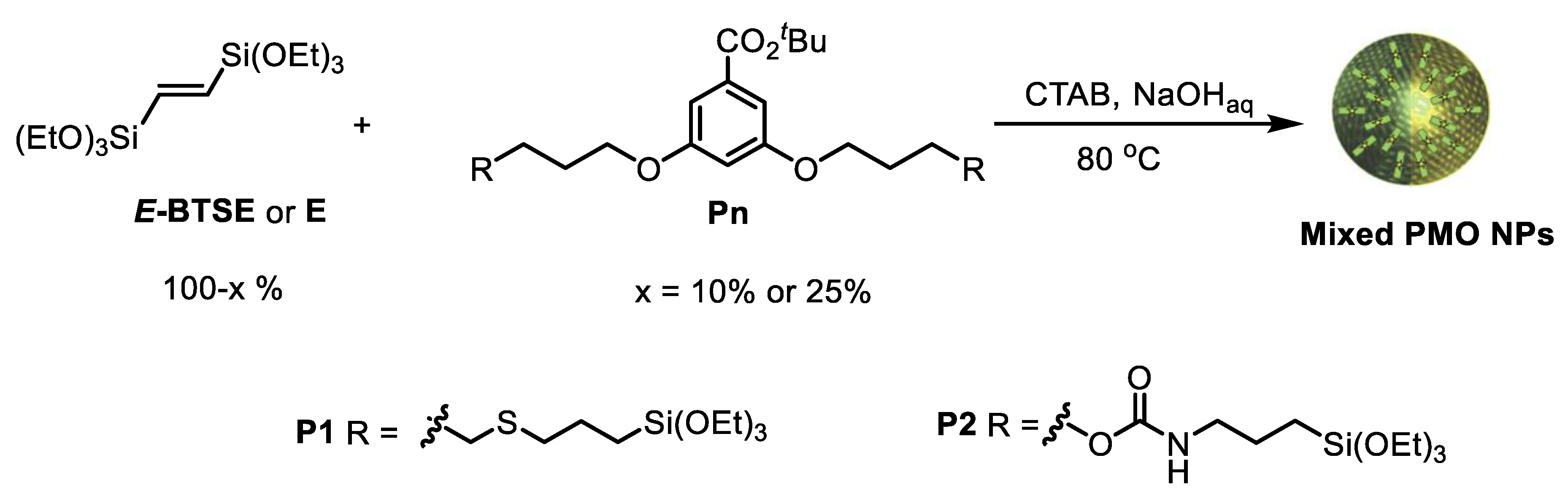
2.14. General Procedure for the Preparation of E-Pn 90/10 or 75/25 PMO NPs
3. Results and Discussion
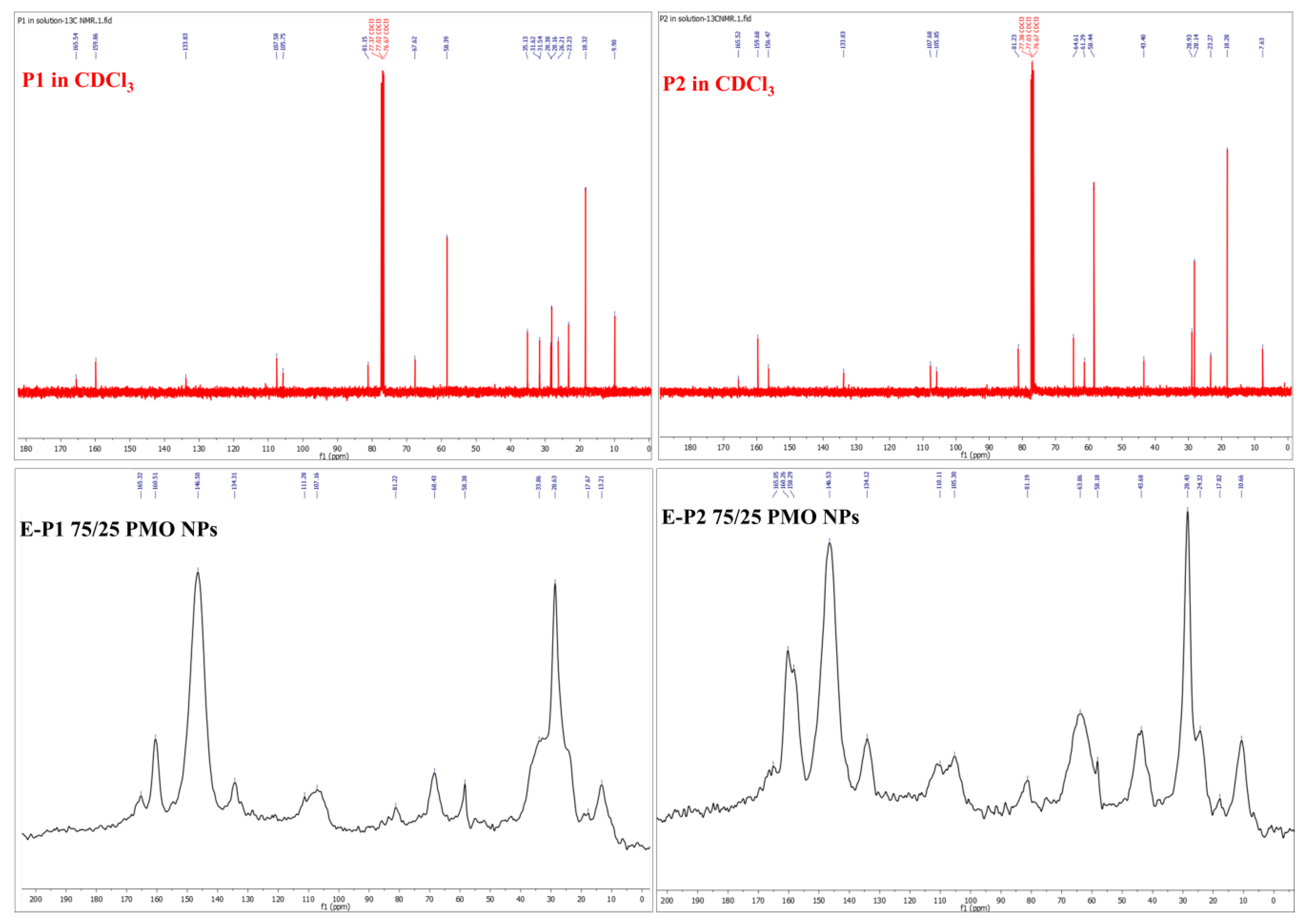
3.1. Synthesis of the Disilylated Precursors P1 and P2
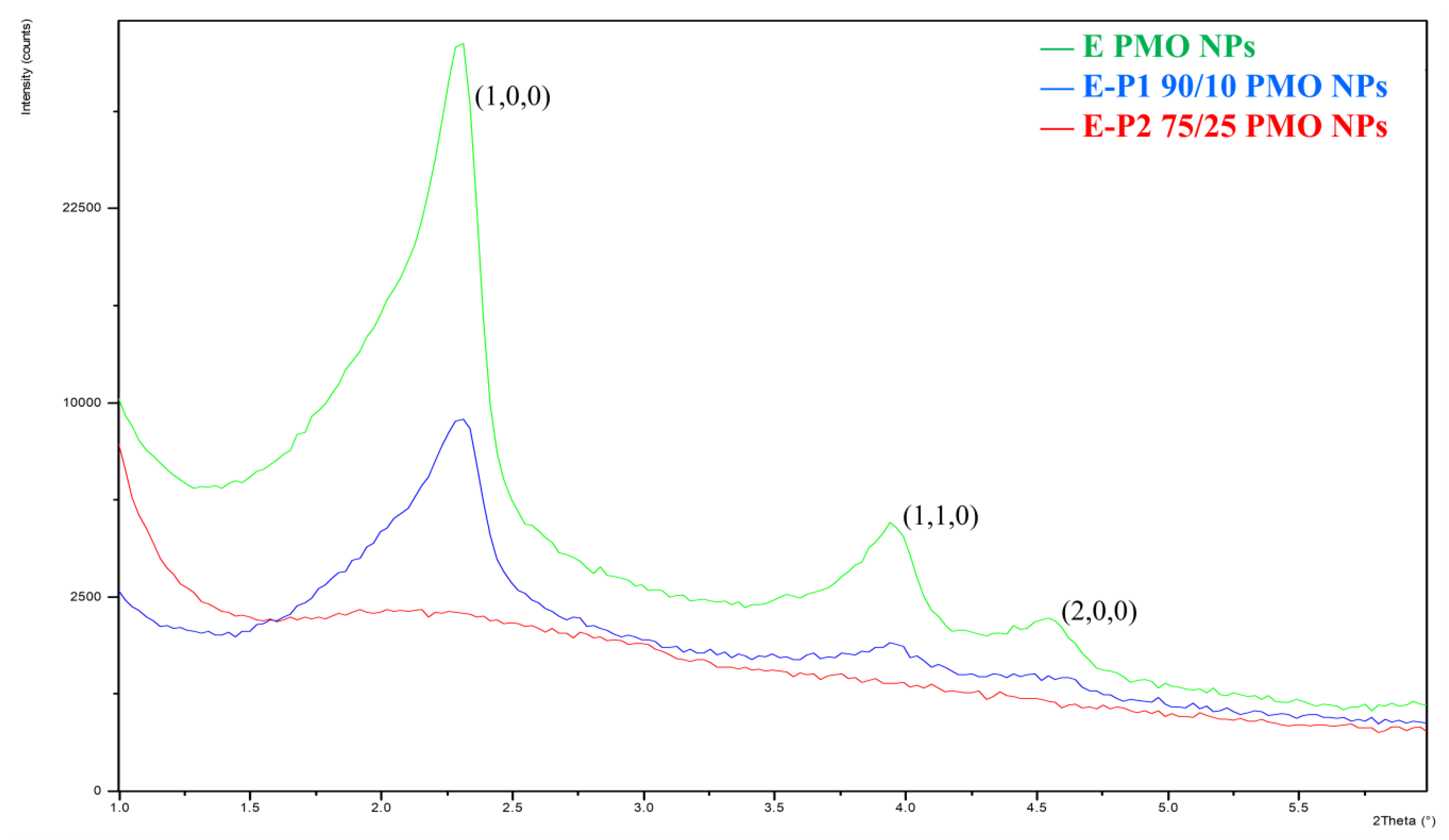
3.2. Preparation and Characterization of E-Pn 90/10 PMO NPs and E-Pn 75/25 PMO NPs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoffmann, F.; Cornelius, M.; Morell, J.; Fröba, M. Silica-Based Mesoporous Organic-Inorganic Hybrid Materials. Angew. Chem. Int. Ed. 2006, 45, 3216–3251. [Google Scholar] [CrossRef]
- Hunks, W.J.; Ozin, G.A. Challenges and Advances in the Chemistry of Periodic Mesoporous Organosilicas (PMOs). J. Mater. Chem. 2005, 15, 3716–3724. [Google Scholar] [CrossRef]
- Shea, K.J.; Loy, D.A. Bridged Polysilsesquioxanes. Molecular-Engineered Hybrid Organic-Inorganic Materials. Chem. Mater. 2001, 13, 3306–3319. [Google Scholar] [CrossRef]
- Manchanda, A.S.; Kruk, M. Synthesis of Large-pore Face-centered-cubic Periodic Mesoporous Organosilicas with Unsaturated Bridging Groups. Microporous Mesoporous Mater. 2016, 222, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Park, S.S.; Moorthy, M.S.; Ha, C.S. Periodic Mesoporous Organosilicas for Advanced Applications. NPG Asia Mater. 2014, 6. [Google Scholar] [CrossRef]
- Mizoshita, N.; Tani, T.; Inagaki, S. Syntheses, Properties and Applications of Periodic Mesoporous Organosilicas Prepared from Bridged Organosilane Precursors. Chem. Soc. Rev. 2011, 40, 789–800. [Google Scholar] [CrossRef]
