Synthesis and Modification by Carbonization of Styrene–Ethylene Glycol Dimethacrylate–Lignin Sorbents and their Sorption of Acetylsalicylic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Eluents
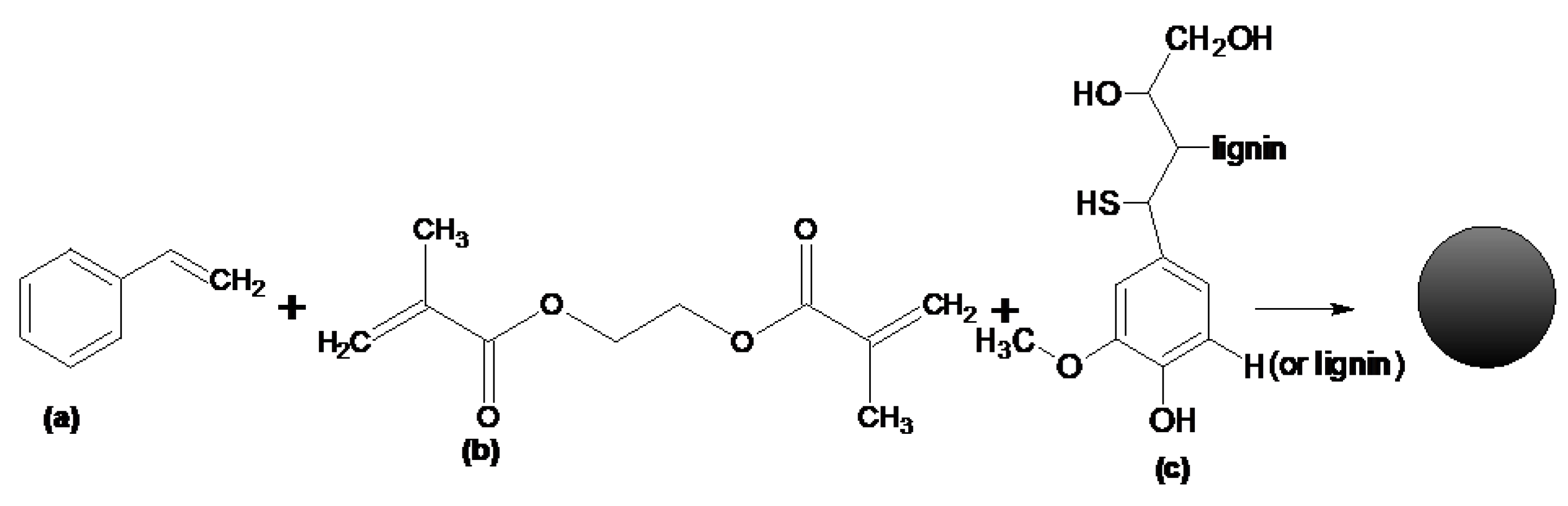
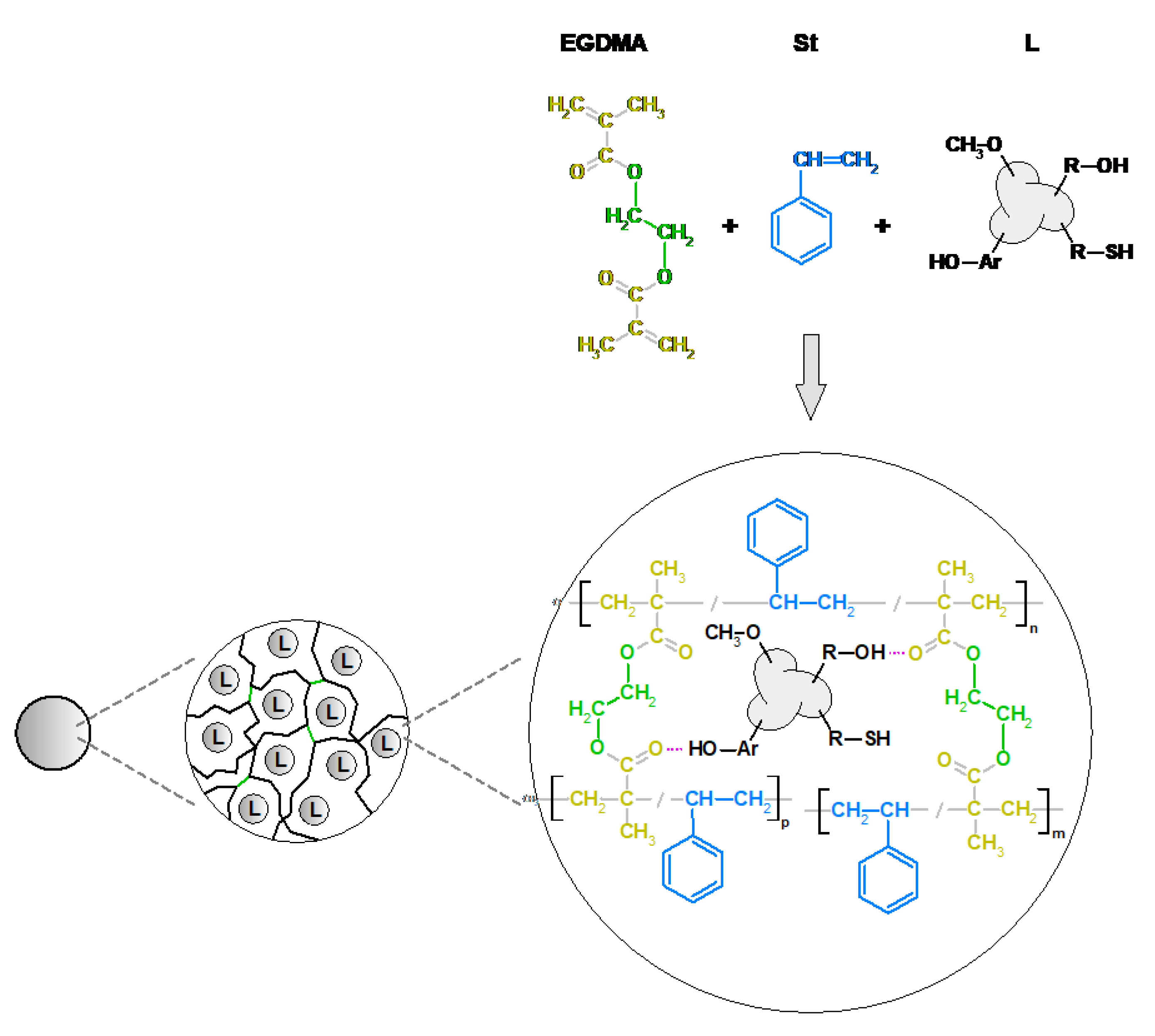
2.2. Synthesis of EGDMA-Based Polymeric Microspheres
2.3. Characterization of Methods
3. Results and Discussion
3.1. Characterization of the EGDMA + St Microspheres with a Lignin Component
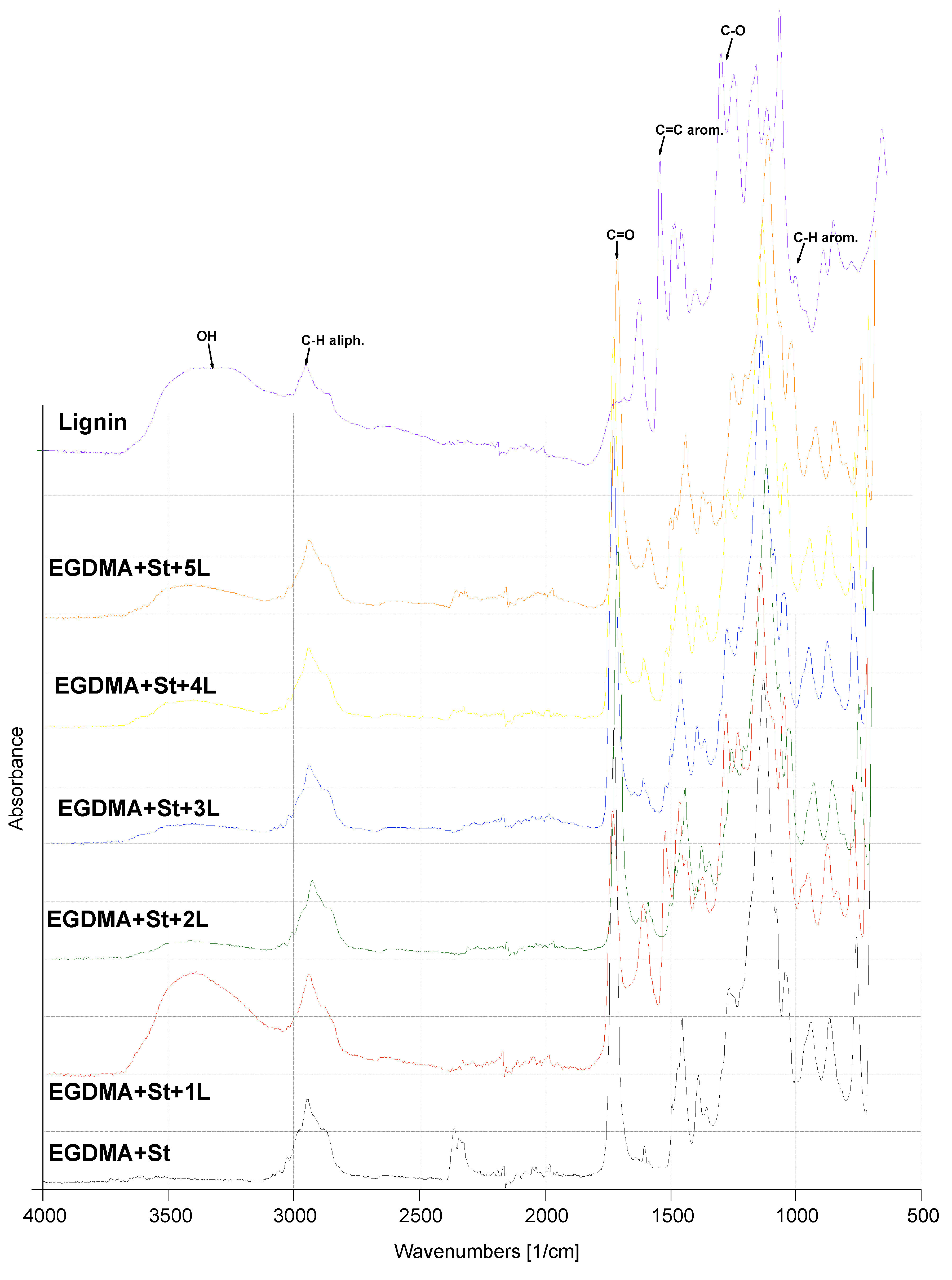
3.2. Spectroscopic Characterization of Copolymers
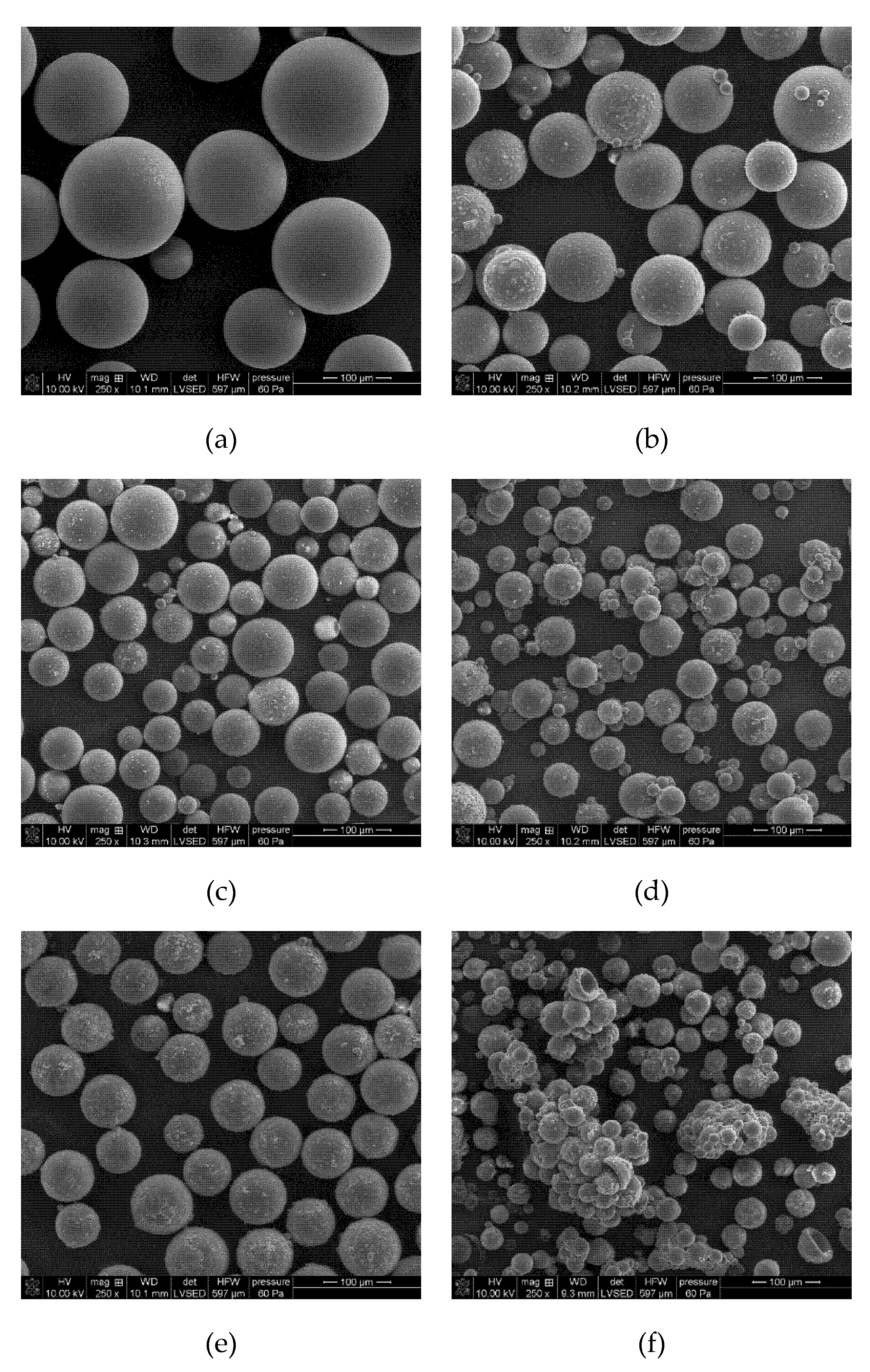
3.3. Morphology of EGDMA + St + lignin Microspheres
3.4. Swelling Studies
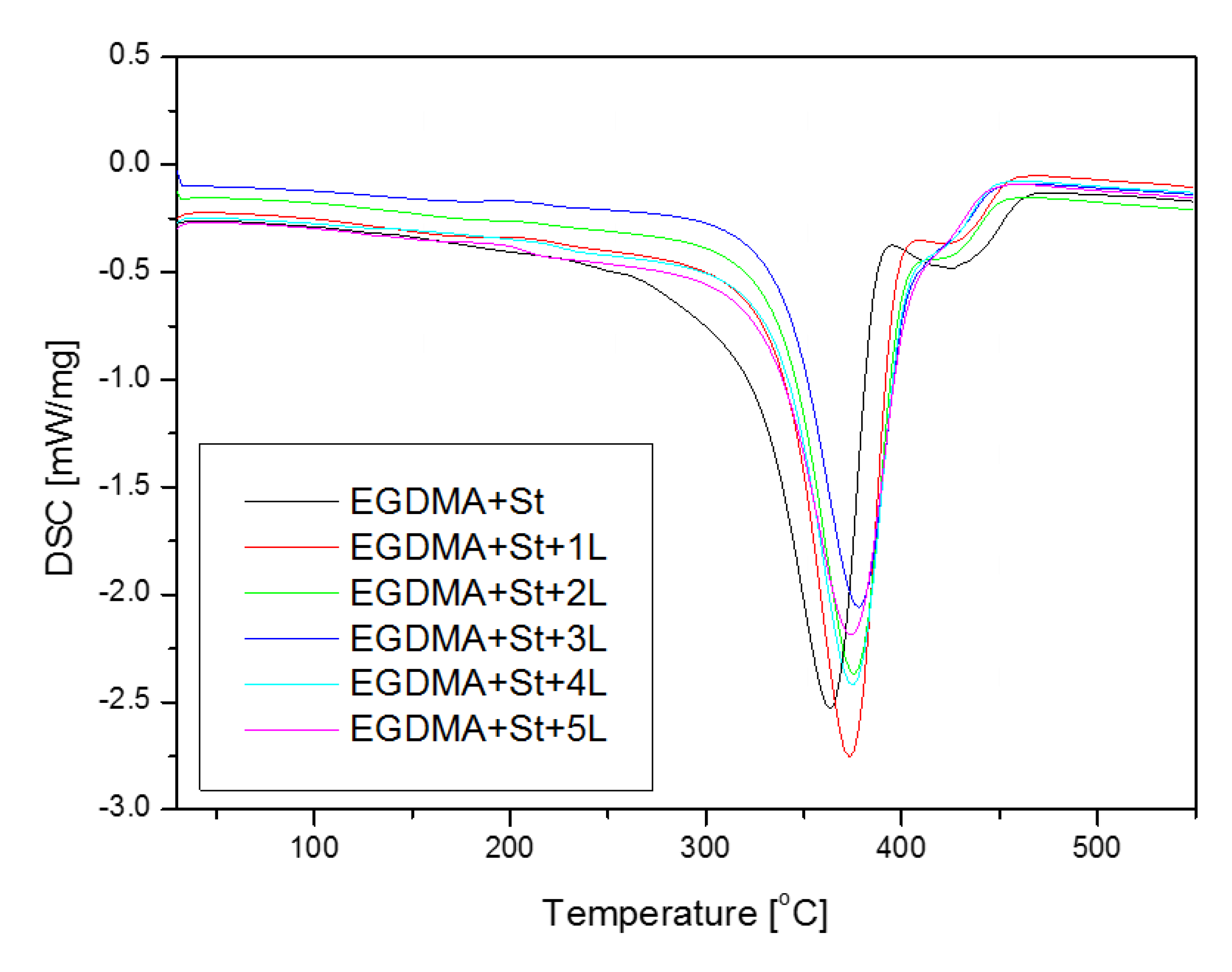
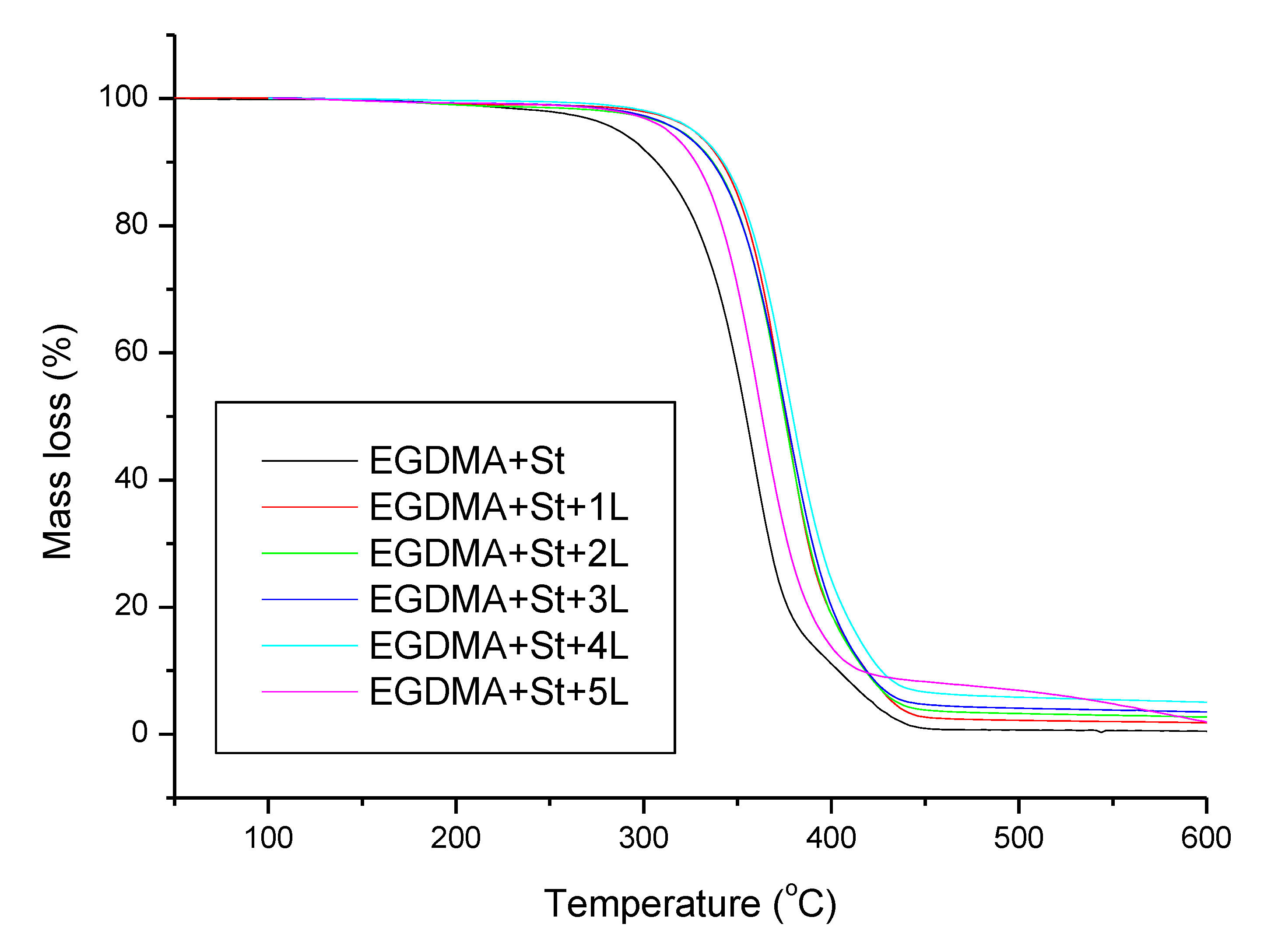
3.5. Thermal Properties of Copolymers
3.6. Carbonization Process
3.7. Porous Structure
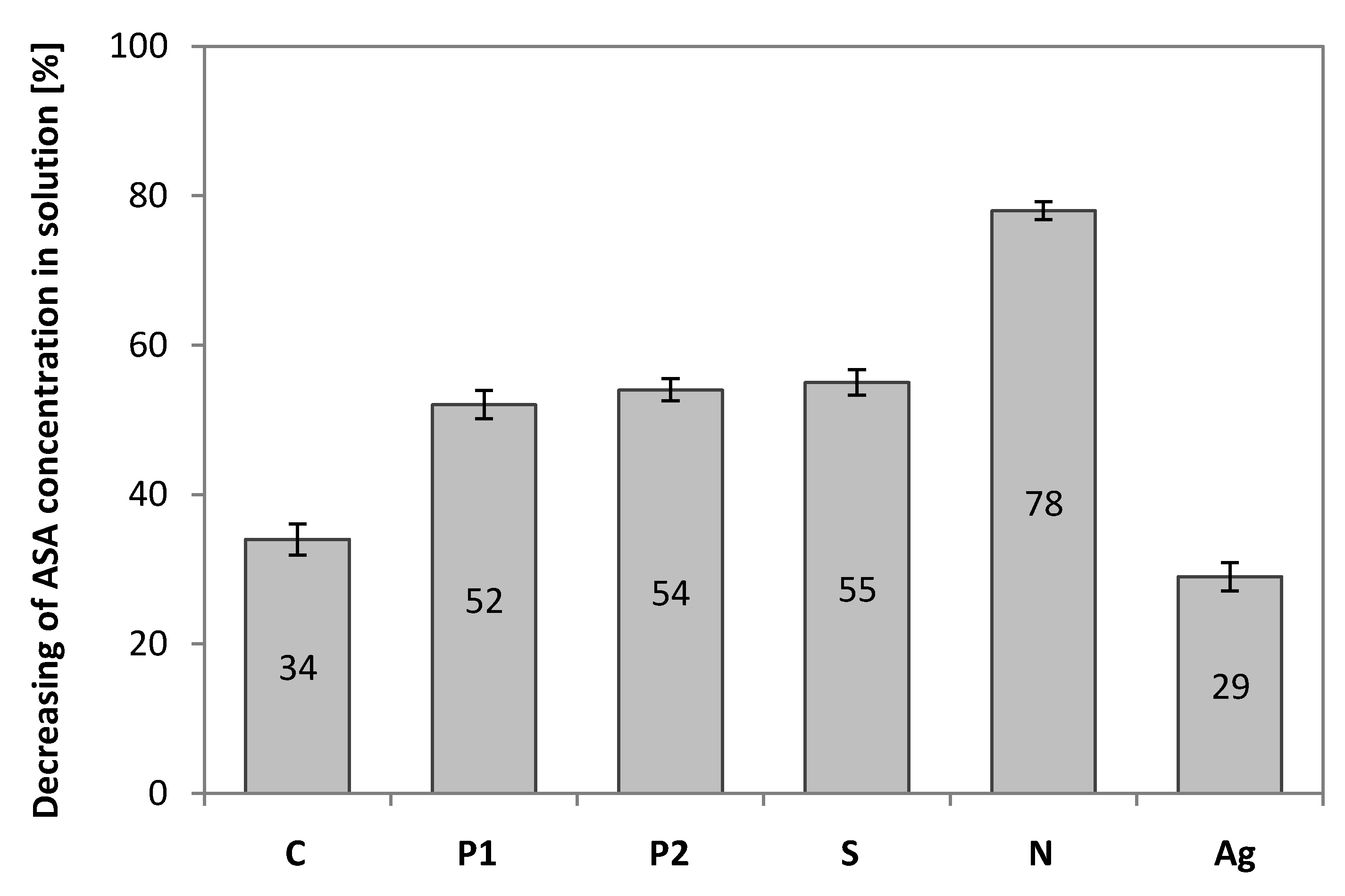
3.8. Chromatography Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Moss, G.; Smith, P.; Tavernier, D. Glossary of class names of organic compounds and reactivity intermediates based on structure. Pure Appl. Chem. 1995, 67, 1307–1375. [Google Scholar] [CrossRef]
- Crespo, E.; Costa, J.; Hanafiah, Z.; Kurnia, K.; Oliveira, M.; Llovell, F.; Vega, L.; Carvalho, P.; Coutinho, J. New measurements and modeling of high pressure thermodynamic properties of glycols. Fluid Phase Equilib. 2017, 436, 113–123. [Google Scholar] [CrossRef]
- Carvalho, P.; Fonseca, C.; Moita, M.; Santos, A.; Coutinho, J. Thermophysical Properties of Glycols and Glymes. J. Chem. Eng. Data. 2015, 60, 3721–3737. [Google Scholar] [CrossRef]
- Rebsdat, S.; Mayer, D. Ullmann’s Encyclopedia of Industrial Chemistry, 7th ed.; Wiley: Weinheim, Germany, 2014; pp. 531–534. [Google Scholar]
