Use of Pyrazole Hydrogen Bonding in Tripodal Complexes to Form Self Assembled Homochiral Dimers
Abstract
:1. Introduction
2. Experimental
2.1. General Information
2.2. Synthesis
2.3. Structure Determinations
3. Results and Discussion
3.1. Synthesis and Initial Characterization
3.2. Structures of the Complexes, General Features
3.3. General Structure of the Dimers/Pseudodimers
3.4. Correlation of Spin State with Structural Parameters
4. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brewer, C.T.; Brewer, G.; Luckett, C.; Marbury, G.S.; Viragh, C.; Beatty, A.M.; Scheidt, W.R. Proton control of oxidation and spin state in a series of iron tripodal imidazole complexes. Inorg. Chem. 2004, 43, 2402–2415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, F.; Policar, C.; Durot, S.; Cesario, M.; Yuwei, L.; Korri-Youssoufi, H.; Keita, B.; Nadjo, L. Imidazole and imidazolate iron complexes: on the way for tuning 3D-structural characteristics and reactivity. Redox Inorg. Chem. 2004, 43, 4178–4188. [Google Scholar] [CrossRef] [PubMed]
- Sunatsuki, Y.; Ikuta, Y.; Matsumoto, N.; Ohta, H.; Kojima, M.; Iijima, S.; Hayami, S.; Maeda, Y.; Kaizaki, S.; Dahan, F.; et al. An unprecedented homochiral mixed-valence spin-crossover compound. Angew. Chem. Int. Ed. 2003, 42, 1614. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, Y.; Ooidemizu, M.; Yamahata, Y.; Yamada, M.; Osa, S.; Matsumoto, N.; Iijima, S.; Sunatsuki, Y.; Kojima, M.; Dahan, F.; et al. A New Family of Spin Crossover Complexes with a Tripod Ligand Containing Three Imidazoles: Synthesis, Characterization, and Magnetic Properties of [FeIIH3LMe](NO3)2·1.5H2O, [FeIIILMe]·3.5H2O, [FeIIH3LMe][FeIILMe]NO3, and [FeIIH3LMe][FeIIILMe](NO3)2 (H3LMe = Tris[2-(((2-methylimidazol-4-yl)methylidene)amino)ethyl]amine). Inorg. Chem. 2003, 42, 7001–7017. [Google Scholar] [PubMed]
- Yamada, M.; Ooidemizu, M.; Ikuta, Y.; Osa, S.; Matsumoto, N.; Iijima, S.; Kojima, M.; Dahan, F.; Tuchagues, J.P. Interlayer Interaction of Two-Dimensional Layered Spin Crossover Complexes [FeIIH3LMe][FeIILMe]X (X− = ClO4−, BF4−, PF6−, AsF6−, and SbF6−; H3LMe = Tris[2-(((2-methylimidazol-4-yl)methylidene)amino)ethyl]amine). Inorg. Chem. 2003, 42, 8406. [Google Scholar] [CrossRef]
- Sunatsuki, Y.; Ohta, H.; Kojima, M.; Ikuta, Y.; Goto, Y.; Matsumoto, N.; Iijima, S.; Akashi, H.; Kaizaki, S.; Dahan, F.; et al. Supramolecular Spin-Crossover Iron Complexes Based on Imidazole−Imidazolate Hydrogen Bonds. Inorg. Chem. 2004, 43, 4154–4171. [Google Scholar] [CrossRef]
- Ohta, H.; Sunatsuki, Y.; Ikuta, Y.; Matsumoto, N.; Ijima, S.; Akashi, H.; Kambee, T.; Kojima, M. Spin crossover in a supramolecular Fe(II)-Fe(III) system. Mater. Sci. 2003, 21, 191. [Google Scholar]