- Qian, K.; Liu, F.; Yang, J.; Huang, X.; Gu, W.; Jambhrunkar, S.; Yuan, P.; Yu, C. Pore Size-optimized Periodic Mesoporous Organosilicas for the Enrichment of Peptides and Polymers. RSC Adv. 2013, 3, 14466–14472. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Meng, Q.; Wu, M.; Wang, S.; Xu, P.; Chen, H.; Li, Y.; Zhang, L.; Wang, L.; Shi, J. Hollow Mesoporous Organosilica Nanoparticles: A Generic Intelligent Framework-Hybridization Approach for Biomedicine. J. Am. Chem. Soc. 2014, 136, 16326–16334. [Google Scholar] [CrossRef]
- Croissant, J.G.; Cattoën, X.; Durand, J.-O.; Wong Chi Man, M.; Khashab, N.M. Organosilica Hybrid Nanomaterials with a High Organic Content: Syntheses and Applications of Silsesquioxanes. Nanoscale 2016, 8, 19945–19972. [Google Scholar] [CrossRef]
- De Canck, E.; Ascoop, I.; Sayari, A.; Van Der Voort, P. Periodic Mesoporous Organosilicas Functionalized with a Wide Variety of Amines for CO2 Adsorption. Phys. Chem. Chem. Phys. 2013, 15, 9792–9799. [Google Scholar] [CrossRef]
- Croissant, J.G.; Cattoën, X.; Wong Chi Man, M.; Gallud, A.; Raehm, L.; Trens, P.; Maynadier, M.; Durand, J.-O. Biodegradable Ethylene-Bis(Propyl)Disulfide-Based Periodic Mesoporous Organosilica Nanorods and Nanospheres for Efficient in-vitro Drug Delivery. Adv. Mater. 2014, 26, 6174–6180. [Google Scholar] [CrossRef] [PubMed]
- Noureddine, A.; Trens, P.; Toquer, G.; Cattoën, X.; Wong Chi Man, M. Tailoring the Hydrophilic/Lipophilic Balance of Clickable Mesoporous Organosilicas by the Copper-Catalyzed Azide-Alkyne Cycloaddition Click-Functionalization. Langmuir 2014, 30, 12297–12305. [Google Scholar] [CrossRef]