- Trochimczuk, A.; Streat, M.; Kolarz, B. Highly polar polymeric sorbents characterization and sorptive properties towards phenol and its derivatives. React. Funct. Polym. 2001, 46, 259–271. [Google Scholar] [CrossRef]
- Salusjärvi, L.; Toivaril, M.; Vehkomäki1, M.; Koivistoinenl, O.; Mojzital, D.; Niemelä1, K.; Penttilä1, M.; Ruohonen, L. Production of ethylene glycol or glycolic acid from D-xylose in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2017, 101, 8151–8163. [Google Scholar]
- Ozdemir, I.; Tekin, N.; Kara, A. Magnetic porous polymer microspheres: Synthesis, characterization and adsorption performance for the removal of phenol. Pure Appl. Chem. 2019, 56, 564–576. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, W.; Luo, Y.; Li, J. Poly(aspartic acid) surface modification of macroporous poly(glycidyl methacrylate) microspheres. J. Appl. Polym. Sci. 2019, 136, 47441. [Google Scholar] [CrossRef]
- Iordache, M.; Dodi, G.; Hritcu, D.; Draganescu, D.; Chiscan, O.; Popa, M. Magnetic chitosan grafted (alkyl acrylate) composite particles: Synthesis, characterization and evaluation as adsorbents. Arab J. Chem. 2018, 11, 1023–1043. [Google Scholar] [CrossRef] [Green Version]
- Lim, K.; Holdsworth, C. Effect of Formulation on the Binding Efficiency and Selectivity of Precipitation Molecularly Imprinted Polymers. Molecules 2018, 23, 2996. [Google Scholar] [CrossRef] [Green Version]
- Killic, G.; Osman, B.; Tuzmen, N. Application of affinity microspheres for effective SPE cleanup before the determination of sulfamerazine by HPLC. Mater. Sci. Eng. C 2018, 91, 55–63. [Google Scholar] [CrossRef]