- Slattery, S.J.; Blaho, J.K.; Lehnes, J.; Goldsby, K.A. pH-Dependent metal-based redox couples as models for proton-coupled electron transfer reactions. Coord. Chem. Rev. 1998, 174, 391–416. [Google Scholar] [CrossRef]
- Hays, A.A.; Vassiliev, I.R.; Golbeck, J.H.; Debus, R.J. Role of D1-His190 in Proton-Coupled Electron Transfer Reactions in Photosystem II: A Chemical Complementation Study. Biochemistry 1998, 37, 11352–11365. [Google Scholar] [CrossRef] [PubMed]
- Aedelroth, P.; Paddock, M.L.; Tehrani, A.; Beatty, J.T.; Feher, G.; Okamura, M.Y. Identification of the Proton Pathway in Bacterial Reaction Centers: Decrease of Proton Transfer Rate by Mutation of Surface Histidines at H126 and H128 and Chemical Rescue by Imidazole Identifies the Initial Proton Donors. Biochemistry 2001, 40, 14538–14546. [Google Scholar] [CrossRef]
- Carina, R.F.; Verzegnassi, L.; Bernardinelli, G.; Williams, A.F. Modulation of iron reduction potential by deprotonation at a remote site. Chem. Comm. 1998, 2681–2682. [Google Scholar] [CrossRef] [Green Version]
- Bond, A.M.; Haga, M. Spectrophotometric and voltammetric characterization of complexes of bis(2,2’-bipyridine)(2,2’-bibenzimidazole)ruthenium and -osmium in oxidation states II, III, and IV in acetonitrile/water mixtures. Inorg. Chem. 1986, 25, 4507–4514. [Google Scholar] [CrossRef]
- Haga, M.; Ano, T.; Kano, K.; Yamabe, S. Proton-induced switching of metal-metal interactions in dinuclear ruthenium and osmium complexes bridged by 2,2’-bis(2-pyridyl)bibenzimidazole. Inorg. Chem. 1991, 30, 3843–3849. [Google Scholar] [CrossRef]
- Real, J.A.; Gasper, A.B.; Muñoz, M.C. Thermal, pressure and light switchable spin-crossover materials. Dalton Trans. 2005, 12, 2062–2079. [Google Scholar] [CrossRef] [PubMed]
- Ohta, H.; Sunatsuki, Y.; Kojima, M.; Iijima, S.; Akashi, H.; Matsumoto, N. A Tripodal Ligand Containing Three Imidazole Groups Inducing Spin Crossover in Both Fe(II) and Fe(III) Complexes; Structures and Spin Crossover Behaviors of the Complexes. Chem. Lett. 2004, 33, 350–351. [Google Scholar] [CrossRef]
- Sunatsuki, Y.; Sakata, M.; Matsuzaki, S.; Matsumoto, N.; Kojima, M. Thermal and Pressure Induced Spin Crossover of a Novel Iron(III) Complex with a Tripodal Ligand Involving Three Imidazole Groups. Chem. Lett. 2001, 1254. [Google Scholar] [CrossRef]
- Brewer, G.; Butcher, R.J.; Viragh, C.; White, G. Supramolecular assemblies prepared from an iron(II) tripodal imidazole complex. A molecular scaffolding for the self assembly of icosahedral complexes of K+, Rb+, Cs+ and NH4+ cations. Dalton Trans. 2007, 4132–4142. [Google Scholar] [CrossRef]