- Corma, A.; Díaz, U.; Arrica, M.; Fernández, E.; Ortega, Í. Organic-Inorganic Nanospheres with Responsive Molecular Gates for Drug Storage and Release. Angew. Chem. Int. Ed. 2009, 48, 6247–6250. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Kleitz, F.; Li, X.; Huang, H.; Zhang, X.; Qiao, S.Z. Disulfide-Bridged Organosilica Frameworks: Designed, Synthesis, Redox-Triggered Biodegradation, and Nanobiomedical Applications. Adv. Funct. Mater. 2018, 28, 1707325. [Google Scholar] [CrossRef] [Green Version]
- Omar, H.; Moosa, B.; Alamoudi, K.; Anjum, D.H.; Emwas, A.H.; El Tall, O.; Vu, B.; Tamanoi, F.; AlMalik, A.; Khashab, N.M. Impact of Pore-Walls Ligand Assembly on the Biodegradation of Mesoporous Organosilica Nanoparticles for Controlled Drug Delivery. ACS Omega 2018, 3, 5195–5201. [Google Scholar] [CrossRef] [PubMed]
- Bürglová, K.; Noureddine, A.; Hodačová, J.; Toquer, G.; Cattoën, X.; Wong Chi Man, M. A General Method for Preparing Bridged Organosilanes with Pendant Functional Groups and Functional Mesoporous Organosilicas. Chem. Eur. J. 2014, 20, 10371–10382. [Google Scholar] [CrossRef]
- Gomes, A.C.; Lourenço, M.A.; Bruno, S.M.; Ferreira, P.; Valente, A.A.; Pillinger, M.; Gonçalves, I.S. Post-synthetic Modification of Crystal-like Periodic Mesoporous Phenylene-silica with Ferrocenyl Groups. J. Organomet. Chem. 2014, 751, 501–507. [Google Scholar] [CrossRef]
- Urata, C.; Yamada, H.; Wakabayashi, R.; Aoyama, Y.; Hirosawa, S.; Arai, S.; Takeoka, S.; Yamauchi, Y.; Kuroda, K. Aqueous Colloidal Mesoporous Nanoparticles with Ethenylene-Bridged Silsesquioxane Frameworks. J. Am. Chem. Soc. 2011, 133, 8102–8105. [Google Scholar] [CrossRef]
