On the Microstructure and Properties of Nb-12Ti-18Si-6Ta-2.5W-1Hf (at.%) Silicide-Based Alloys with Ge and Sn Additions
Abstract
:1. Introduction
2. Alloy Design and Selection
3. Experimental
4. Results
4.1. Alloy JZ1
4.2. Alloy JZ2
4.3. Oxidation
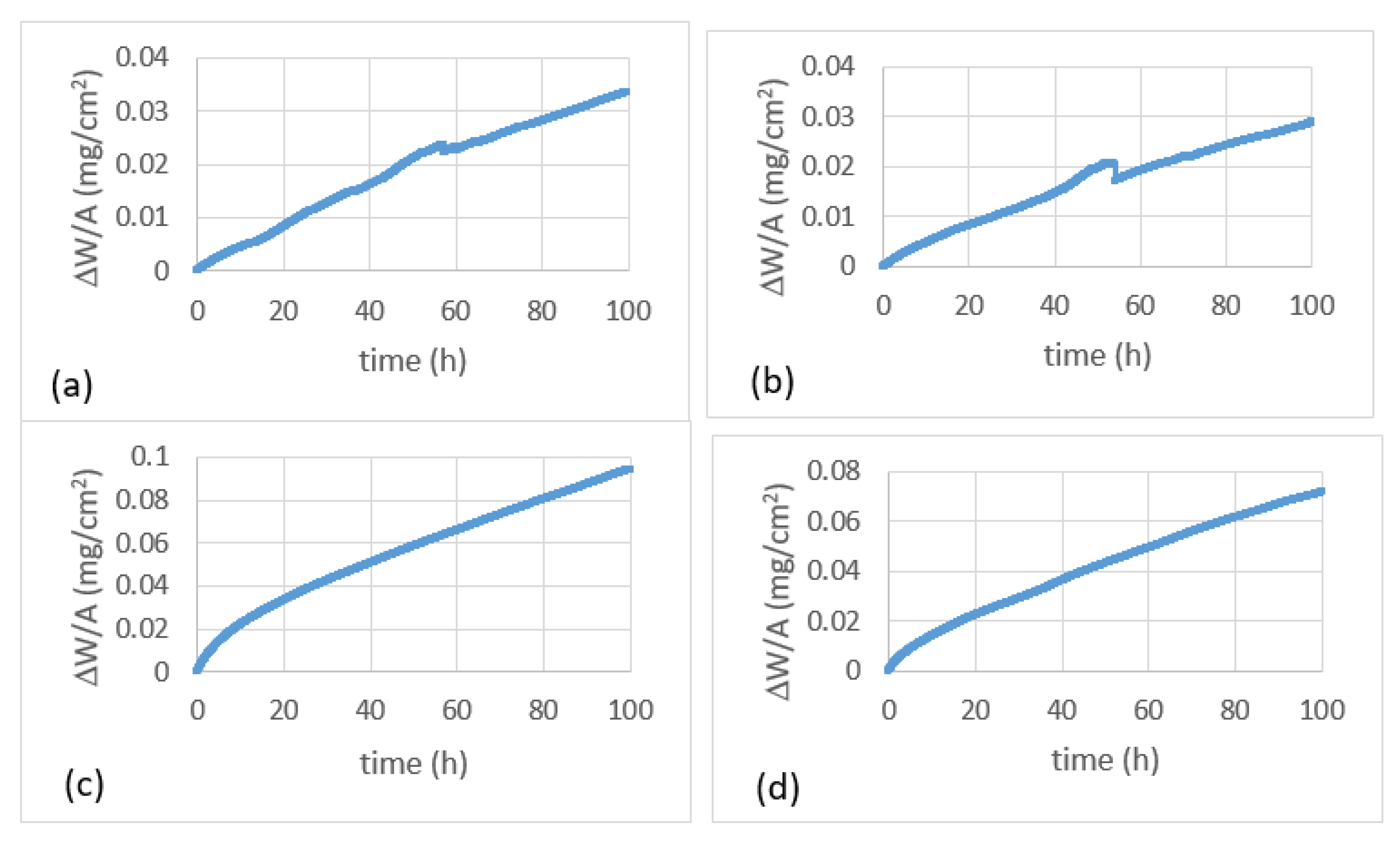
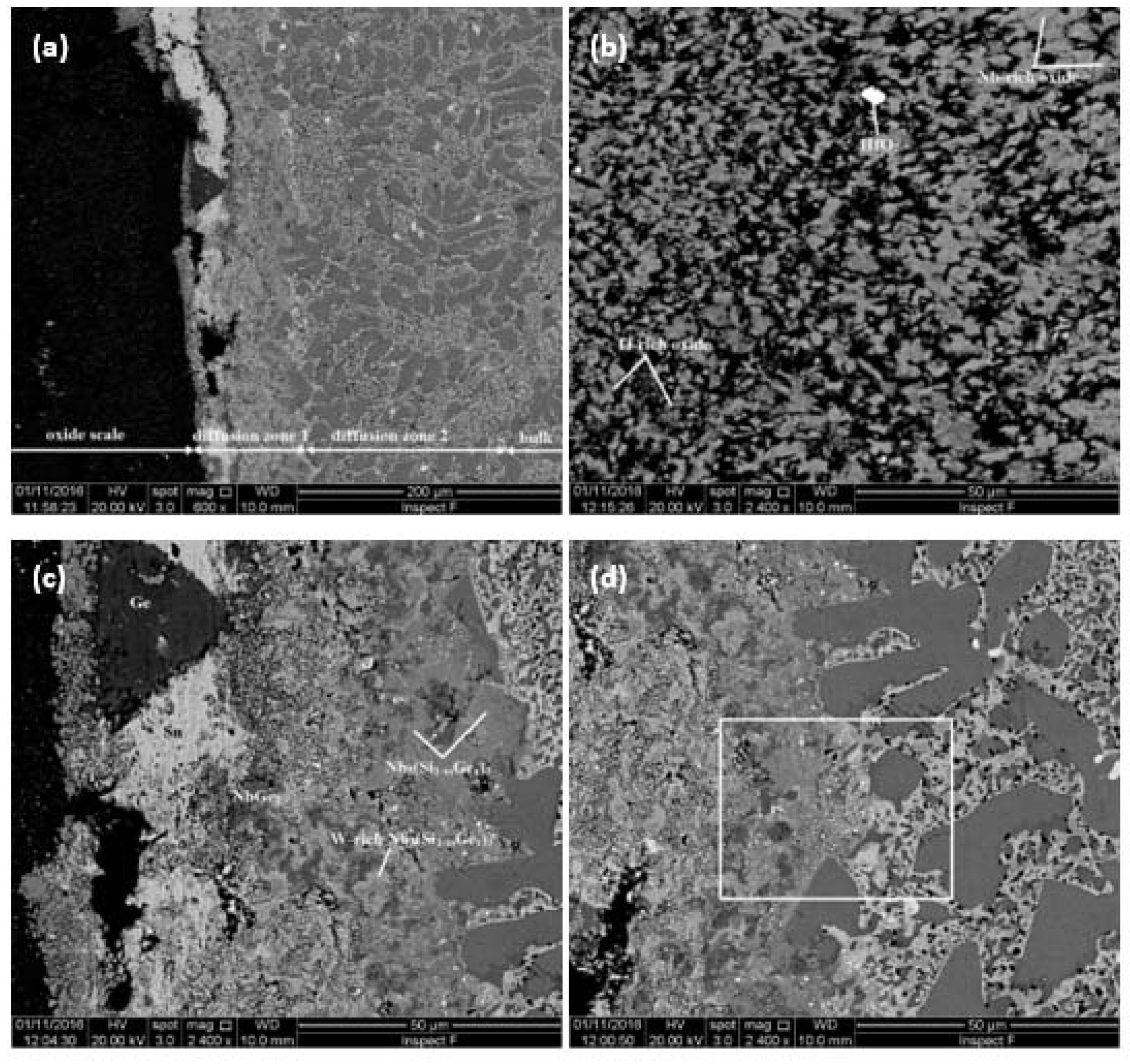
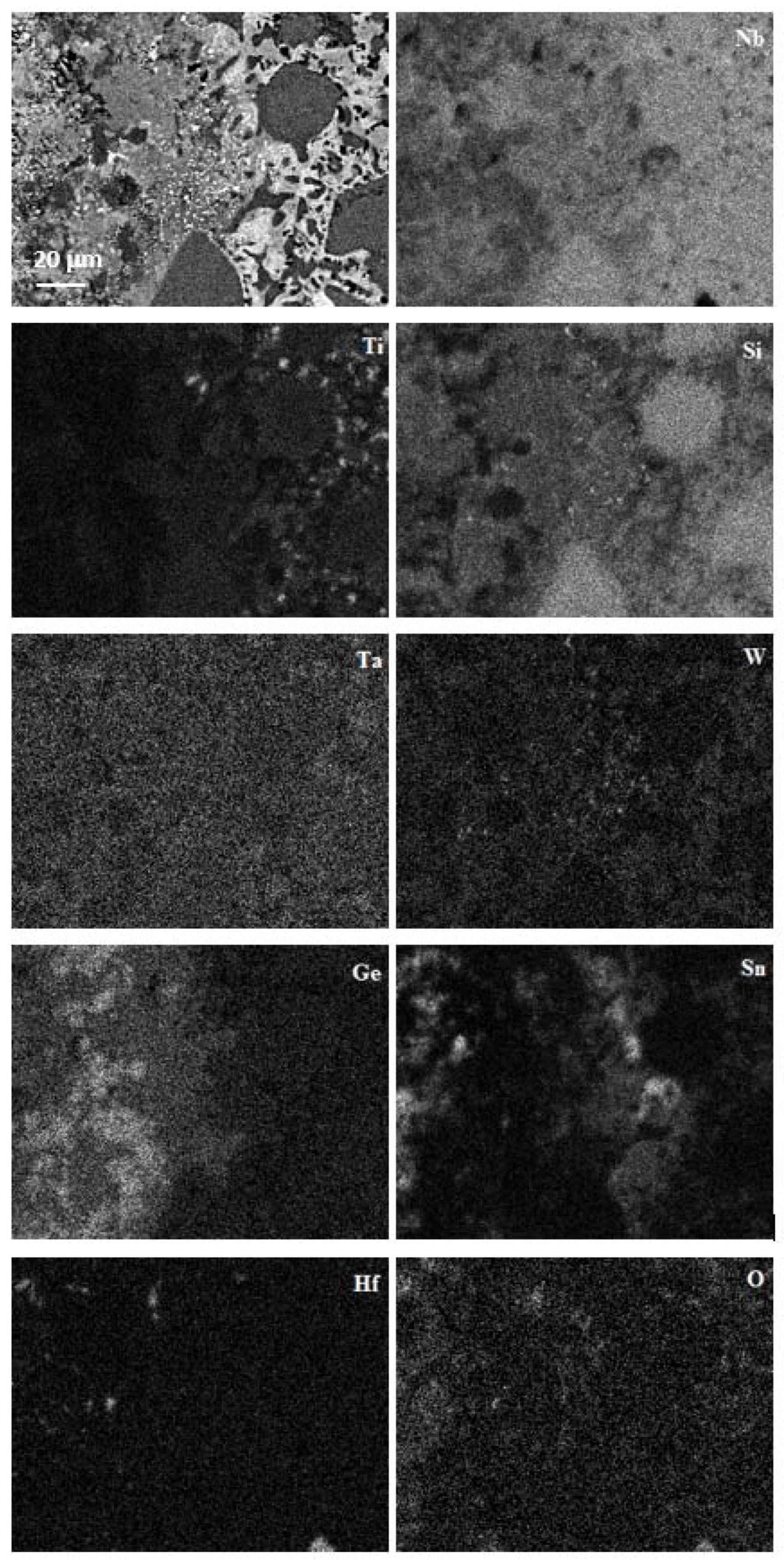
4.3.1. Oxidation at 800 °C
4.3.2. Oxidation at 1200 °C
5. Discussion
5.1. Densities
5.2. Macrosegregation
5.3. Microstructures
5.4. Oxidation
5.4.1. Oxidation at 800 °C
5.4.2. Oxidation at 1200 °C
5.5. Comparisons of Experimental Data with NICE
Creep
6. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- MacKay, R.A.; Gabb, T.P.; Smialek, J.L.; Nathal, M.V. Alloy Design Challenge: Development of Low Density Superalloys for Turbine Blade Applications; NASA/TM-2009-215819; NASA Technical Reports Server: Hampton, VA, USA, 2009.