- Łyszczek, R.; Gil, M.; Głuchowksa, K.; Podkościelna, B.; Lipke, A.; Mergo, P. Hybrid materials based on PEGDMA matrix and europium(III) carboxylates -thermal and luminescent investigations. Eur. Polym. J. 2018, 106, 318–328. [Google Scholar] [CrossRef]
- Fernandes, R.; Dinc, M.; Raimundo, I.; Mizaikoff, B. Synthesis and characterization of porous surface molecularly imprinted silica microsphere for selective extraction of ascorbic acid. Micropor mesopor mat. 2018, 264, 28–34. [Google Scholar] [CrossRef]
- Yang, M.; Huang, Y.; Cao, H.; Lin, Y.; Song, A.; Sun, Q. Synthesis of a Chemically Organic/Inorganic Hybrid Adsorbent and Its Adsorption to Phenol in Aqueous Solution. Polym. Korea 2017, 3, 394–403. [Google Scholar]
- Xu, W.; Zhang, W.; Li, Y.; Zhang, S. Adsorption of Methyl Orange Dye from Aqueous Solutions by Acrylic Composite Resin Chemically Modified with Calcium Lignosulphonate. Acta Polym. Sin. 2016, 3, 307–314. [Google Scholar]
- Feng, Q.; Li, J.; Cheng, H.; Chen, F.; Xie, Y. Synthesis and Characterization of Porous Hydrogel Based on Lignin and Polyacrylamide. Bioresources 2014, 3, 4369–4381. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Zhao, Y.; Li, J. From hollow lignin microsphere preparation to simultaneous preparation of urea encapsulation for controlled release using industrial kraft lignin via slow and exhaustive acetone-water evaporation. Holzforschung 2020, 4, 77–87. [Google Scholar] [CrossRef]
- Menéndez-Díaza, J.; Martín-Gullónb, I. Types of carbon adsorbents and their production published in Activated carbon surfaces in environmental remediation. Interface Sci. Technol. 2006, 1–48. [Google Scholar]
- Ahmaruzzaman, M. Adsorption of phenolic compounds on low-cost adsorbents: A review. Adv. Colloid Interface Sci. 2008, 143, 48–67. [Google Scholar] [CrossRef] [PubMed]