- Doustkhah, E.; Mohtasham, H.; Farajzadeh, M.; Rostamnia, S.; Wang, Y.; Arandiyan, H.; Assadi, M.H.N. Organosiloxane Tunability in Mesoporous Organosilica and Punctuated Pd Nanoparticles Growth; Theory and Experiment. Microporous Mesoporous Mater. 2020, 293, 109832. [Google Scholar] [CrossRef]
- Du, X.; Li, X.; Xiong, L.; Zhang, X.; Kleitz, F.; Qiao, S.Z. Mesoporous Silica Nanoparticles with Organo-bridged Silsesquioxane Framework as Innovative Platforms for Bioimaging and Therapeutic Agent Delivery. Biomaterials 2016, 91, 90–127. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Almalik, A.; Khashab, N.M. Mesoporous Silica and Organosilica Nanoparticles: Physical Chemistry, Biosafety, Delivery Strategies, and Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1700831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, P.; Guliants, V.V. Periodic Mesoporous Organic-inorganic Hybrid Materials: Applications in Membrane Separations and Adsorption. Microporous Mesoporous Mater. 2010, 132, 1–14. [Google Scholar] [CrossRef]
- Croissant, J.G.; Cattoën, X.; Man, M.W.C.; Durand, J.-O.; Khashab, N.M. Syntheses and Applications of Periodic Mesoporous Organosilica Nanoparticles. Nanoscale 2015, 7, 20318–20334. [Google Scholar] [CrossRef] [PubMed]
- Djojoputro, H.; Zhou, X.F.; Qiao, S.Z.; Wang, L.Z.; Yu, C.Z.; Lu, G.Q. Periodic Mesoporous Organosilica Hollow Spheres with Tunable Wall Thickness. J. Am. Chem. Soc. 2006, 128, 6320–6321. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.-B.; Kim, D.; Jaroniec, M. Preparation of Mesoporous Benzene-silica Nanoparticles. Microporous Mesoporous Mater. 2009, 120, 252–256. [Google Scholar] [CrossRef]
- Liu, J.; Bai, S.; Zhong, H.; Li, C.; Yang, Q. Tunable Assembly of Organosilica Hollow Nanospheres. J. Phys. Chem. C 2010, 114, 953–961. [Google Scholar] [CrossRef]