- Jackson, M.R.; Bewlay, B.P.; Rowe, R.G.; Skelly, D.W.; Lipsitt, H.A. High temperature refractory metal intermetallic composites. JOM 1996, 48, 9–44. [Google Scholar] [CrossRef]
- Jackson, M.R.; Bewlay, B.P.; Zhao, J.-C. Niobium Silicide Based Composites Resistant to Low Temperature Pesting. U.S. Patent 6,419,765, 16 July 2002. [Google Scholar]
- Jackson, M.R.; Bewlay, B.P.; Briant, C.L. Creep Resistant Nb-Silicide Based Two Phase Composites. U.S. Patent 6,447,623 B1, 9 October 2002. [Google Scholar]
- Jackson, M.R.; Bewlay, B.P.; Zhao, J.-C. Niobium-Silicide Based Composites Resistant to High Temperature Oxidation. U.S. Patent 6,913,655, 1 August 2002. [Google Scholar]
- Tsakiropoulos, P. On Nb silicide based alloys: Part II. J. Alloys Compd. 2018, 748, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Gorsse, S.; Miracle, D.B.; Senkov, O.N. Mapping the world of complex concentrated alloys. Acta Mater. 2017, 135, 177–187. [Google Scholar] [CrossRef] [Green Version]
- Senkov, O.N.; Miracle, D.B.; Chaput, K.J. Development and exploration of refractory high entropy alloys—A review. J. Mater. Res. 2018, 33, 3092–3128. [Google Scholar] [CrossRef] [Green Version]
- Senkov, O.N.; Senkova, S.V.; Woodward, C.; Miracle, D.B. Low-density, refractory multi-principle element alloys of the Cr-Nb-Ti-V-Zr system: Microstructure and phase analysis. Acta Mater. 2013, 61, 1545–1557. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Yurchenko, N.Y.; Panina, E.S.; Tikhonovsky, M.A.; Zherebtsov, S.V. Precipitation strengthened refractory Al0.5CrNbTi2V0.5 high entropy alloy. Mater. Lett. 2017, 188, 162–164. [Google Scholar] [CrossRef]
- Muller, F.; Gorr, B.; Christ, H.-J.; Chen, H.; Kauffmann, A.; Heilmaier, M. Effect of micro alloying with silicon on high temperature oxidation resistance of novel refractory high entropy alloy Ta-Mo-Cr-Ti-Al. Mater. High Temp. 2018, 35, 168–176. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Hou, X.; Wang, X.; Meng, Y.; Zheng, X.; Zheng, L. Isothermal oxidation mechanism of a newly developed Nb-Ti-V-Cr-Al-W-Mo-Hf alloy at 800–1200 °C. Intern. J. Refract. Met. Hard Mater. 2016, 54, 322–329. [Google Scholar] [CrossRef]
- Tsakiropoulos, P. On Nb silicide based alloys: Alloy design and selection. Materials 2018, 11, 844. [Google Scholar] [CrossRef] [Green Version]
- Tsakiropoulos, P. On the Nb silicide based alloys: Part I-The bcc Nb solid solution. J. Alloys Compd. 2017, 708, 961–971. [Google Scholar] [CrossRef] [Green Version]
- Chang, W.H. Strengthening of refractory metals. In Refractory Metal and Alloys, Proceedings of the Conference Refractory Metals and Alloys, Detroit, MI, USA, 25–26 May 1960; Semchyshen, M., Harwood, J.J., Eds.; Interscience Publishers: New York, NY, USA, 1961; pp. 83–118. [Google Scholar]
- Begley, R.T.; Bechtold, J.H. Effect of alloying on the mechanical properties of Niobium. J. Less Common Met. 1961, 3, 1–12. [Google Scholar] [CrossRef]
- Prokoshkin, D.A.; Vasileva, E.V. Alloys of Niobium; Samarin, A.M., Ed.; Israel Program for Scientific Translations: Jerusalem, Israel, 1965. [Google Scholar]