- Alvarado, L.; Brewer, C.; Brewer, G.; Butcher, R.J.; Straka, A.; Viragh, C. Supramolecular assemblies prepared from an iron(II) tripodal complex, tetrafluoroborate, and alkali metal cations. The effect of cation size on coordination number, anion disorder and hydrogen bonding. Cryst. Eng. Comm. 2009, 11, 2297–2307. [Google Scholar] [CrossRef]
- Ohba, M.; Yoneda, K.; Agusti, G.; Munoz, M.C.; Gasbar, A.B.; Real, J.A.; Yamasaki, M.; Ando, H.; Nakao, Y.; Sakaki, S.; et al. Bidirectional Chemo-Switching of Spin State in a Microporous Framework. Angew. Chem. Int. Ed. 2009, 48, 4767. [Google Scholar] [CrossRef]
- Hauser, A.; Jeftic, J.; Romstedt, H.; Hinek, R.; Spiering, H. Cooperative phenomena and light-induced bistability in iron(II) spin-crossover compounds. Coord. Chem. Rev. 1999, 190, 471–491. [Google Scholar] [CrossRef] [Green Version]
- Kahn, T.O.; Jay-Martinez, C. Spin-Transition Polymers: From Molecular Materials Toward Memory Devices. Science 1998, 279, 44–48. [Google Scholar] [CrossRef]
- Miller, T.J.S. Magnetically ordered molecule-based assemblies. Dalton Trans. 2006, 2742–2749. [Google Scholar] [CrossRef]
- Batten, S.R.; Murray, K.S. Structure and magnetism of coordination polymers containing dicyanamide and tricyanomethanide, Coord. Chem. Rev. Coord. Chem. Rev. 2003, 246, 103–130. [Google Scholar] [CrossRef]
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry; John Wiley and Sons: Weinheim, Germany, 2000. [Google Scholar]
- Saalfrank, R.W.; Demleitner, B.; Sauvage, J.P. Transition Metals in Supramolecular Chemistry; Sauvage, J.P., Ed.; John Wiley and Sons: Weinheim, Germany, 1999. [Google Scholar]
- Dodziuk, H. Introduction to Supramolecular Chemistry; Kluwer Academic Publishers: Boston, MA, USA, 2001. [Google Scholar]
- Alvarado, L.; Brewer, G.; Carpenter, E.E.; Viragh, C.; Zavalij, P. Use of acid–base and redox chemistry to synthesize cobalt(III) and iron(III) complexes of a partially deprotonated triprotic imidazole-containing Schiff base ligand: Hydrogen bound 1D linear homochiral and zig-zag heterochiral supramolecular complexes. Inorg. Chim. Acta 2010, 363, 817–822. [Google Scholar] [CrossRef]
- Brewer, G.; Alvorado, L.; Lear, S.; Viragh, C.; Zavalij, P. Synthesis, Structure and Supramolecular Features of Heteronuclear Iron(III)-Copper(II) Complexes of a tripodal imidazole containing ligand. Inorg. Chim. Acta 2016, 439, 111–116. [Google Scholar] [CrossRef]
- Garcia-Terán, J.P.; Castillo, O.; Luque, A.; Garcia-Couceiro, U.; Beobide, G.; Román, P. Supramolecular architectures assembled by the interaction of purine nucleobases with metal-oxalato frameworks. Non-covalent stabilization of the 7H-adenine tautomer in the solid-state. Dalton Trans. 2006, 902–911. [Google Scholar]
- Maurizot, V.; Yoshizawa, M.; Kawano, M.; Fujita, M. Control of molecular interactions by the hollow of coordination cages. Dalton. Trans. 2006, 2750–2756. [Google Scholar] [CrossRef]