- Guan, B.; Cui, Y.; Ren, Z.; Qiao, Z.; Wang, L.; Liu, Y.; Huo, Q. Highly Ordered Periodic Mesoporous Organosilica Nanoparticles with Controllable Pore Structures. Nanoscale 2012, 4, 6588–6596. [Google Scholar] [CrossRef]
- Qiao, S.Z.; Yu, C.Z.; Xing, W.; Hu, Q.H.; Djojoputro, H.; Lu, G.Q. Synthesis and Bio-adsorptive Properties of Large-pore Periodic Mesoporous Organosilica Rods. J. Am. Chem. Soc. 2005, 17, 6172–6176. [Google Scholar] [CrossRef]
- Cho, E.-B.; Kim, D.; Jaroniec, M. Monodisperse Particles of Bifunctional Periodic Mesoporous Organosilica. J. Phys. Chem. C 2008, 112, 4897–4902. [Google Scholar] [CrossRef]
- Mohanty, P.; Landskron, K. Periodic Mesoporous Organosilica Nanorice. Nanoscale Res. Lett. 2009, 4, 169–172. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, P.; Landskron, K. Simple Systematic Synthesis of Periodic Mesoporous Organosilica Nanoparticles with Adjustable Aspect Ratios. Nanoscale Res. Lett. 2009, 4, 1524–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Li, Y.; Bi, L.; Zhuang, W.; Wang, S.; Chen, Y.; Li, B.; Yang, Y. Preparation of Helical Mesoporous Ethenylene-silica Nanofibers with Lamellar Mesopores on Their Surface. Chin. J. Chem. 2011, 29, 933–941. [Google Scholar] [CrossRef]
- Fatieiev, Y.; Croissant, J.G.; Alamoudi, K.; Khashab, N.M. Cellular Internalization and Biocompatibility of Periodic Mesoporous Organosilica Nanoparticles with Tunable Morphologies: From Nanospheres to Nanowires. ChemPlusChem 2017, 82, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Croissant, J.G.; Cattoën, X.; Man, M.W.C.; Dieudonne, P.; Charnay, C.; Raehm, L.; Durand, J.-O. One-Pot Construction of Multipodal Hybrid Periodic Mesoporous Organosilica Nanoparticles with Crystal-Like Architectures. Adv. Mater. 2015, 27, 145–149. [Google Scholar] [CrossRef]