- Fujikara, M.; Kasama, A.; Tanaka, R.; Hanada, S. Effect of alloy chemistry on the high temperature strengths and room temperature fracture toughness of advanced Nb-based alloys. Mater. Trans. 2004, 45, 493–501. [Google Scholar] [CrossRef] [Green Version]
- Hirai, H.; Tabaru, T.; Sha, J.; Ueno, H.; Kitahara, A.; Hanada, S. High temperature compression strength of directionally solidified Nb-Mo-W-Ti-Si in situ composites. Mat. Res. Soc. Symp. Proc. 2001, 646, N5.41.1–N5.41.6. [Google Scholar] [CrossRef]
- Ma, C.L.; Li, J.G.; Tan, Y.; Tanaka, R.; Hanada, S. Microstructure and mechanical properties of Nb/Nb5Si3 in situ composites in Nb-Mo-Si and Nb-W.-Si systems. Mater. Sci. Eng. A 2004, 386, 375–383. [Google Scholar] [CrossRef]
- Menon, E.S.K.; Mendiratta, M.G.; Dimiduk, D.M. Oxidation behavior of complex niobium based alloys. In Niobium Science & Technology, Proceedings of the International Symposium Niobium 2001, Orlando, FL, USA, 2–5 December 2001; Niobium 2001 Limited: Bridgeville, PA, USA, 2002; pp. 121–145. ISBN 9780971206809. [Google Scholar]
- Sarah, E.; Menon, K.; Mendiratta, M.G.; Dimiduk, D.M. High temperature oxidation mechanisms in Nb-silicide bearing multicomponent alloys. In Structural Intermetallics; Hemker, K.J., Dimiduk, D.M., Clemens, H., Darolia, R., Inui, H., Larsen, J.M., Sikka, V.K., Thomas, M., Whittenberger, J.D., Eds.; TMS: Warrendale, PA, USA, 2001; pp. 591–600. [Google Scholar]
- Bewlay, B.P.; Jackson, M.R.; Zhao, J.-C.; Subramanian, P.R.; Mendiratta, M.G.; Lewandowski, J.J. Ultrahigh temperature Nb-silicide based composites. MRS Bull. 2003, 28, 646–653. [Google Scholar] [CrossRef]
- Knittel, S.; Mathieu, S.; Portebois, L.; Vilasi, M. Effect of tin addition on Nb-Si based in situ composites. Part II: Oxidation behavior. Intermetallics 2014, 47, 43–52. [Google Scholar] [CrossRef]
- Li, Z.; Tsakiropoulos, P. The effect of Ge addition on the oxidation of Nb-24Ti-18Si silicide based alloys. Materials 2019, 12, 3120. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Utton, C.; Tsakiropoulos, P. A study of the effect of 5 at.% Sn on the microstructure and isothermal oxidation at 800 and 1200 °C of Nb-24Ti-18Si based alloys with Al and/or Cr additions. Materials 2020, 13, 245. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Negrete, O.; Tsakiropoulos, P. On the microstructure and isothermal oxidation at 800 and 1200 °C of the Nb-24Ti-18Si-5Al-5Cr-5Ge-5Sn (at.%) silicide based alloy. Materials 2020, 13, 722. [Google Scholar] [CrossRef] [Green Version]
- Bewlay, B.P.; Jackson, M.R.; Gigliotti, M.F.X. Niobium silicide high temperature in situ composites. In Intermetallic Compounds: Principles and Practice; Fleischer, R.L., Westbrook, J.H., Eds.; John Wiley: New York, NY, 2001; Volume 3, p. 541. [Google Scholar]
- Chan, K.S. Alloying effects on the fracture toughness of Nb-based silicides and Laves phases. Mater. Sci. Eng. 2005, A409, 257–269. [Google Scholar] [CrossRef]