- Brewer, G.; Alvorado, L.J.; Brewer, C.T.; Butcher, R.J.; Cipresi, J.; Viragh, C.; Zavalij, P.Y. Synthesis, structure and supramolecular features of homonuclear iron(III) and heteronuclear iron(III)–cobalt(III) complexes, [FeH3L][ML](ClO4)3 (M = Fe(III) or Co(III). 2D sheet and tetrahedral arrays. Inorg. Chim. Acta 2014, 421, 100–109. [Google Scholar] [CrossRef]
- Brewer, C.T.; Brewer, G.; Butcher, R.J.; Carpenter, E.E.; Schmiedekamp, A.M.; Schmiedekamp, C.; Straka, A.; Viragh, C.; Yuzafpolskiy, Y.; Zavalij, P. Synthesis and Characterization of Homo- and Heterodinuclear M(II)-M(III)’ (M(II) = Mn or Fe, M(III)’ = Fe or Co) Mixed Valence Supramolecular Pseudo-dimers. The Effect of Hydrogen Bonding on Spin State Selection of M(II). Dalton Trans. 2011, 40, 181. [Google Scholar] [CrossRef]
- Paul, S.; Barik, A.K.; Peng, S.M.; Kar, S.K. Novel Copper(II) Induced Formation of a Porphyrinogen Derivative: X-ray Structural, Spectroscopic, and Electrochemical Studies of Porphyrinogen Complexes of Cu(II) and Co(III) Complex of a Trispyrazolyl Tripodal Ligand. Inorg. Chem. 2002, 41, 5803–5809. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Phase Annealing in SHELX-90: Direct Methods for Larger Structures. Acta Crystallogr. 1990, A46, 467–473. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-97: FORTRAN Program for Crystal Structure Refinement, 1997; Göttingen University: Göttingen, Germany, 2001. [Google Scholar]
- Sheldrick, G.M. SHELXL-97: FORTRAN Program for Crystal Structure Analysis, Göttingen University, Instit; Fr Anorganisheche Chemie der Universitat, Tammanstrasse 4, D-3400; Gőttingen University: Göttingen, Germany, 1998. [Google Scholar]
- Brewer, C.T.; Brewer, G.; Butcher, R.J.; Carpenter, E.E.; Schmiedekamp, A.M.; Viragh, C. Synthesis and characterization of a spin crossover iron(ii)–iron(iii) mixed valence supramolecular pseudo-dimer exhibiting chiral recognition, hydrogen bonding, and π–π interactions. Dalton Trans. 2007, 21, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.J.H. The Chemistry and spectroscopy of Mixed-Valence complexes. Chem. Soc. Rev. 1984, 13, 219. [Google Scholar] [CrossRef]
- Katsuki, I.; Motoda, Y.; Sunatsuki, Y.; Matsumoto, N.; Nakashima, T.; Kojima, M. Spontaneous Resolution Induced by Self-Organization of Chiral Self-Complementary Cobalt(III) Complexes with Achiral Tripod-Type Ligands Containing Three Imidazole Groups. J. Am. Chem. Soc. 2002, 124, 629–640. [Google Scholar] [CrossRef]