- Teng, Z.; Li, W.; Tang, Y.; Elzatahry, A.; Lu, G.; Zhao, D. Mesoporous Organosilica Hollow Nanoparticles: Synthesis and Applications. Adv. Mater. 2019, 31, 1707612. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, J. Chemistry of Mesoporous Organosilica in Nanotechnology: Molecularly Organic-Inorganic Hybridization into Frameworks. Adv. Mater. 2016, 28, 3235–3272. [Google Scholar] [CrossRef]
- Doustkhah, E.; Rostamnia, S.; Imura, M.; Ide, Y.; Mohammadi, S.; Hyland, C.J.T.; You, J.; Tsunoji, N.; Zeynizadeh, B.; Yamauchi, Y. Thiourea Bridged Periodic Mesoporous Organosilica with Ultra-small Pd Nanoparticles for Coupling Reactions. RSC Adv. 2017, 7, 56306–56310. [Google Scholar] [CrossRef] [Green Version]
- Vercaemst, C.; Ide, M.; Wiper, P.V.; Jones, J.T.A.; Khimyak, Y.Z.; Verpoort, F. Ethenylene-Bridged Periodic Mesoporous Organosilicas: From E to Z. Chem. Mater. 2009, 21, 5792–5800. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Gascό, C.; Delalande, A.; Charnay, C.; Raehm, L.; Midoux, P.; Pichon, C.; Pleixats, R.; Durand, J.-O. Periodic Mesoporous Organosilica Nanoparticles with BOC Group, towards HIFU Responsive Agents. Molecules 2020, 25, 974. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Nguyen, K.T.; Jiang, V.C.; Lofland, D.; Moser, H.E.; Pei, D. Macrocyclic Inhibitors for Peptide Deformylase: A Structure-Activity Relationship Study of the Ring Size. J. Med. Chem. 2004, 47, 4941–4949. [Google Scholar] [CrossRef]