- Grycova, B.; Pryszcz, A.; Lestinsky, P.; Chamradova, K. Preparation and characterization of sorbents from food waste. Green Process Synth. 2017, 6, 287–293. [Google Scholar]
- Wang, J.; Chen, C. Biosorbents for Heavy Metal Removal and Their Future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef]
- Joshi, N. Heavy metals, conventional methods for heavy metal removal, biosorption and the development of low cost adsorbent. Eur. J. Pharm. Med. Res. 2017, 4, 388–393. [Google Scholar]
- Gunatilake, S. Methods of removing heavy metals from industrial wastewater. J. Multidiscip. Eng. Sci. 2015, 1, 12–18. [Google Scholar]
- Mesfin-Yeneneh, A.; Maitra, S.; Eldemerdasch, U. Study on Biosorption of Heavy Metals by Modified Lignocellulosic Waste. J. Appl. Sci. 2011, 11, 3555–3562. [Google Scholar]
- Van Son, T.; Huu Hao, N.; Wenshan, G.; Jian, Z.; Shuang, L.; Cuong, T.; Xinbo, Z. Typical low cost biosorbents for adsorptive removal of specific organic pollutants from water. Bioresour. Technol. 2015, 182, 353–363. [Google Scholar]
- Budnyak, T.; Aminzadeh, S.; Pylypchuk, I.; Sternik, D.; Tertykh, V.; Lindström, M.; Sevastyanova, O. Methylene Blue dye sorption by hybrid materials from technical lignins. J. Environ. Chem. Eng. 2018, 6, 4997–5007. [Google Scholar] [CrossRef]
- Lapo, B.; Demey, H.; Zapata, J.; Romero, C.; Sastre, A. Sorption of Hg(II) and Pb(II) Ions on Chitosan-Iron(III) from Aqueous Solutions: Single and Binary Systems. Polymers 2018, 10, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klapiszewski, Ł.; Siwińska-Stefańska, K.; Kołodyńska, D. Preparation and characterization of novel TiO2/lignin and TiO2-SiO2/lignin hybrids and their use as functional biosorbents for Pb(II). Chem. Eng. 2017, 314, 169–181. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K. Biomedical applications of polymer-composite materials: A review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Duval, A.; Lawoko, M. A review on lignin-based polymeric, micro- and nano-structured materials. React. Funct. Polym. 2014, 85, 78–96. [Google Scholar] [CrossRef]
- Kent, J.; Bommaraju, T.; Barnicki, S. Handbook of Industrial Chemistry and Biotechnology, 13th ed.; Springer: Cham, Switzerland, 2012; pp. 2015–2019. [Google Scholar]
- Holmberg, A.; Nguyen, N.; Karavolias, M.; Reno, K.; Wool, R.; Epps, T. Softwood Lignin-Based Methacrylate Polymers with Tunable Thermal and Viscoelastic Properties. Macromolecules 2016, 49, 1286–1295. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, A.; Ulbrich, A.; Coon, J.; Stahl, S. Formic-acid-induced depolymerization of oxidized lignin to aromatics. Nat. Lett. 2014, 515, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Upton, B.; Kasko, A. Strategies for the Conversion of Lignin to High-Value Polymeric Materials: Review and Perspective. Chem. Rev. 2016, 116, 2275–2306. [Google Scholar] [CrossRef]
- Podkościelna, B.; Goliszek, M.; Sevastyanova, O. New approach in the application of lignin for the synthesis of hybrid materials. Pure Appl. Chem. 2017, 89, 161–171. [Google Scholar] [CrossRef]
- Goliszek, M.; Podkościelna, B.; Fila, K.; Riazanova, A.; Aminzadeh, S.; Sevastyanova, O.; Gun’ko, V. Synthesis and structure characterization of polymeric nanoporous microspheres with lignin. Cellulose 2018, 25, 5843–5862. [Google Scholar] [CrossRef] [Green Version]
- Naseem, A.; Tabasum, S.; Zia, K.M.; Zuber, M.; Ali, M.; Noreen, A. Lignin-derivatives based polymers, blends and composites: A review. Int. J. Biol. Macromol. 2016, 93, 296–313. [Google Scholar] [CrossRef]