- Mimura, M.; Matsuo, T.; Motoda, Y.; Matsumoto, N.; Nakashima, T.; Kojima, M. Deprotonated Copper(II) Complex with a Tripod Ligand Involving Three Imidazole Groups: A Two-Dimensional Chiral Honey-Comb Structure Made by Hydrogen Bonds. Chem. Lett. 1998, 691. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 3rd ed.; John Wiley and Sons: New York, NY, USA, 1978. [Google Scholar]
- Novak, A. Hydrogen bonding in solids correlation of spectroscopic and crystallographic data. Struc. Bond. 1973, 18, 177–216. [Google Scholar]
- Brewer, G.; Brewer, C.; White, G.; Butcher, R.J.; Viragh, C.; Carpenter, E.E.; Schmiedekamp, A. Synthesis and Characterization of Iron(II) and Iron(III) Complexes Derived from Tris(2-aminoethyl)methane. Inorg. Chim. Acta 2009, 362, 4158. [Google Scholar] [CrossRef]
- Hardie, M.J.; Kilner, C.A.; Halcrow, M.A. Tris[4-(1H-pyrazol-3-yl)-3-azabut-3-enyl]amine iron(II) diperchlorate monohydrate. Acta Crystallogr. Sect. C. 2004, C60, m177. [Google Scholar] [CrossRef]
- Sczepanik, J.; Kaminsky, E.B.K.W.; Dechert, S.; Meyer, F. Non-Macrocyclic Schiff Base Complexes of Iron(II) as ParaCEST Agents for MRI. Eur. J. Inorg. Chem. 2019, 2019, 2404–2411. [Google Scholar] [CrossRef]
- Lazar, H.C.; Barrett, T.F.S.A.; Kliner, C.A.; Letard, J.F.; Halcrow, M.A. Thermal and light-induced spin-crossover in salts of the heptadentate complex [tris(4-{pyrazol-3-yl}-3-aza-3-butenyl)amine]iron(ii). Dalton Trans. 2007, 4276–4285. [Google Scholar] [CrossRef]
- Paul, S.; Barik, A.K.; Butcher, R.J.; Kar, S.K. Synthesis and characterisation of nickel(II) complexes with tripodal ligand tris[4-(3-(5-methylpyrazolyl)-3-aza-3-butenyl] amine (MPz3tren): X-ray crystal structure of [Ni(MPz3tren)](BF4)2·0.5H2O. Polyhedron 2000, 19, 2661. [Google Scholar] [CrossRef]
- Plass, W.; Pohlmann, A.; Rautengarten, J. Magnetic Interactions as Supramolecular Function: Structure and Magnetic Properties of Hydrogen-Bridged Dinuclear Copper(II) Complexes. Angew. Chem. Int. Ed. 2001, 40, 4207. [Google Scholar] [CrossRef]
- Yi, J.; Che, Y.X.; Zheng, J.M. Magnetic Interactions as Supramolecular Function: Structure and Magnetic Properties of Hydrogen-Bridged Dinuclear Copper(II) Complexes. J. Coord. Chem. 2007, 60, 2067. [Google Scholar]
- Sun, H.; Steeb, J.; Kaifer, A.E.J. Efficient Electronic Communication between Two Identical Ferrocene Centers in a Hydrogen-Bonded Dimer. Am. Chem. Soc. 2006, 128, 2820–2821. [Google Scholar] [CrossRef]
- Lenartson, A. Absolute Asymmetric Synthesis. Ph.D. Thesis, University of Gothenberg, Gothenberg, Sweden, 2009. [Google Scholar]
- Brewer, G.; Butcher, R.J.; Lear, S.; Noll, B.; Zavalij, P.Y. Synthesis, Structure and Supramolecular Features of Cobalt(III) and Cobalt(II) Complexes, [CoHxL](ClO4)y (x = 3, 2, 1.50, 1.45, 1 and 0) and x = 3, y = 2) of a Triprotic Imidazole Containing Schiff Base Ligand. Effect of protonation state on supramolecular structure. Inorg. Chim. Acta 2014, 410, 94–105. [Google Scholar]