- Beil, J.B.; Zimmerman, S.C. Synthesis of Nanosized “Cored” Star Polymers. Macromolecules 2004, 37, 778–787. [Google Scholar] [CrossRef]
- Dalcanale, E.; Montanari, F. Selective Oxidation of Aldehydes to Carboxylic Acids with Sodium Chlorite-hydrogen Peroxide. J. Org. Chem. 1986, 51, 567–569. [Google Scholar] [CrossRef]
- Takeda, K.; Akiyama, A.; Nakamura, H.; Takizawa, S.; Mizuno, Y.; Takayanagi, H.; Harigaya, Y. Dicarbonates: Convenient 4-Dimethylaminopyridine Catalyzed Esterification Reagents. Synthesis 1994, 10, 1063–1066. [Google Scholar] [CrossRef]
- Killops, K.L.; Campos, L.M.; Hawker, C.J. Robust, Efficient, and Orthogonal Synthesis of Dendrimers via Thiol-ene “Click” Chemistry. J. Am. Chem. Soc. 2008, 130, 5062–5064. [Google Scholar] [CrossRef]
- Sun, F.G.; Li, M.; Gu, Z.H. Pd/norbornene-Catalyzed Sequential ortho-C-H Alkylation and ipso-Alkynylation: A 1,1-dimethyl-2-alkynol Strategy. Org. Chem. Front. 2016, 3, 309–313. [Google Scholar] [CrossRef]
- Heathcock, C.H.; Ratcliffe, R. A Stereoselective Total Synthesis of the Guaiazulenic Sesquiterpenoids α-Bulnesene and Bulnesol. J. Am. Chem. Soc. 1971, 93, 1746–1757. [Google Scholar] [CrossRef]
- Park, S.S.; Jung, M.H.; Lee, Y.S.; Bae, J.H.; Kim, S.H.; Ha, C.S. Functionalised Mesoporous Silica Nanoparticles with Excellent Cytotoxicity against Various Cancer Cells for pH-Responsive and Controlled Drug Delivery. Mater. Des. 2019, 184, 108187. [Google Scholar] [CrossRef]
- Xie, M.; Shi, H.; Ma, K.; Shen, H.J.; Li, B.; Shen, S.; Wang, X.S.; Jin, Y. Hybrid Nanoparticles for Drug Delivery and Bioimaging: Mesoporous Silica Nanoparticles Functionalized with Carboxyl Groups and a Near-Infrared Fluorescent Dye. J. Colloid Interface Sci. 2013, 395, 306–314. [Google Scholar] [CrossRef]