- Grammenos, I.; Tsakiropoulos, P. Study of the role of Hf, Mo and W additions in the microstructure of Nb-20Si silicide based alloy. Intermetallics 2011, 19, 1612–1621. [Google Scholar] [CrossRef]
- McCaughey, C.; Tsakiropoulos, P. Type of primary Nb5Si3 and precipitation of Nbss in αNb5Si3 in a Nb-8.3Ti-21.1Si-5.4Mo-4W-0.7Hf (at.%) near eutectic Nb-silicide based alloy. Materials 2018, 11, 967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendersky, L.; Biancaniello, F.S.; Boettinger, W.J.; Perepezko, J.H. Microstructural characterisation of rapidly solidified Nb-Si alloys. Mater. Sci. Eng. 1987, A89, 151–159. [Google Scholar] [CrossRef]
- Schlesinger, M.E.; Okamoto, H.; Gokhale, A.B.; Abbaschian, R. The Nb-Si (Niobium-Silicon) System. J. Phase Equilibria 1993, 14, 502–5099. [Google Scholar] [CrossRef]
- Zacharis, E.; Utton, C.; Tsakiropoulos, P. A study of the effects of Hf and Sn on the microstructure, hardness and oxidation of Nb-18Si silicide based alloys without Ti addition. Materials 2018, 11, 2447. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, H. Phase Diagrams for Binary Alloys: Desk Handbook; ASM International: Metals Park, OH, USA, 2000. [Google Scholar]
- David, N.; Cartigny, Y.; Belmonte, T.; Fiorani, J.M.; Vilasi, M. Thermodynamic description of the Cr-Nb-Si isothermal section at 1473 K. Intermetallics 2006, 14, 464–473. [Google Scholar] [CrossRef]
- Han, X.; Wei, B. Microstructural characteristics of Ni-Sb eutectic alloys under substantial undercooling and containerless solidification conditions. Metall. Mater. Trans. A 2002, 33, 1221–1228. [Google Scholar] [CrossRef]
- Li, M.; Kuribayashi, K. Further discussion on the free growth behaviour in the solidification of undercooled eutectic melts. Metall. Mater. Trans. 2003, 34, 1393–1396. [Google Scholar] [CrossRef]
- Knittel, S.; Mathieu, S.; Vilasi, M. Effect of tin addition on Nb-Si based in situ composites: Part, I. Structural modifications. Intermetallics 2014, 47, 36–42. [Google Scholar] [CrossRef]
- Stewart, G.; Newkirk, L.R.; Valencia, F.A. Impurity stabilized A15 Nb3Nb—A new Superconductor. Phys. Rev. B 1980, 21, 5055–5064. [Google Scholar] [CrossRef]
- Devantay, H.; Jorda, J.; Decroux, M.; Muller, J.; Flukiger, R. The physical and structural properties of superconducting A15-type Nb-Sn alloys. J. Mater. Sci. 1981, 16, 2145–2153. [Google Scholar] [CrossRef]
- Straumanis, M.; Zyszczynsk, S. Lattice parameters, thermal expansion coefficients and densities of Nb, and of solid solutions Nb-O and Nb-N-O and their defect structure. J. Appl. Cryst. 1970, 3, 1–6. [Google Scholar] [CrossRef]
- Tafto, J.; Suenaga, M.; Welch, D. Crystal site determination of dilute alloying elements in polycrystalline Nb3Sn superconductors using a transmission electron microscope. J. Appl. Phys. 1984, 55, 4330–4333. [Google Scholar] [CrossRef]
- Zelenitsas, K.; Tsakiropoulos, P. Study of the role of Ta and Cr additions in the microstructure of Nb-Ti-Si-Al in situ composites. Intermetallics 2006, 14, 639–659. [Google Scholar] [CrossRef]