- Brewer, C.; Brewer, G.; Butcher, R.J.; Carpenter, E.E.; Cuenca, L.; Noll, B.C.; Scheidt, W.R.; Viragh, C.; Zavalij, P.Y.; Zielaski, D. Synthesis and characterization of manganese(ii) and iron(iii) d5 tripodal imidazole complexes. Effect of oxidation state, protonation state and ligand conformation on coordination number and spin state. Dalton. Trans. 2006, 28, 1009–1019. [Google Scholar] [CrossRef] [Green Version]
- Brewer, G.; Olida, M.J.; Schmiedekamp, A.M.; Viragh, C.; Zavalij, P.Y. A DFT Computational Study of Spin Crossover in Iron(III) and Iron(II) Tripodal Imidazole Complexes. A Comparison of Experiment with Calculations. Dalton Trans. 2006, 5617–5629. [Google Scholar] [CrossRef]
- Brewer, G.; Olida, M.J.; Schmiedekamp, A.M.; Viragh, C.; Zavalij, P.Y. A DFT Computational Study of Spin Crossover in Iron(III) and Iron(II) Tripodal Imidazole Complexes. A Comparison of Experiment with Calculations, 2nd printing. Dalton Trans. 2007, 697. [Google Scholar] [CrossRef]

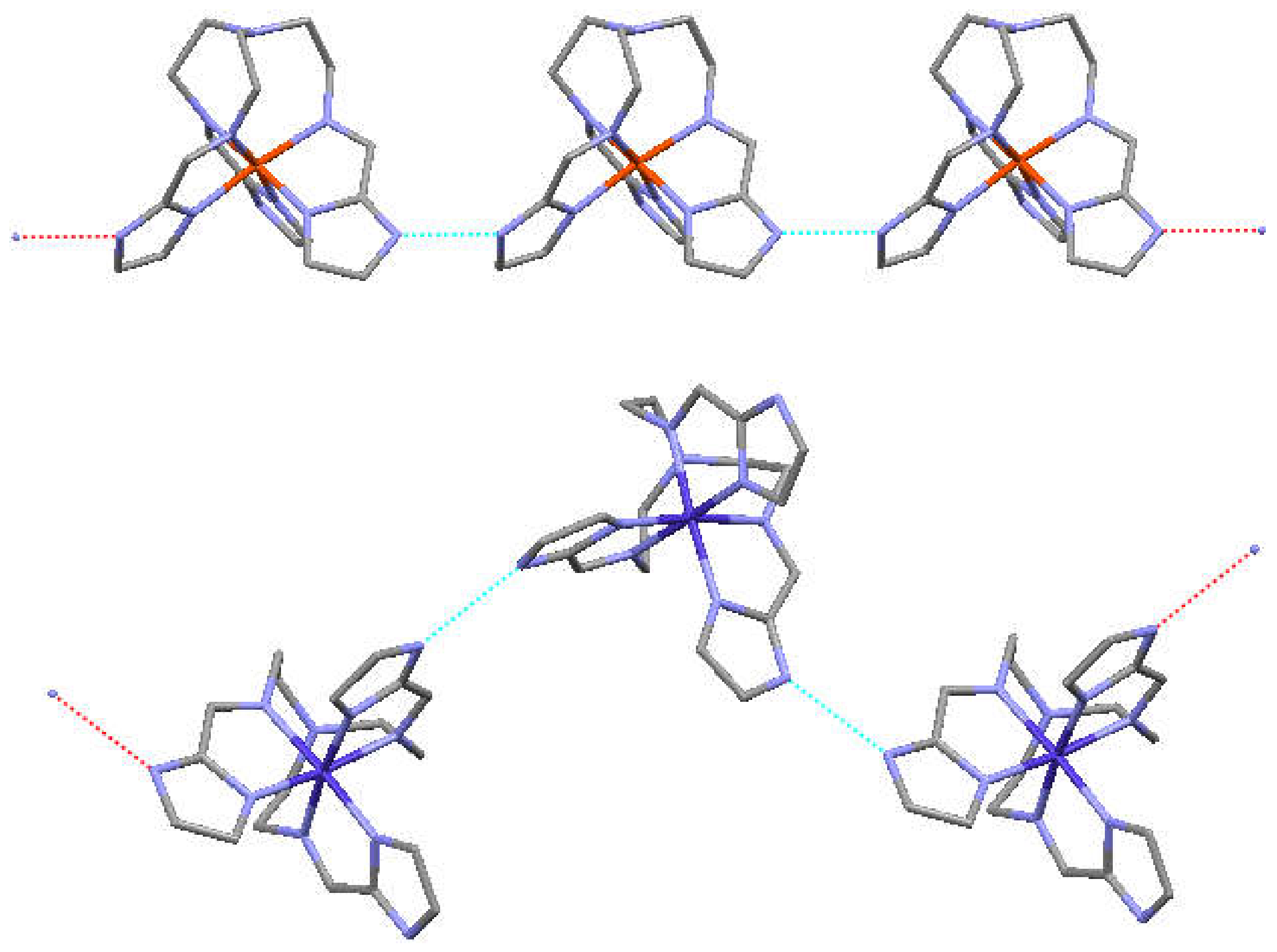

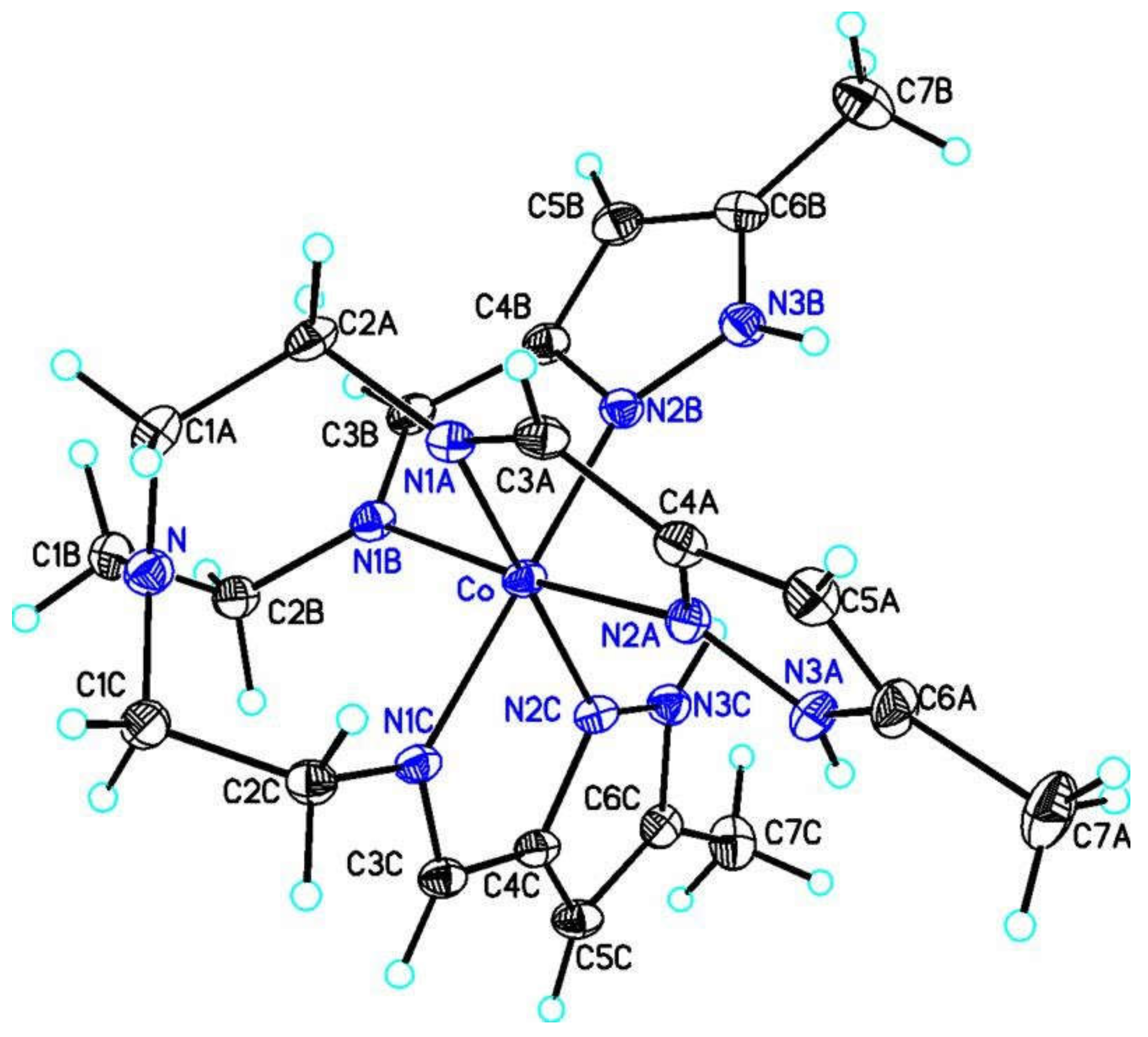
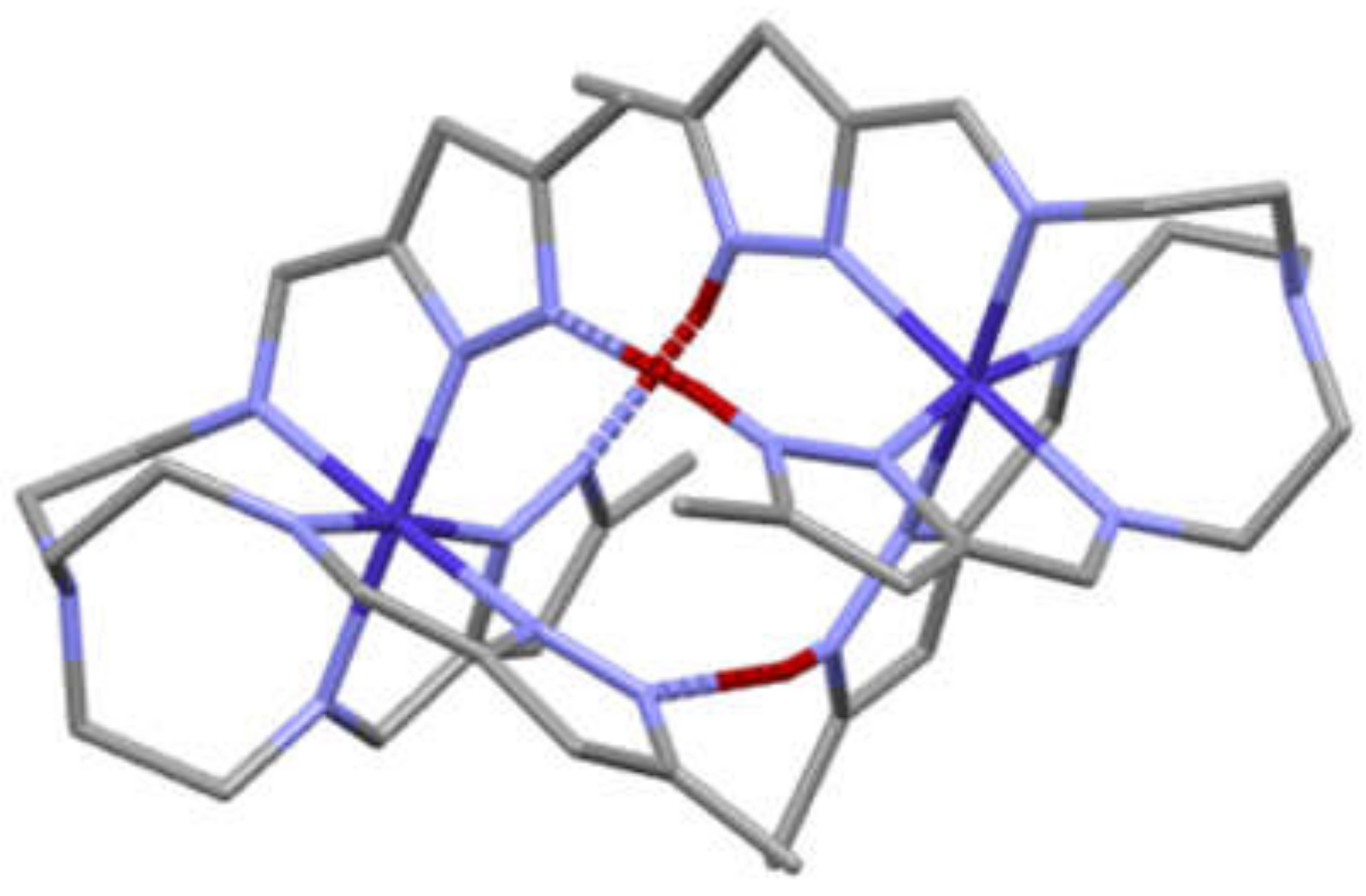


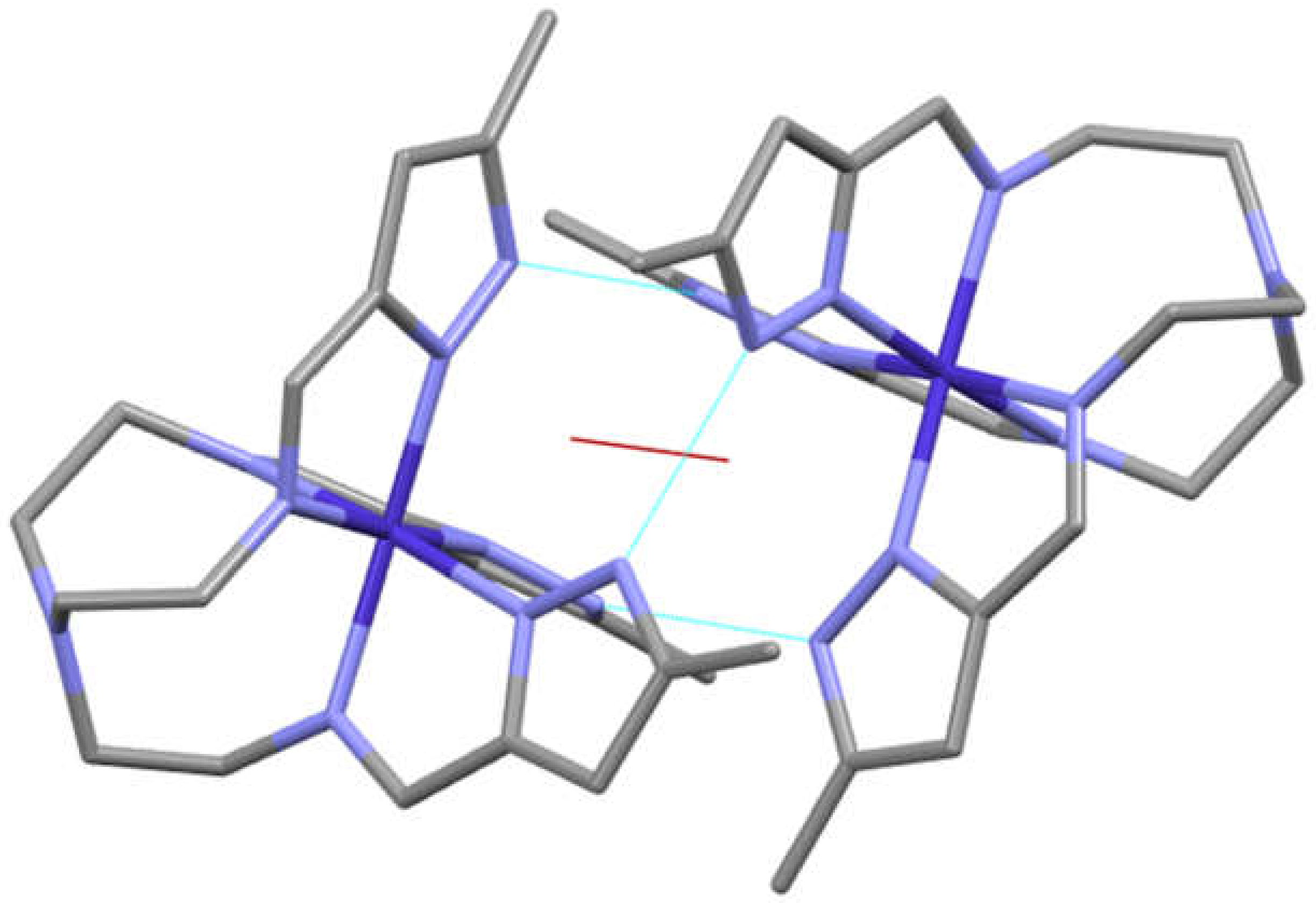
| a) | b) | c) | d) | e) | f) | g) | |
|---|---|---|---|---|---|---|---|
| Empirical formula | C21H37Cl3Co N10O15.5 | C42H57Cl3Co2 N20O12 | C42H57B3Co2 F12 N20 | C42H64.62B3Co2 F12N20O3.81 | C58H75Cl3Co2 N22O12 | C58H75Cl3Co2 N22O12 | C42H57Co2 N20I2 |
| M/g mol−1 | 842.89 | 1258.28 | 1220.37 | 1292.43 | 1496.61 | 1496.61 | 1213.74 |
| Temperature/K | 295(2) | 123(2) | 123(2) | 150(2) | 123(2) | 150(2) | 150(2) |
| λ/Å | 0.71073 | 0.71073 | 1.54178 | 0.71073 | 0.71073 | 0.71073 | 0.71073 |
| Crystal System | Monoclinic | Trigonal | Trigonal | Monoclinic | Tetragonal | Tetragonal | Monoclinic |
| Space group | P21//c | R32 | R32 | Cc | P43212 | P43212 | Cc |
| Unit cell dimensions | a = 12.9005(9) Å | a = 16.7663(2) Å | a = 16.6003(3) Å | a = 16.268(2) Å | a = 11.82570(10) Å | a = 11.8445(18) Å | a = 21.1716(10) Å |
| b = 17.8994(10) Å | b = 16.766(3) Å | b = 16.6003(3) Å | b = 17.394(2) Å | b = 11.82570(10) Å | b = 11.8445(18) Å | b = 12.3416(6) Å | |
| c = 15.5785(11) Å | c = 20.0641(3) Å | c = 20.0513(6) Å | c = 20.188(3) Å | c = 48.5723(11) Å | c = 48.694(8) Å | c = 19.6850(9) Å | |
| α = 90° | α = 90° | α = 90° | α = 90° | α = 90° | α = 90° | α = 90° | |
| β = 99.931(7)° | β = 90° | β =90° | β = 106.941(2)° | β = 90° | β = 90° | β = 93.1640(10) ° | |
| γ = 90° | γ = 120° | γ = 120° | γ = 90° | γ = 90° | γ = 90° | γ = 90° | |
| Volume/Å3 | 3543.3(4) | 4884.55(14) | 4785.25(19) | 5464.7(13) | 6792.70(17) | 6831.4(18) | 5135.7(4)) |
| Z | 4 | 3 | 3 | 4 | 4 | 4 | 4 |