- Olson, E. Particle Shape Factors and Their Use In Image Analysis–Part 1: Theory. J. GxP Compliance 2011, 15, 85–96. [Google Scholar]
- Mikli, V.; Käerdi, H.; Kulu, P.; Besterci, M. Characterization of Powder Morphology. Proc. Estonian Acad. Sci. Eng. 2001, 7, 22–34. [Google Scholar]
- Malvern Instruments Ltd. Morphologi G3 User Manual; Enigma Business Park: Malvern, UK, 2013; pp. 10–15. [Google Scholar]
- Tuncel, A.; Pişkin, A. Nonswellable and swellable poly(EGDMA) microspheres. J. Appl. Polym. Sci. 1996, 62, 789–798. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Wan Daud, W.M.A.; Houshmand, A.; Shamiri, A. A review on surface modification of activated carbon for carbon dioxide adsorption. J. Anal. Appl. Pyrol. 2010, 89, 143–151. [Google Scholar] [CrossRef]

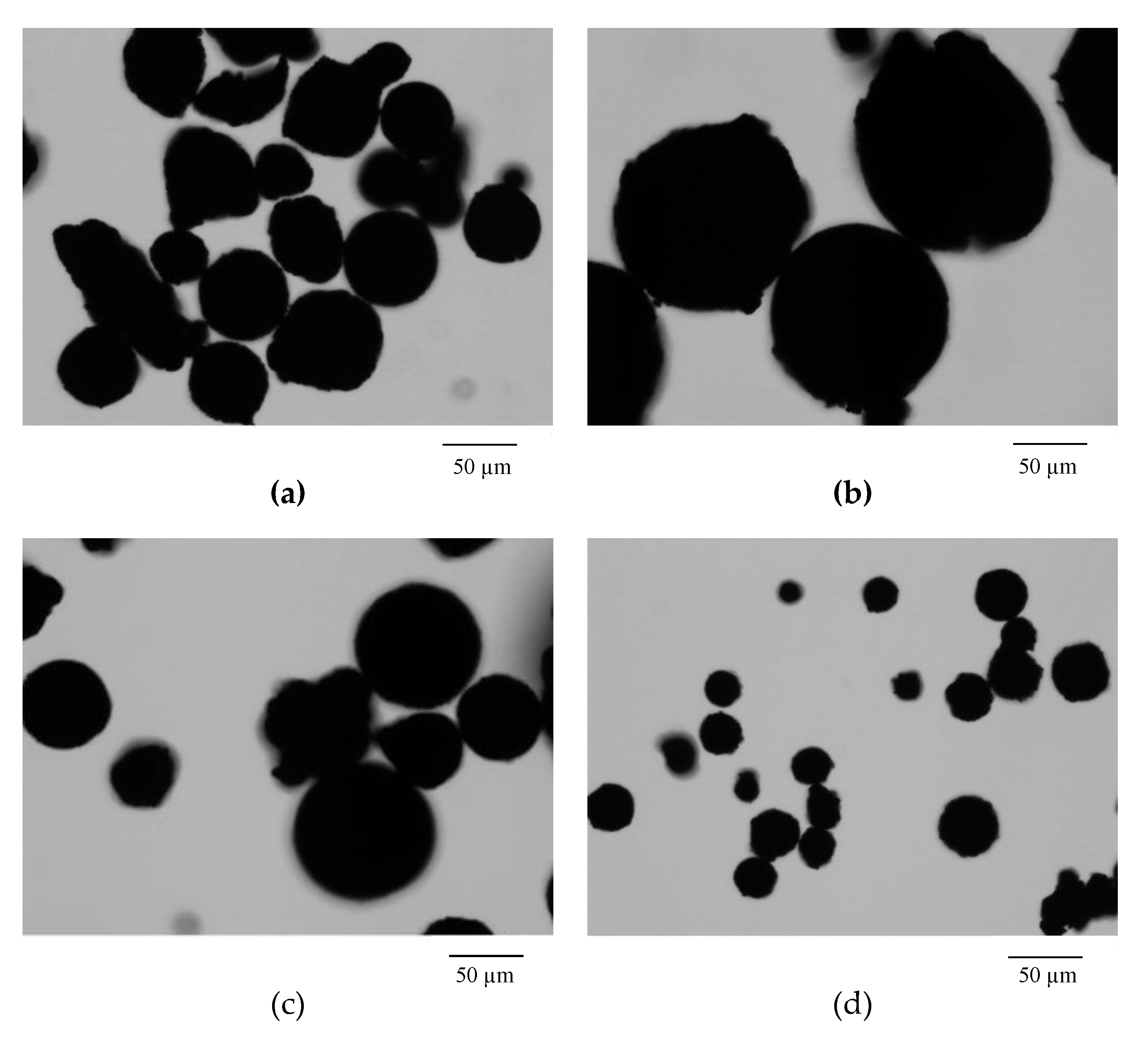


| Kraft Lignin [g] | St [g] | EGDMA [g] | AIBN [g] | Yield [%] |
|---|---|---|---|---|
| 0 | 4 | 3.8 | 0.18 | 74 |
| 1 | 4 | 3.8 | 0.20 | 90 |
| 2 | 4 | 3.8 | 0.22 | 83 |
| 3 | 4 | 3.8 | 0.24 | 80 |
| 4 | 4 | 3.8 | 0.26 | 81 |
| 5 | 4 | 3.8 | 0.28 | 87 |
| C–H aliph. | C–H arom. | C=C arom. | C–O | C=O | –OH | |
|---|---|---|---|---|---|---|
| EGDMA + St | 2943 1386 | 941 862 | 1453 | 1259 1127 | 1723 | — |
| EGDMA + St + 1L | 2939 | 941 860 | 1597 | 1265 1128 | 1720 | 3402 |
| EGDMA + St + 2L | 2943 | 940 861 | 1599 | 1263 1127 | 1722 | 3434 |
| EGDMA + St + 3L | 2939 | 937 861 | 1599 | 1262 1178 | 1722 | 3420 |
| EGDMA + St + 4L | — | 939 860 | 1598 | 1262 1128 | 1722 | 3500 |
| EGDMA + St + 5L | 2941 | 937 860 | 1598 | 1263 1128 | 1722 | 3402 |
| Lignin | 2950 | 930 | 1620 | 1300 | 1680 | 3510 |
| EGDMA | 2951 | — | — | 1293 | 1717 | — |
| St | — | 990 | 1600 | — | — | — |