- Westbrook, J.; Wood, D. Pest degradation in beryllides, silicides, aluminides, and related compounds. J. Nucl. Mater. 1964, 12, 208–215. [Google Scholar] [CrossRef]
- Geng, J.; Tsakiropoulos, P.; Shao, G. A Thermo-gravimetric and microstructural study of the oxidation of Nbss/Nb5Si3 based in situ composites with Sn addition. Intermetallics 2007, 15, 270–281. [Google Scholar] [CrossRef]

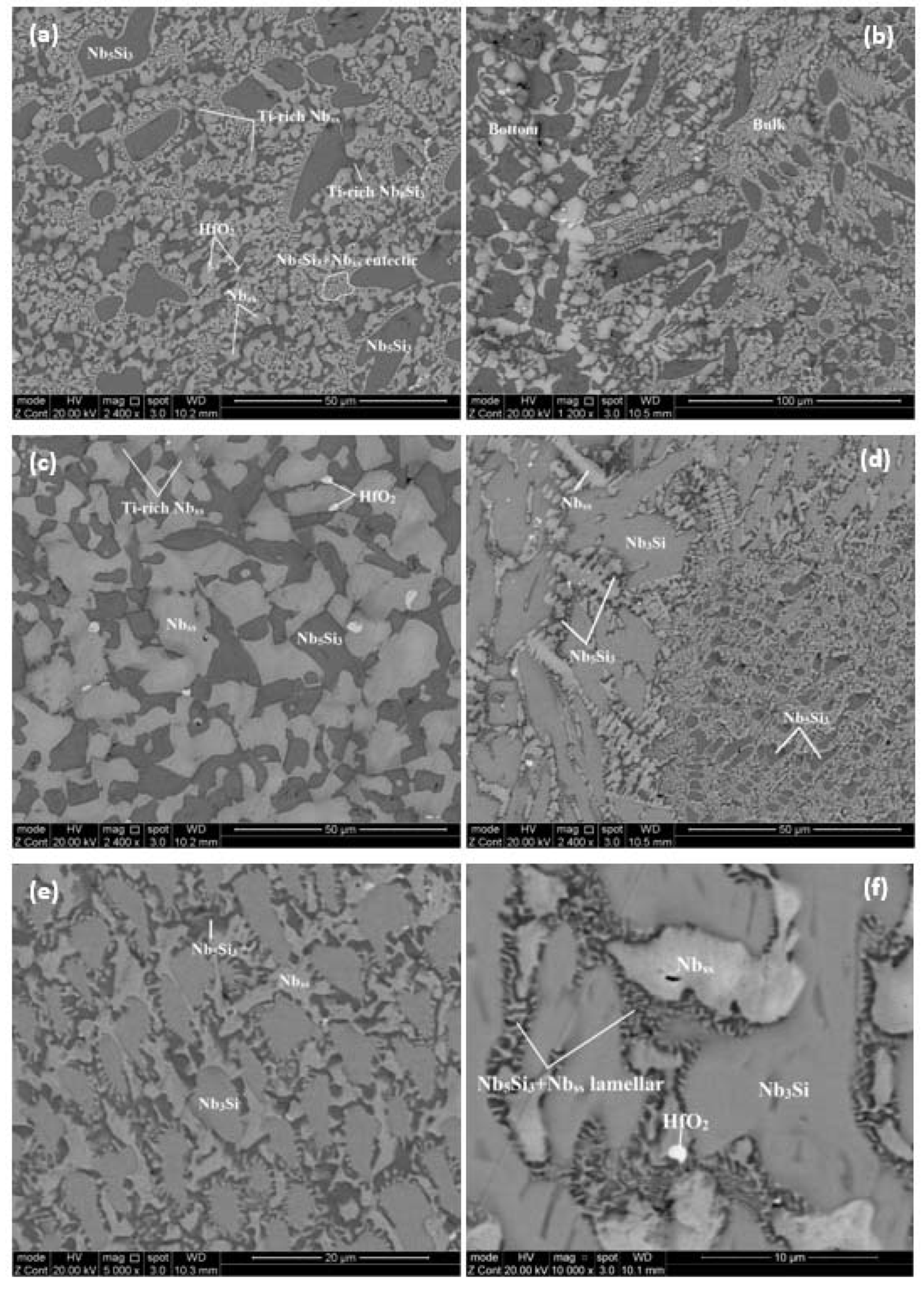
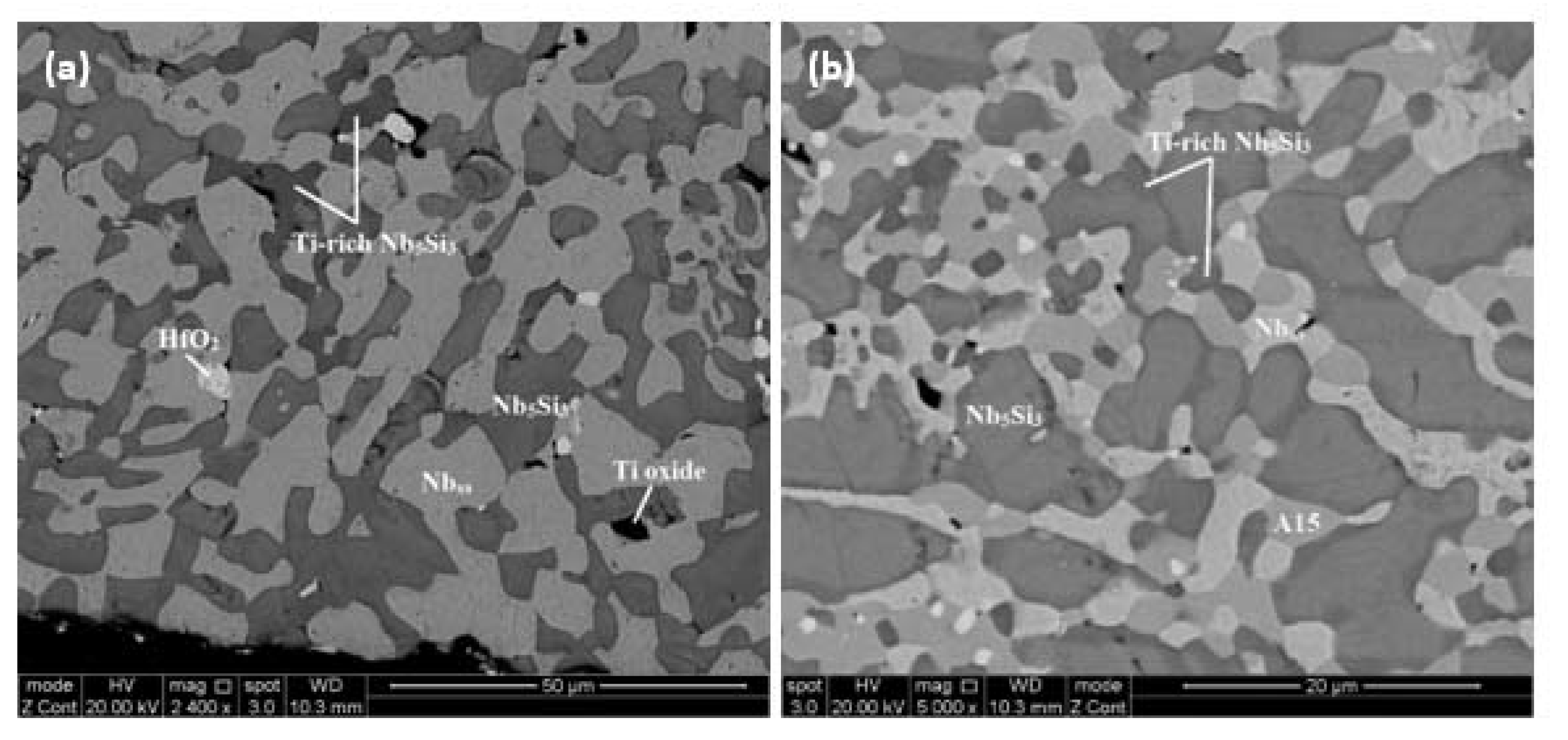
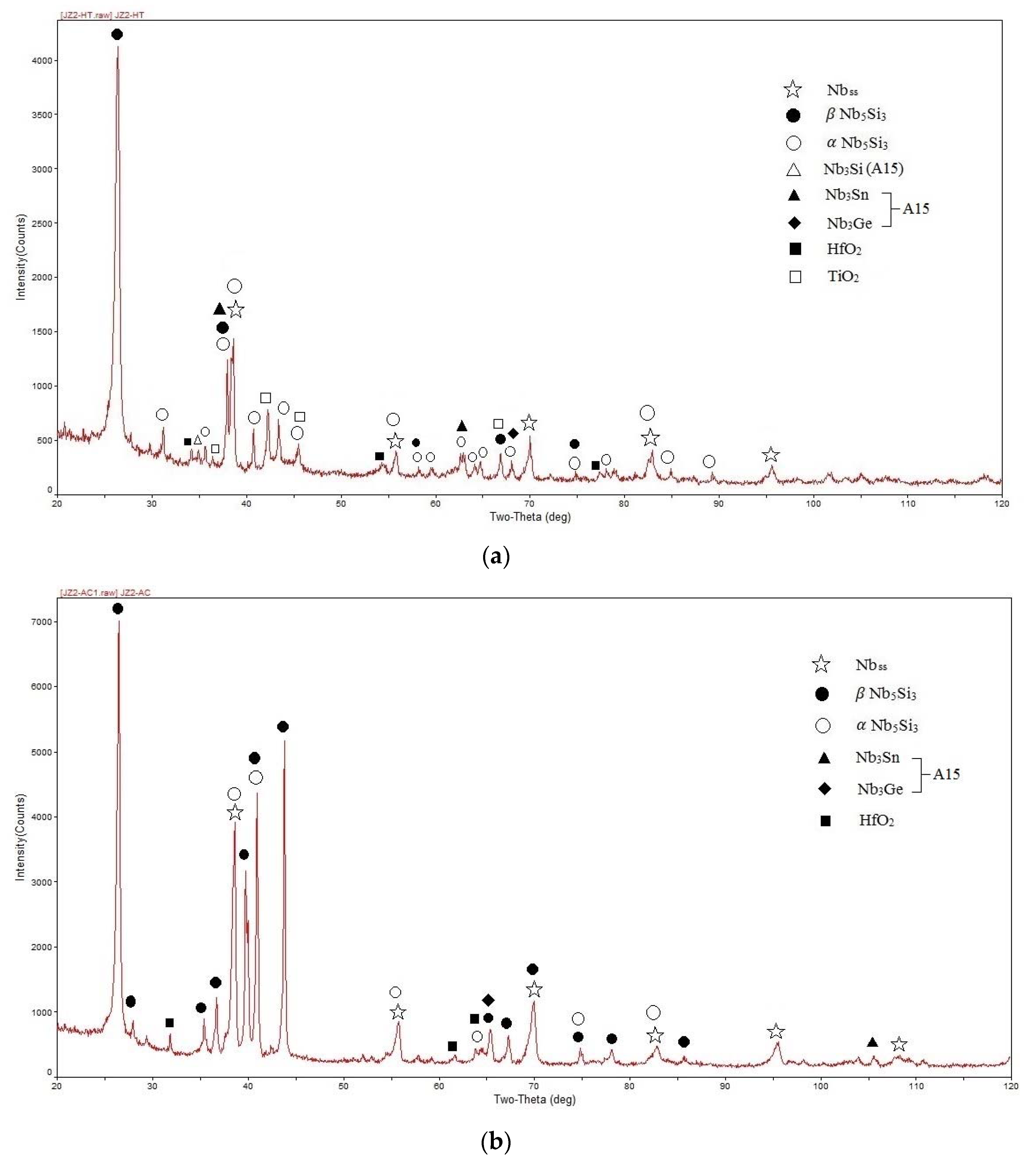
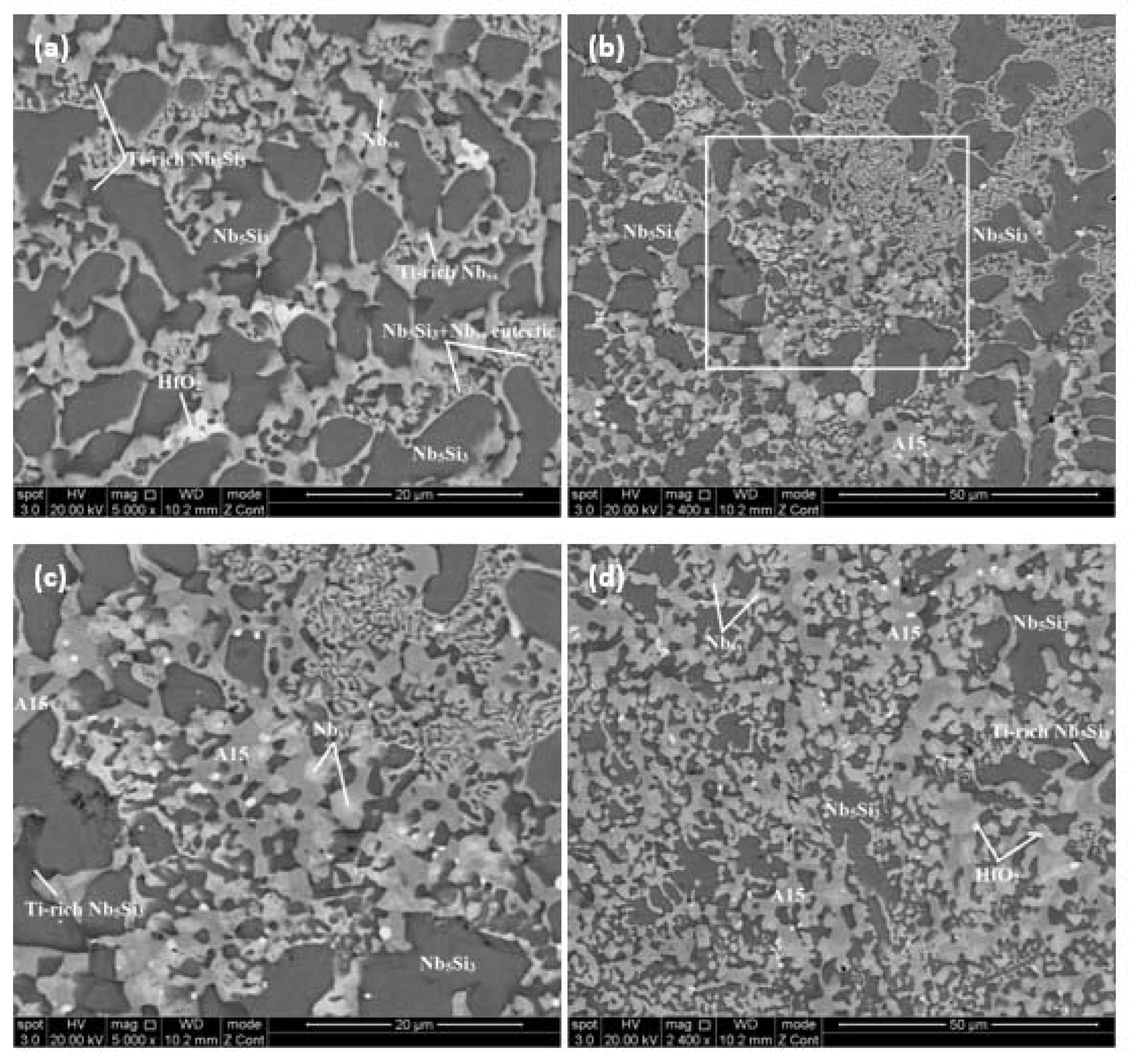


| Area Phase | Nb | Ti | Si | Ta | W | Sn | Ge | Hf |
|---|---|---|---|---|---|---|---|---|
| As-cast | ||||||||
| Top | 55.1 ± 0.8 54.0–55.9 | 11.9 ± 0.6 11.3–12.7 | 22.3 ± 1.7 20.4–24.4 | 5.4 ± 0.3 5.2–5.8 | 1.6 ± 0.3 1.4–2.0 | 1.1 ± 0.2 0.9–1.3 | 1.8 ± 0.1 1.7–2.0 | 0.8 ± 0.1 0.7–0.9 |
| Bulk | 55.1 ± 0.9 53.5–56.0 | 11.4 ± 0.2 11.1–11.6 | 22.9 ± 1.6 21.2–23.0 | 5.1 ± 0.4 4.6–5.7 | 1.6 ± 0.3 1.3–1.9 | 1.2 ± 0.1 1.1–1.3 | 1.8 ± 0.2 1.6–2.1 | 0.9 ± 0.2 0.8–1.1 |
| Bottom | 55.4 ± 2.0 53.5–57.6 | 11.4 ± 0.2 11.3–11.7 | 22.3 ± 2.6 19.0–24.6 | 5.5 0.4 5.1–6.2 | 1.7 0.4 1.3–2.3 | 1.1 ± 0.2 0.9–1.3 | 1.8 0.2 1.6–2.0 | 0.8 0.1 0.6–0.9 |
| Nbss | 68.2 0.6 67.1–69.4 | 11.8 1.7 9.4–13.9 | 4.4 0.9 3.6–5.4 | 8.5 0.5 7.7–9.2 | 4.5 0.4 3.8–5.3 | 1.7 0.3 1.2–2.0 | 0.5 0.1 0.3–0.7 | 0.4 0.1 0.2–0.6 |
| Ti–rich Nbss | 61.1± 1.6 59.6–64.5 | 22.7 1.8 21.6–24.8 | 4.2 1.1 3.1–5.6 | 5.5 0.7 4.4–6.9 | 1.8 0.4 1.0–2.4 | 3.0 0.2 2.7–3.5 | 0.6 0.1 0.4–0.7 | 1.1 0.2 0.9–1.4 |
| Nb5Si3 | 48.4 0.3 47.7–48.6 | 7.8 0.2 7.4–8.0 | 36.3 0.6 35.4–37.2 | 3.9 0.2 3.6–4.2 | 0.3 0.1 0.1–0.5 | 0.5 0.1 0.4–0.7 | 2.3 0.1 2.1–2.6 | 0.5 0.1 0.4–0.6 |
| Ti-rich Nb5Si3 | 46.3 0.7 45.1–47.0 | 11.9 0.9 10.9–12.9 | 34.5 0.7 34.0–35.7 | 2.9 0.2 2.5–3.1 | 0.1 | 0.6 0.1 0.5–0.7 | 2.8 0.2 2.6–3.0 | 0.9 0.2 0.7–1.1 |
| Nb3Si | 55.9 0.4 55.2–56.1 | 8.0 0.1 7.8–8.1 | 26.20.5 25.7–26.9 | 6.5 0.2 6.2–6.8 | 1.5 0.1 1.3–1.6 | 0 | 1.4 0.1 1.4–1.5 | 0.5 0.1 0.4–0.6 |
| Eutectic | 58.8 0.6 58.0–59.8 | 10.8 0.7 9.9–11.9 | 18.2 0.6 16.1–18.1 | 6.2 0.3 6.1–6.8 | 2.8 0.2 2.7–3.2 | 1.3 0.1 1.2–1.6 | 1.2 0.2 0.9–1.5 | 0.7 0.1 0.4–0.8 |
| Heat-treated | ||||||||
| Large area | 55.0 1.3 53.3–56.9 | 12.0 0.4 11.5–12.8 | 22.4 1.6 20.1–24.5 | 5.2 0.3 4.6–5.5 | 1.6 0.3 1.1–2.1 | 1.2 0.2 1.0–1.5 | 1.8 0.2 1.5–2.1 | 0.8 0.1 0.7–0.9 |
| Nbss | 69.3 0.5 68.7–69.8 | 12.3 0.2 12.1–12.6 | 3.8 0.7 3.1–4.7 | 8.0 0.3 7.5–8.3 | 4.0 0.2 3.8–4.3 | 2.3 0.1 2.2–2.4 | 0.3 0.1 0.2–0.3 | 0 |
| Nb5Si3 | 48.3 0.4 47.9–48.8 | 7.9 0.2 7.7–8.2 | 35.9 0.4 35.4–36.4 | 4.1 0.1 3.9–4.2 | 0.4 0.1 0.2–0.4 | 0.6 0.1 0.5–0.7 | 2.3 0.2 2.0–2.4 | 0.5 0.1 04–0.5 |
| Ti-rich Nb5Si3 | 44.8 0.5 44.0–45.2 | 12.5 0.4 12.1–13.0 | 35.3 0.6 34.3–35.8 | 2.8 0.2 2.4–3.1 | 0 | 0.7 0.1 0.5–0.8 | 2.9 0.3 2.7–3.3 | 1.0 0.2 0.8–1.2 |
| Alloy | Density (g/cm3) | % Area Nbss a | ||
|---|---|---|---|---|
| Top | Bulk | Bottom | ||
| JZ1-AC a | 8.240.02 8.2–8.26 | 42.3 0.7 41.3–43 | 46.5 0.8 45.4–47.6 | 46.51.0 45.4–48.2 |
| JZ1-HT | - | - | 46.5 1.5 44–48.2 | - |
| JZ2-AC a | 8.31 0.02 8.28–8.33 | 32.9 1.1 32.1–34.1 | 34.5 1.0 33.9–35.6 | 21.9 1.1 20.7–22.7 |