| Abs. Coeff./mm−1 | 0.791 | 0.697 | 4.776 | 0.710 | 0.682 | 0.679 | 1.900 |
| F(000) | 1740 | 1950 | 1878 | 2664 | 3112 | 3112 | 2436 |
| Crystal size/mm3 | 0.53 × 0.47 × 0.08 | 0.47 × 0.43 × 0.28 | 0.26 × 0.24 × 0.19 | 0.295 × 0.05 × 0.045 | 0.39 × 0.39 × 0.32 | 0.21 × 0.30 × 0.44 | 0.22 × 0.135 × 0.11 |
| Theta range/o | 5.05 to 26.37 | 5.164 to 32.828 | 5.33 to 75.57 | 1.76 to 22.50 | 3.05 to 35.17 | 2.13 to 25.00 | 1.91 to 27.50 |
| Index ranges | −15 ≤ h ≤ 16 | −24 ≤ h ≤ 25 | −20 ≤ h ≤ 20 | −17 ≤ h ≤ 17 | −19 ≤ h ≤ 18 | −14 ≤ h ≤ 13 | −27 ≤ h ≤ 27 |
| −22 ≤ k ≤ 19 | −25 ≤ k ≤ 25 | −19 ≤ k ≤ 20 | −18 ≤ k ≤ 18 | −18 ≤ k ≤ 18 | −14 ≤ k ≤ 14 | −16 ≤ k ≤ 16 | |
| −18 ≤ l ≤ 19 | −30 ≤l ≤ 30 | −14 ≤ l ≤ 25 | −21 ≤ l ≤ 21 | −76 ≤ l ≤ 51 | −57 ≤ l ≤ 57 | −25 ≤ l ≤ 25 | |
| Reflections Collected | 20,906 | 31,672 | 11,282 | 15,317 | 99,409 | 57,503 | 36,049 |
| Independent Reflections | 7193 | 3862 | 2195 | 6970 | 14,527 | 6006 | 11,652 |
| R1 | 0.0853 | 0.0294 | 0.0814 | 0.0775 | 0.0724 | 0.0537 | 0.0371 |
| WR2 | 0.2031 | 0.0847 | 0.2203 | 0.1913 | 0.1515 | 0.1189 | 0.0774 |
| GOF on F2 | 1.031 | 1.123 | 1.075 | 1.074 | 1.194 | 1.000 | 1005 |
| a) | b) | c) | d) [CoH2L]2+ and [CoHL]+ | e) | f) | g) [CoH3L]2+ and [CoL] | |
|---|---|---|---|---|---|---|---|
| SG and sym | P21/c C2h | R32 D3 | R32 D3 | Cc, Cs | P43212 D4 | P43212 D4 | Cc, Cs |
| T(K) | 295(2) | 123(2) | 123(2) | 150(2) | 123(2) | 150(2) | 150(2) |
| Co-Nap non-bonded | 3.339 | 3.468 | 3.468 | 3.432 3.462 | 3.492 | 3.492 | 3.306 3.300 |
| M-M’non-bonded | NA | 5.853 | 5.853 | 5.773 | 5.861 | 5.872 | 5.847 |
| Co-N1(imine) | 1.954(2) | 1.9591(14) | 1.962(4) | 1.954(18) 1.947(18) | 1.951(2) | 1.963(4) | 2.031(8) 2.060(7) |
| 1.955(2) | 1.940(17) 1.982(17) | 1.961(2) | 1.951(4) | 2.049(7) 2.067(8) | |||
| 1.970(3) | 1.974(18) 1.921(18) | 1.924(2) | 1.962(4) | 2.064(9) 2.038(10) | |||
| average | 1.960 | 1.9591 | 1.9624 | 1.956 1.950 | 1.945 | 1.959 | 2.048 2.055 |
| Co-N2(pyrazole) | 1.908(3) | 1.9141(15) | 1.910(4) | 1.896(15) 1.918(15) | 1.908(2) | 1.928(4) | 1.979(7) 2.017(8) |
| 1.919(2) | 1.893(16) 1.8959(16) | 1.917(2) | 1.913(4) | 2.019(8) 2.048(7) | |||
| 1.928(2) | 1.954(17) 1.883(17) | 1.924(2) | 1.915(4) | 2.145(5) 1.865(5) | |||
| average | 1.918 | 1.9141 | 1.910 | 1.914 1.899 | 1.916 | 1.919 | 2.048 1.977 |
| N1-Co-N2 (bite) | 81.32(10) | 81.66(6) | 81.52(17) | 81.6(8) 83.6(8) | 82.16(10) | 80.94(15) | 77.3(3) 80.0(3) |
| 81.53(11) | 82.5(7) 81.3(7) | 81.32(10) | 82.00(15) | 78.3(3) 80.6(3) | |||
| 81.46(11) | 80.2(8) 80.1(7) | 81.25(9) | 81.17(16) | 76.1(3) 82.2(3) | |||
| average | 81.44 | 81.66 | 81.52 | 81.4 81.7 | 81.58 | 81.37 | 77.2 80.9 |
| N1-Co-N2’(trans) | 175.82(11) | 175.23(6) | 175.43(17) | 175.0(7) 176.0(8) | 174.95(11) | 173.78(17) | 174.2(4) 175.8(3) |