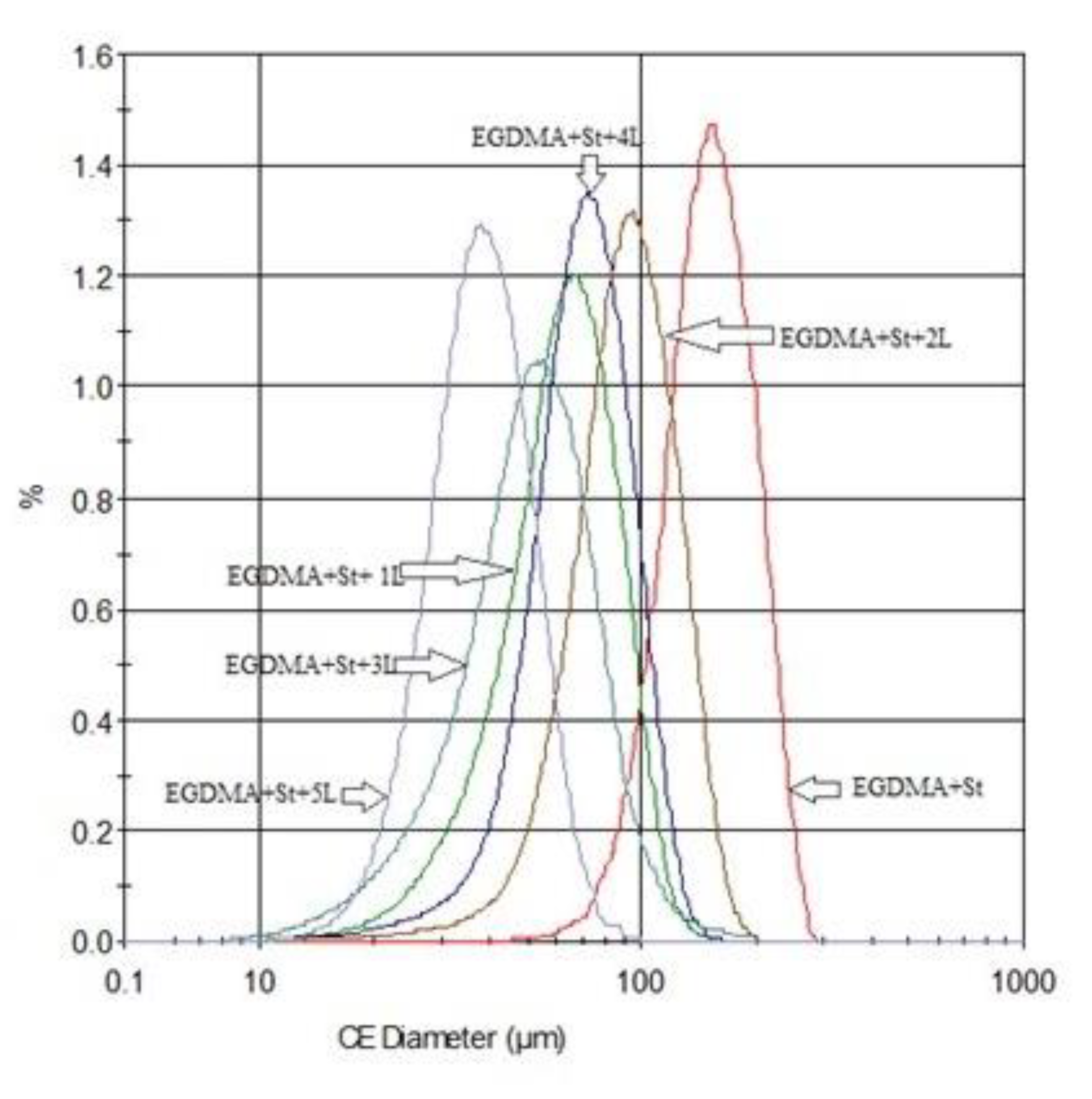
| CEdiam ± SD [μm] | C ± SD | E ± SD | S ± SD | |
|---|---|---|---|---|
| EGDMA + St | 130.79 ± 48.30 | 0.996 ± 0.003 | 0.006 ± 0.012 | 1.000 ± 0.003 |
| EGDMA + St + 1L | 52.66 ± 32.44 | 0.992 ± 0.005 | 0.021 ± 0.023 | 0.999 ± 0.002 |
| EGDMA + St + 2L | 41.86 ± 20.29 | 0.992 ± 0.005 | 0.023 ± 0.024 | 0.999 ± 0.002 |
| EGDMA + St + 3L | 30.87 ± 9.33 | 0.987 ± 0.004 | 0.047 ± 0.032 | 0.998 ± 0.002 |
| EGDMA + St + 4L | 43.25 ± 23.80 | 0.990 ± 0.005 | 0.034 ± 0.029 | 0.998 ± 0.002 |
| EGDMA + St + 5L | 27.91 ± 15.54 | 0.990 ± 0.005 | 0.037 ± 0.029 | 0.999 ± 0.002 |
| Solvent | EGDMA + St | EGDMA + St + 1L | EGDMA + St + 2L | EGDMA + St + 3L | EGDMA + St + 4L | EGDMA + St + 5L | EGDMA + St + 4L-S |
|---|---|---|---|---|---|---|---|
| B [%] | |||||||
| THF | 6 | 8 | 8 | 13 | 27 | 38 | 0 |
| ACE | 6 | 13 | 13 | 17 | 27 | 38 | 0 |
| ACN | 0 | 5 | 8 | 13 | 27 | 27 | 0 |
| TOL | 0 | 15 | 20 | 25 | 25 | 38 | 0 |
| TCM | 0 | 6 | 8 | 10 | 17 | 27 | 0 |
| MeOH | 0 | 6 | 6 | 6 | 8 | 8 | 0 |
| Water | 0 | 0 | 6 | 6 | 8 | 8 | 0 |
| Material | T5% (°C) | T10% (°C) | T50% (°C) | Tmax1 (°C) | Tmax2 (°C) | RM (%) |
|---|---|---|---|---|---|---|
| EGDMA + St | 286 | 307 | 355 | 358 | 407 | 0.32 |
| EGDMA + St + 1L | 326 | 342 | 376 | 376 | 416 | 1.00 |
| EGDMA + St + 2L | 319 | 337 | 375 | 376 | — | 1.67 |
| EGDMA + St + 3L | 319 | 336 | 376 | 377 | — | 2.52 |
| EGDMA + St + 4L | 326 | 342 | 379 | 378 | — | 3.92 |
| EGDMA + St + 5L | 313 | 328 | 363 | 362 | — | 0.46 |
| Reagents and Concentration | Yield (%) | Carbon Name Acronym (Abbreviation) |
|---|---|---|
| Temperature only | 8.3% | EGDMA + St + 4L-C (C) |
| H3PO4 (85%) | 9.1% | EGDMA + St + 4L-P1 (P1) |