| JZ2-HT | - | - | 23.52.7 20.4–25.4 | - |
| Area Phase | Nb | Ti | Si | Ta | W | Sn | Ge | Hf |
|---|---|---|---|---|---|---|---|---|
| As-cast | ||||||||
| Top | 50.7 0.9 49.0–50.5 | 11.6 0.2 11.4–12.0 | 21.6 1.7 19.2–23.7 | 5.4 0.5 4.8–6.2 | 1.9 0.2 1.7–2.1 | 2.6 0.2 2.3–2.9 | 5.3 0.4 4.8–5.9 | 0.9 0.1 0.8–1.0 |
| Bulk | 50.3 0.2 50.2–50.7 | 11.7 0.4 11.3–12.4 | 21.7 1.2 20.0–23.0 | 5.3 0.3 5.0–5.7 | 2.0 0.2 1.8–2.2 | 2.5 0.3 2.1–2.8 | 5.4 0.7 5.0–6.6 | 1.1 0.1 0.9–1.3 |
| Bottom | 50.8 0.9 50.1–52.1 | 12.1 0.5 11.4–12.7 | 21.1 1.7 18.8–22.5 | 4.9 0.4 4.5–5.6 | 2.0 0.2 1.9–2.4 | 3.2 0.3 2.9–3.6 | 4.9 0.2 4.7–5.1 | 1.0 0.2 0.8–1.3 |
| Nbss | 63.7 0.7 62.9–64.7 | 11.5 1.0 10.6–12.9 | 3.4 0.4 3.1–4.1 | 9.3 0.5 8.7–9.8 | 6.5 0.4 5.9–7.0 | 4.1 0.4 3.9–4.8 | 1.0 0.2 0.8–1.2 | 0.5 0.1 0.3–0.6 |
| Ti–rich Nbss | 61.4 0.9 60.3–62.4 | 15.5 1.1 14.7–17.4 | 4.0 0.5 3.5–4.7 | 7.6 0.5 7.0–8.1 | 4.4 0.4 3.7–4.7 | 5.5 0.4 5.1–6.1 | 1.0 0.1 0.8–1.2 | 0.6 0.1 0.5–0.7 |
| Nb5Si3 | 46.7 0.2 46.4–47.0 | 8.2 0.3 8.1–8.6 | 32.5 0.7 31.4–33.2 | 4.3 0.1 4.2–4.4 | 0.7 0.1 0.6–0.8 | 1.0 0.1 0.9–1.1 | 6.0 0.2 5.9–6.2 | 0.6 0.2 0.4–0.7 |
| Ti–rich Nb5Si3 | 44.0 1.1 42.1–45.1 | 13.0 1.2 12.2–15.2 | 29.2 0.8 28.3–30.4 | 3.1 0.4 2.3–3.4 | 0.2 | 1.9 0.4 1.5–2.5 | 7.6 0.6 7.1–8.7 | 1.0 0.1 0.9–1.2 |
| A15 | 56.5 0.9 55.4–57.8 | 16.8 1.3 15.0–18.5 | 6.6 0.5 5.8–7.1 | 4.7 0.5 4.0–5.2 | 2.2 0.4 1.7–2.7 | 9.5 0.4 8.9–10.0 | 3.1 0.2 2.9–3.4 | 0.6 0.2 0.3–0.9 |
| Eutectic | 53.2 0.9 52.2–54.3 | 13.5 0.6 12.9–14.3 | 15.6 0.4 15.1–16.1 | 5.9 0.1 5.9–5.9 | 3.0 0.2 2.9–3.0 | 3.4 0.2 3.2–3.6 | 4.3 0.2 4.2–4.6 | 1.1 0.3 0.8–1.6 |
| Heat-treated | ||||||||
| Large area | 51.0 0.6 50.3–51.8 | 12.0 0.3 11.8–12.5 | 21.1 1.1 19.5–22.6 | 5.4 0.3 4.9–5.8 | 2.1 0.2 1.8–2.2 | 2.7 0.1 2.7–2.8 | 4.9 0.2 4.6–5.2 | 0.8 0.1 0.7–0.8 |
| Nbss | 65.6 0.3 65.3–66.2 | 10.7 0.4 10.4–11.3 | 3.5 1.0 2.5–4.9 | 10.0 0.4 9.5–10.4 | 7.5 0.2 7.3–7.7 | 2.2 0.2 1.9–2.4 | 0.5 0.1 0.4–0.6 | 0 |
| Nb5Si3 | 46.7 0.4 46.2–47.1 | 8.4 0.5 7.9–9.1 | 32.3 0.7 31.2–32.8 | 4.6 0.2 4.4–4.8 | 0.7 0.3 0.2–0.9 | 0.8 0.1 0.8–1.0 | 5.9 0.2 5.6–6.1 | 0.6 0.1 05–0.7 |
| Ti–rich Nb5Si3 | 37.9 4.6 34.3–43.3 | 19.4 4.6 14.3–22.8 | 28.6 2.4 26.5–31.8 | 2.6 0.3 2.2–2.9 | 0 | 0.7 0.4 0.2–1.2 | 9.3 2.1 6.8–10.9 | 1.5 0.6 0.7–2.2 |
| A15 | 58.8 0.4 58.3–59.4 | 14.5 0.3 14.2–14.8 | 5.7 0.6 5.2–6.6 | 5.3 0.2 4.9–5.4 | 2.3 0.1 2.2–2.5 | 10.4 0.2 10.2–10.7 | 2.9 0.1 2.7–3 | 0 |
| Alloy | 800 °C | 1200 °C | |||
|---|---|---|---|---|---|
| Weight Gain (mg/cm2) | Rate Constant | Weight Gain (mg/cm2) | Rate Constant | ||
| kl (g cm−2 s−1) | kl (g cm−2 s−1) | kp (g2 cm−4 s−1) | |||
| JZ1 | 33.6 (100 h) | 1.14 10−7 (0–58 h) 7.5 10−8 (58−100 h) | 91.3 (100 h) | 2.16 10−7 (9–100 h) | 1.4 10−8 (0–9 h) |
| JZ2 | 28.9 (100 h) | 1 10−7 (0–53 h) 6.8 10−8 (53–100 h) | 71.7 (100 h) | 1.8 10−7 (9–100 h) | 5.5 10−9 (0–9 h) |
| Area | Phase | O | Nb | Ti | Si | Ta | W | Sn | Ge | Hf |
|---|---|---|---|---|---|---|---|---|---|---|