| 175.22(11) | 174.0(8) 177.5(8) | 174.19(10) | 174.71(17) | 170.6(3) 176.2(3) | |||
| 175.19(11) | 172.6(8) 173.7(8) | 175.16(10) | 175.12(16) | 171.4(3) 172.9(3) | |||
| average | 175.4 | 175.23 | 175.43 | 173.9 175.7 | 174.77 | 174.54 | 172.1 174.97 |
| C1-Nap-C1 | 118.6(3) | 119.991(5) | 119.996(14) | 121(2) 130(2) | 120.0(2) | 120.3(4) | 119.6(80 116.2(8) |
| 120.8(3) | 119.990(6) | 119.991(12) | 114(2) 116.0(19) | 120.6(2) | 120.0(4) | 121.1(8) 120.7(7) | |
| 119.1(3) | 119.984(5) | 119.995(14) | 123(2) 114(2) | 119.4(3) | 119.7(4) | 116.6(8) 119.1(9) | |
| average | 119.5 | 119.988 | 119.994 | 119.3 120.0 | 120.0 | 120.0 | 119.1 118.7 |
| Compound | Interaction | d(D-H) | d(H …A) | d(D …A) | <(DHA) |
|---|---|---|---|---|---|
| [Cotren(MepyrzH0.5]2 (ClO4)3 | N-H….N’ (three) | 0.88 | 1.83 | 2.670(3) | 160.1 |
| T = 123(2)K | |||||
| Average | 0.88 | 1.83 | 2.670 | 160.1 | |
| [Cotren(MepyrzH0.5]2 (BF4)3 | N-H….N’ (three) | 0.88 | 1.83 | 2.674(8) | 158.9 |
| T = 123(2)K | |||||
| Average | 0.88 | 1.83 | 2.67 | 158.9 | |
| [Cotren(MepyrzH)2(Mepyrz)}[Cotren(MepyrzH)(Mepyrz)2](BF4)3.3.81 H2O | N-H….N’ N-H …N’ N-H …N’ | 0.88 | 1.88 | 2.73(2) | 162 |
| 0.88 | 1.87 | 2.72(3) | 161 | ||
| T = 150(2) K | 0.88 | 1.87 | 2.714(10) | 160. | |
| Average | 0.88 | 1.87 | 2.72 | 161 | |
| {[Cotren(MepyrzH0.5)3]2(ClO4)3.(C6H6)2.(CH3CN)2@123K | N-H….N’ N-H …N’ N-H….N’ | 0.88 | 1.83 | 2.679(3) | 161.9 |
| 0.88 | 1.83 | 2.679(3) | 161.5 | ||
| T = 123(2) K | 0.88 | 1.80 | 2.643(4) | 160.5 | |
| Average | 0.88 | 1.82 | 2.667 | 161.3 | |
| {[Cotren(MepyrzH0.5)3]2 (ClO4)3.(C6 H6)2. (CH3CN)2 @150K, | N-H….N’ N-H …N’(twice) | 0.88 | 1.81 | 2.655(7) | 160.0 |
| 0.88 | 1.84 | 2.685(5) | 161.0 | ||
| T = 150(2) K | |||||
| Average | 0.88 | 1.83 | 2.675 | 160.7 | |
| [Cotren(MepyrzH)3][Cotren(Mepyrz)3] I2 | N-H….N’ N-H …N’ N-H ….N’ | 0.88 | 1.98 | 2.819(12) | 160. |
| 0.88 | 1.89 | 2.744(12) | 162 | ||
| T = 150(2) K | 0.88 | 1.90 | 2.754(8) | 162 | |
| Average | 0.88 | 1.92 | 2.772 | 152 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brewer, G.; Butcher, R.J.; Zavalij, P. Use of Pyrazole Hydrogen Bonding in Tripodal Complexes to Form Self Assembled Homochiral Dimers. Materials 2020, 13, 1595. https://doi.org/10.3390/ma13071595
Brewer G, Butcher RJ, Zavalij P. Use of Pyrazole Hydrogen Bonding in Tripodal Complexes to Form Self Assembled Homochiral Dimers. Materials. 2020; 13(7):1595. https://doi.org/10.3390/ma13071595
Chicago/Turabian StyleBrewer, Greg, Raymond J. Butcher, and Peter Zavalij. 2020. "Use of Pyrazole Hydrogen Bonding in Tripodal Complexes to Form Self Assembled Homochiral Dimers" Materials 13, no. 7: 1595. https://doi.org/10.3390/ma13071595