| H3PO4 (85% diluted in volume ratio 1:1) | 10.2% | EGDMA + St + 4L-P2 (P2) |
| H2SO4 (95% diluted in volume ratio 1:1) | 10.5% | EGDMA + St + 4L-S (S) |
| HNO3 (65% diluted in volume ratio 1:1) | 23.9% | EGDMA + St + 4L-N (N) |
| AgNO3 (9%) | 26.2% | EGDMA + St + 4L-Ag (Ag) |
| Specific Surface Area SBET [m2/g] | Total Pore Volume VTOT [cm3/g] | Micropore Volume [cm3/g] | Average Pore Diameter [nm] | |
|---|---|---|---|---|
| EGDMA + St + 4L | 0.55 | 0.0046 | 0.0011 | 40.68 |
| EGDMA + St + 4L-C | 12.41 | 0.0112 | 0.0064 | 3.89 |
| EGDMA + St + 4L-P1 | 4.21 | 0.0216 | 0.0008 | 19.78 |
| EGDMA + St + 4L-P2 | 21.29 | 0.0403 | 0.0013 | 7.19 |
| EGDMA + St + 4L-S | 299.04 | 0.1492 | 0.0960 | 2.00 |
| EGDMA + St + 4L-N | 1.02 | 0.0054 | 0.0006 | 22.12 |
| EGDMA + St + 4L-Ag | 28.46 | 0.0309 | 0.0091 | 4.45 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wnuczek, K.; Podkościelna, B.; Sobiesiak, M.; Szajnecki, Ł.; Goliszek, M. Synthesis and Modification by Carbonization of Styrene–Ethylene Glycol Dimethacrylate–Lignin Sorbents and their Sorption of Acetylsalicylic Acid. Materials 2020, 13, 1761. https://doi.org/10.3390/ma13071761
Wnuczek K, Podkościelna B, Sobiesiak M, Szajnecki Ł, Goliszek M. Synthesis and Modification by Carbonization of Styrene–Ethylene Glycol Dimethacrylate–Lignin Sorbents and their Sorption of Acetylsalicylic Acid. Materials. 2020; 13(7):1761. https://doi.org/10.3390/ma13071761
Chicago/Turabian StyleWnuczek, Krystyna, Beata Podkościelna, Magdalena Sobiesiak, Łukasz Szajnecki, and Marta Goliszek. 2020. "Synthesis and Modification by Carbonization of Styrene–Ethylene Glycol Dimethacrylate–Lignin Sorbents and their Sorption of Acetylsalicylic Acid" Materials 13, no. 7: 1761. https://doi.org/10.3390/ma13071761