- Dutta, S.; Kao, H.M.; Wu, K.C.W. Effect of Carboxylic Acid of Periodic Mesoporous Organosilicas on the Fructose-to-5-Hydroxymethylfurfural Conversion in Dimethylsulfoxide Systems. APL Mater. 2014, 2, 113314. [Google Scholar] [CrossRef]
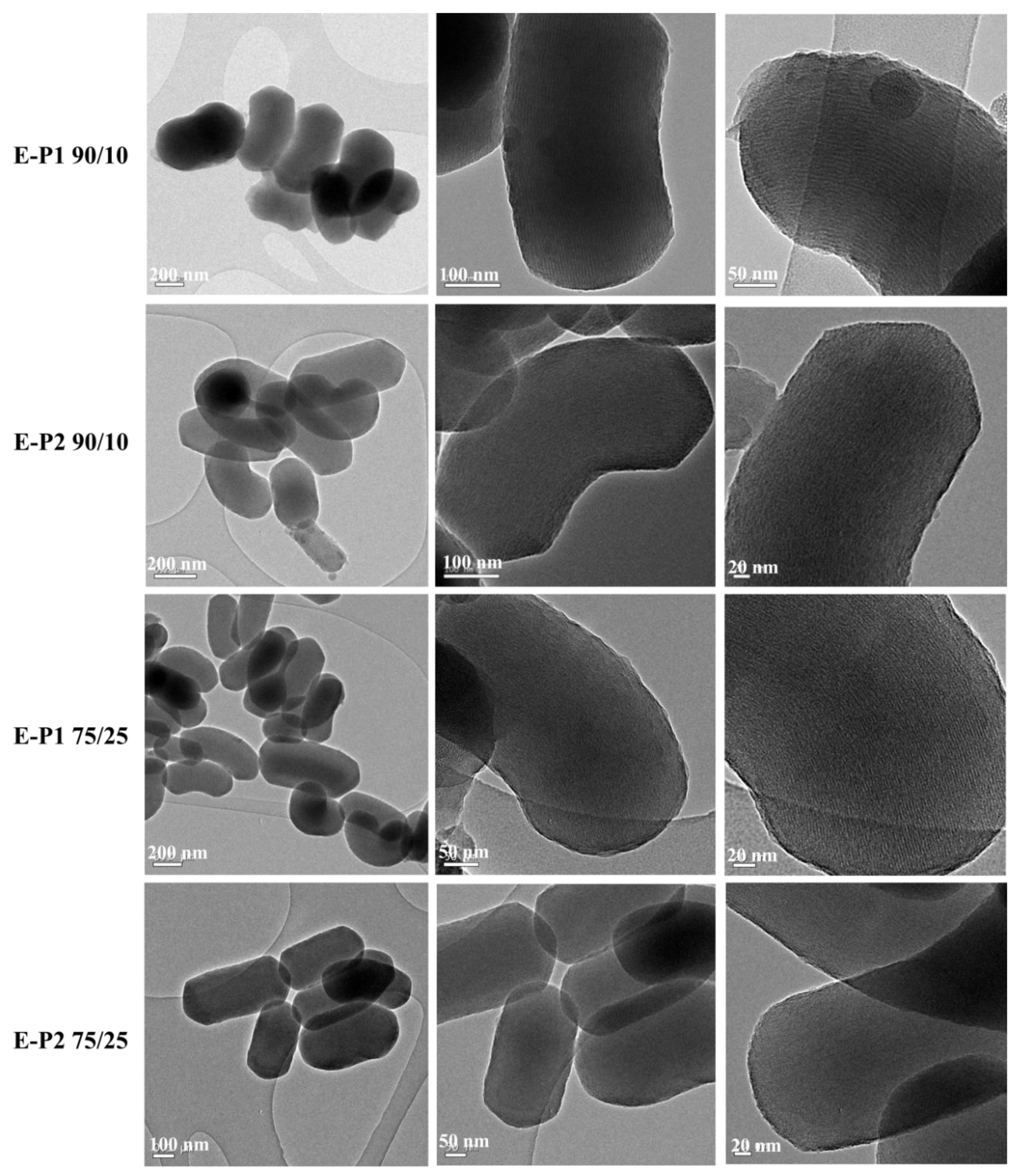
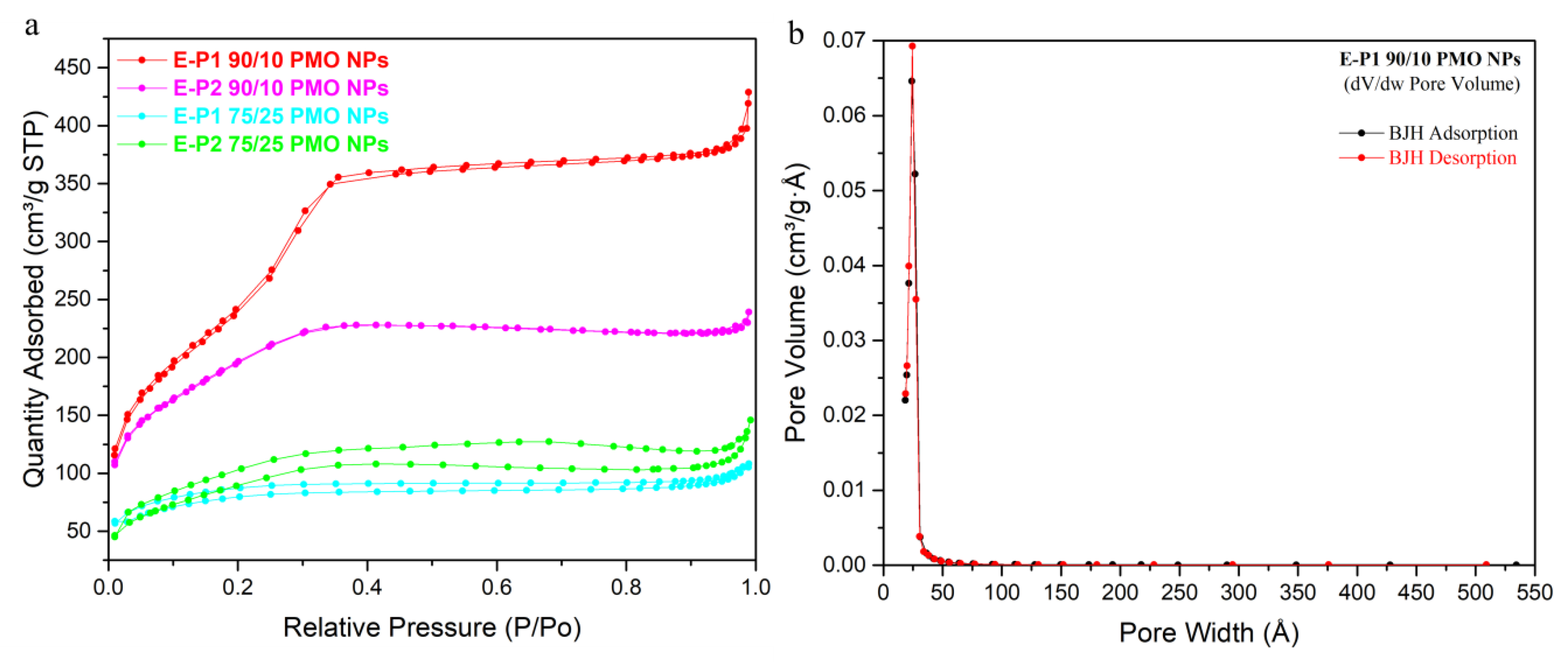
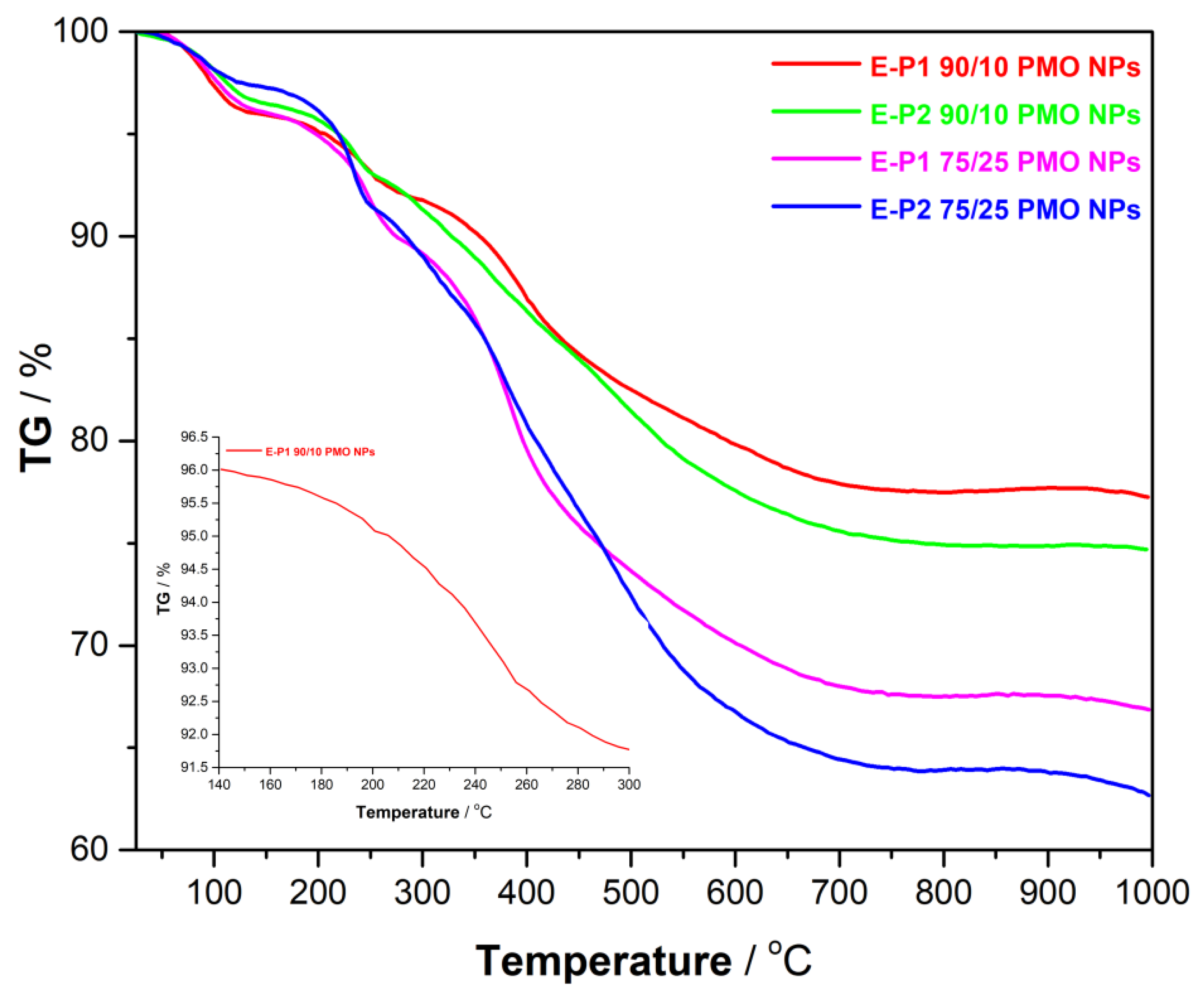

| Material | N2-Sorption Measurements | Particles Size (nm) c | Zeta Potential | TGA d | |||
|---|---|---|---|---|---|---|---|
| SBET (m2g−1) | Vpore (cm3g−1) a | ∅ pore (nm) b | pH | (mV) | |||
| E-P190/10 PMO NPs | 973 | 0.66 | 2.7 | 1122 | 6.80 | −30.9 | 77% |
| E-P290/10 PMO NPs | 696 | 0.37 | 2.1 | 694 | 6.72 | −38.2 | 75% |
| E-P175/25 PMO NPs | 259 | 0.17 | 2.6 | 742 | 9.07 | −25.6 | 67% |
| E-P275/25 PMO NPs | 329 | 0.23 | 2.7 | 562 | 6.85 | −42.3 | 63% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Raehm, L.; Charnay, C.; Durand, J.-O.; Pleixats, R. Preparation and Characterization of Novel Mixed Periodic Mesoporous Organosilica Nanoparticles. Materials 2020, 13, 1569. https://doi.org/10.3390/ma13071569
Li H, Raehm L, Charnay C, Durand J-O, Pleixats R. Preparation and Characterization of Novel Mixed Periodic Mesoporous Organosilica Nanoparticles. Materials. 2020; 13(7):1569. https://doi.org/10.3390/ma13071569
Chicago/Turabian StyleLi, Hao, Laurence Raehm, Clarence Charnay, Jean-Olivier Durand, and Roser Pleixats. 2020. "Preparation and Characterization of Novel Mixed Periodic Mesoporous Organosilica Nanoparticles" Materials 13, no. 7: 1569. https://doi.org/10.3390/ma13071569