| Oxide scale | Nb-rich oxide | 74.4 0.7 73.3–75.0 | 16.6 0.9 15.6–17.7 | 5.7 1.9 3.6–7.5 | 0.7 0.5 0.3–1.4 | 1.8 0.4 1.3–2.3 | 0.4 0.2 0.3–0.7 | – | – | 0.3 0.2 0.2–0.6 |
| Ti–rich oxide | 73.8 0.8 72.8–74.6 | 4.0 0.7 3.5–5.2 | 20.9 0.9 19.7–22.2 | 0.3 0.2 0.1–0.5 | 0.6 0.1 0.6–0.7 | – | – | – | 0.4 0.1 0.3–0.4 | |
| HfO2 | 71.8 1.7 69.7–73.2 | 1.6 0.4 1.2–1.9 | 1.1 0.1 1.0–1.1 | 0.5 0.9 0–1.6 | – | – | – | – | 24.9 0.3 24.6–25.1 | |
| Diffusion zone 1 | Nb5(Si1–x,Gex)3 | - | 54.3 0.9 52.8–54.8 | 1.6 0.6 1.1–2.7 | 15.3 0.5 14.6–15.7 | 5.2 0.3 4.7–5.4 | 1.1 0.2 0.9–1.4 | - | 22.4 1.0 21.6–24.1 | - |
| W–rich Nb5(Si1–x,Gex)3 | - | 47.4 1.2 45.7–48.7 | 1.2 0.2 1.0–1.5 | 12.8 1.0 12.2–14.6 | 7.4 0.3 7.2–7.9 | 6.2 0.5 5.7–6.9 | 0.5 0.4 0.3–1.0 | 24.8 1.5 22.7–26.6 | - | |
| NbGe2 | - | 33.0 0.9 32.0–34.1 | 1.0 0.5 0.2–1.4 | 4.4 1.2 2.8–5.8 | 2.7 0.4 2.3–3.4 | 0.9 0.3 0.6–1.5 | 0.2 0.1 0.1–0.4 | 57.8 1.0 56.5–5.89 | - | |
| Diffusion zone 2 | Nb5Si3 | - | 48.3 0.3 47.8–48.7 | 8.0 0.2 7.6–8.2 | 31.4 0.7 30.7–32.4 | 4.5 0.3 4.1–4.9 | 0.7 0.1 0.6–0.9 | 1.0 0.1 0.9–1.1 | 5.5 0.2 5.3–5.7 | 0.6 0.2 0.5–0.9 |
| Ti–rich Nb5Si3 | - | 45.8 1.2 44.1–46.6 | 15.0 1.8 13.7–17.7 | 24.9 0.6 24.2–25.6 | 3.4 0.5 2.7–3.7 | 0.3 0.1 0.2–0.4 | 3.2 0.4 2.8–3.7 | 6.8 0.5 6.3–7.4 | 0.9 0.1 0.7–1.0 | |
| A15 | - | 61.0 0.8 59.9–61.9 | 14.2 1.0 12.9–15.4 | 4.7 0.9 3.7–5.7 | 4.6 0.3 4.2–4.9 | 2.0 0.4 1.4–2.5 | 10.3 0.3 10.0–10.8 | 3.2 0.1 3.1–3.3 | - | |
| Nbss a | - | 69.7 | 5.5 | 4.6 | 10.2 | 7.1 | 2.0 | 0.8 | 0.2 | |
| Bulk | Nb5Si3 | - | 47.0 1.0 45.9–48.3 | 8.2 0.2 8.1–8.6 | 31.9 0.8 30.9–32.7 | 4.6 0.3 4.2–5.0 | 0.7 0.10 0.6–0.8 | 1.0 0.1 0.9–1.1 | 5.9 0.5 5.3–6.4 | 0.7 0.1 0.5–0.8 |
| Ti–rich Nb5Si3 | - | 42.9 1.5 40.6–44.6 | 17.0 2.2 13.8–19.4 | 26.2 0.9 25.0–27.3 | 2.9 0.6 2.4–3.9 | – | 2.4 0.4 1.8–2.9 | 7.6 0.7 6.5–8.6 | 1.1 0.1 1.0–1.3 | |
| A15 | - | 58.9 0.9 57.2–59.3 | 16.6 0.5 16.2–17.3 | 3.9 1.0 3.1–5.5 | 5.0 0.4 4.6–5.6 | 2.0 0.2 1.8–2.2 | 10.8 0.5 10.4–11.7 | 2.8 0.2 2.4–2.9 | - | |
| Nbss | - | 66.1 1.0 64.9–67.5 | 10.3 0.4 9.7–10.7 | 3.9 1.4 2.1–5.4 | 10.1 0.5 9.6–10.9 | 6.8 0.5 6.2–7.3 | 1.9 0.6 1.2–2.7 | 1.0 0.3 0.6–1.3 | - |
| Theory * | Element | ||||
|---|---|---|---|---|---|
| A | Si | Sn | Ge | Hf | Ti |
| B | - | Sn | Ge | - | Ti |
| C | Si | Sn | Ge | - | - |
| D | Si | Sn | Ge | Hf | Ti |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Utton, C.; Tsakiropoulos, P. On the Microstructure and Properties of Nb-12Ti-18Si-6Ta-2.5W-1Hf (at.%) Silicide-Based Alloys with Ge and Sn Additions. Materials 2020, 13, 1778. https://doi.org/10.3390/ma13071778
Zhao J, Utton C, Tsakiropoulos P. On the Microstructure and Properties of Nb-12Ti-18Si-6Ta-2.5W-1Hf (at.%) Silicide-Based Alloys with Ge and Sn Additions. Materials. 2020; 13(7):1778. https://doi.org/10.3390/ma13071778
Chicago/Turabian StyleZhao, Jiang, Claire Utton, and Panos Tsakiropoulos. 2020. "On the Microstructure and Properties of Nb-12Ti-18Si-6Ta-2.5W-1Hf (at.%) Silicide-Based Alloys with Ge and Sn Additions" Materials 13, no. 7: 1778. https://doi.org/10.3390/ma13071778





