Characterization of Platinum-Based Thin Films Deposited by Thermionic Vacuum Arc (TVA) Method
Abstract
:1. Introduction
2. Experimental Setup
2.1. Deposition Method
2.2. Experimental Parameters
2.3. Surface Structural Analysis and Elemental Analysis
2.4. Mechanical Properties Characterization
2.5. Surface Free Energy (SFE) Evaluation
2.6. Surface Morphology and Structure Investigation
3. Results and Discussion
3.1. SEM/EDS Analysis
3.2. Friction Coefficient
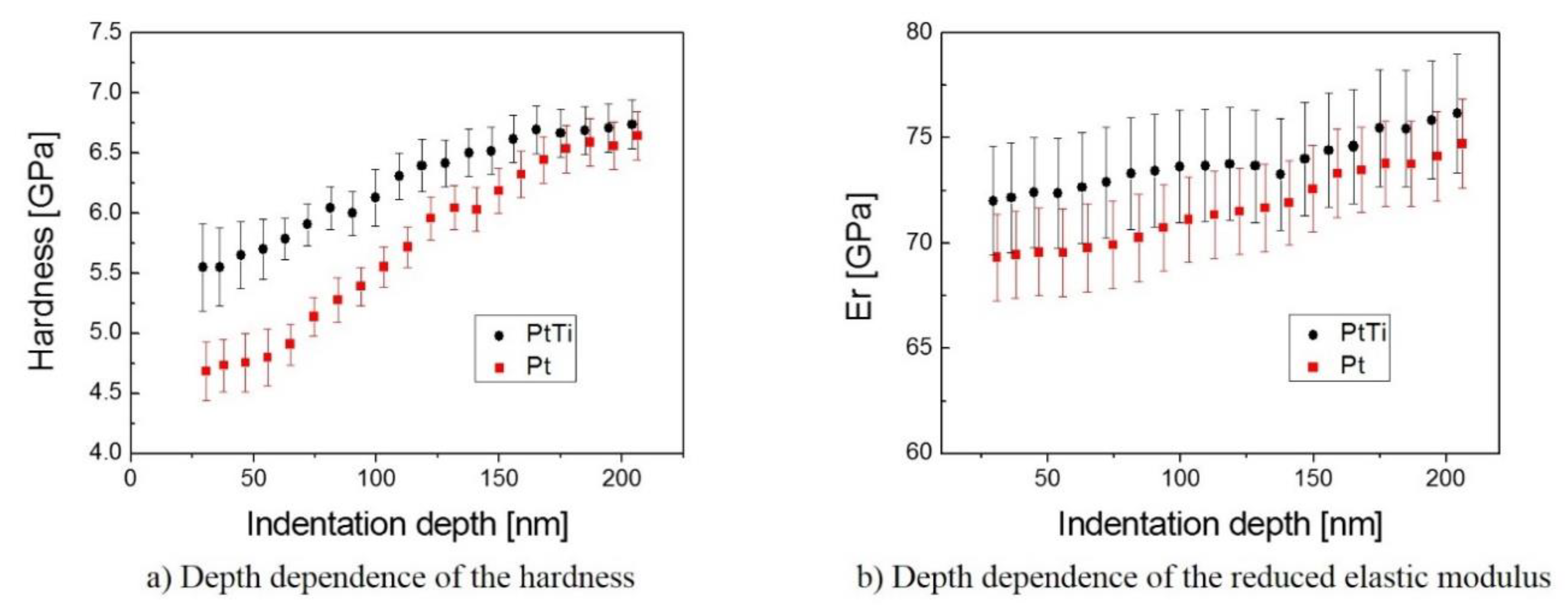
3.3. Mechanical Properties
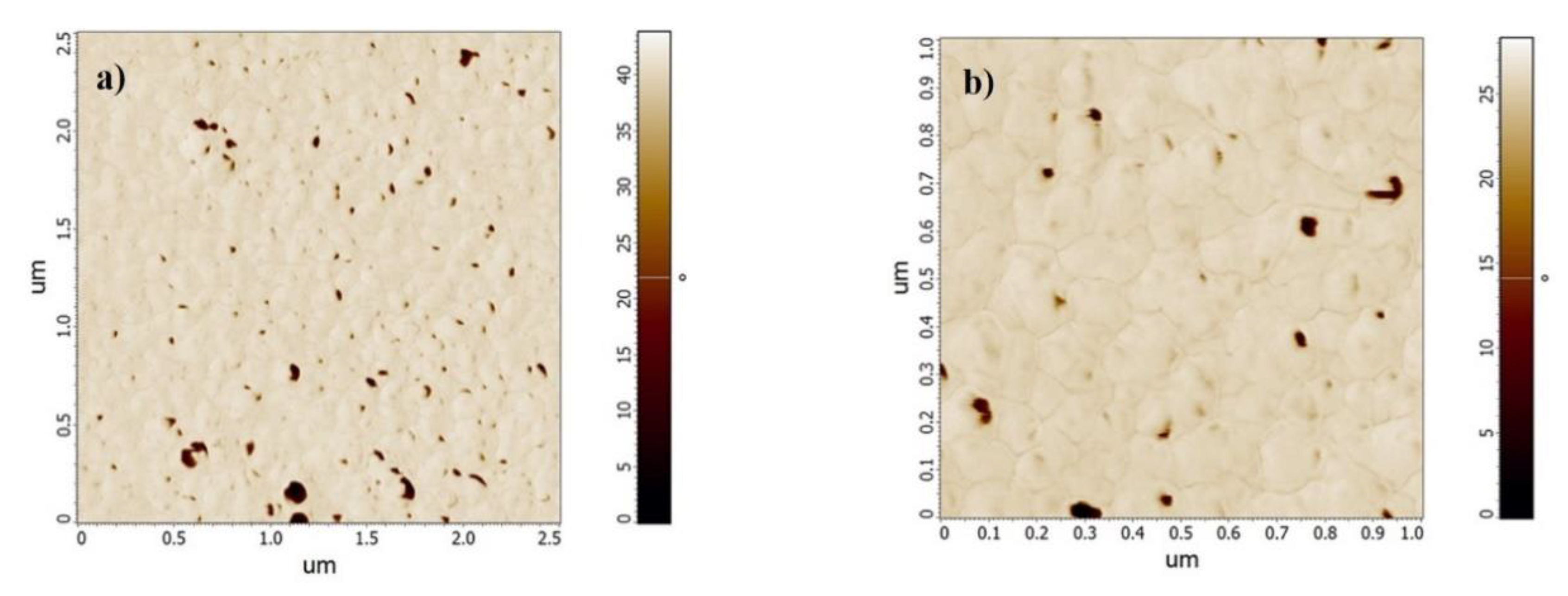
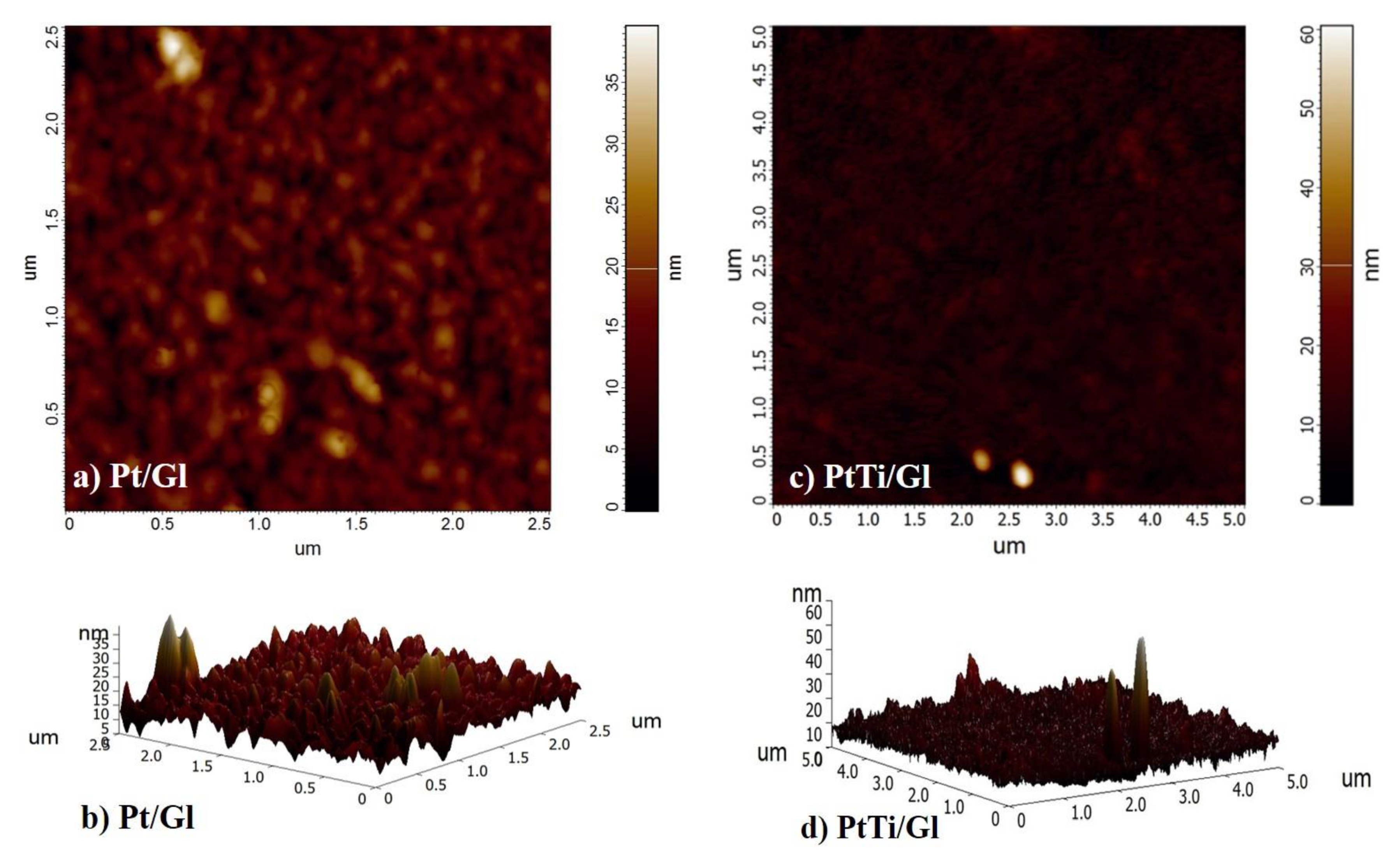
3.4. AFM Analysis
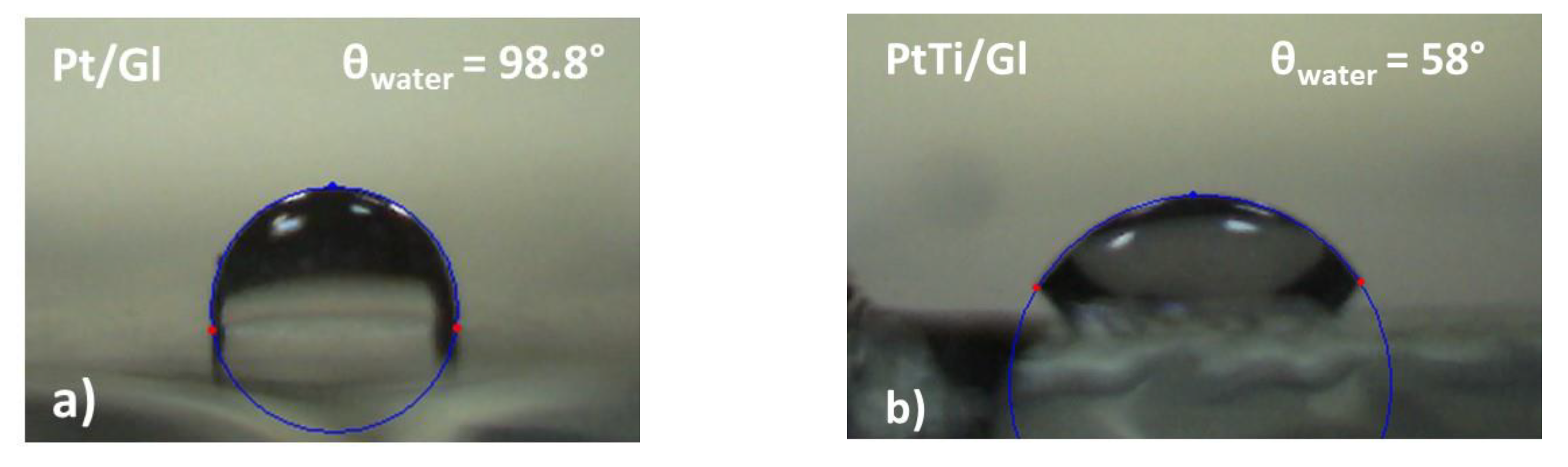
3.5. Wettability
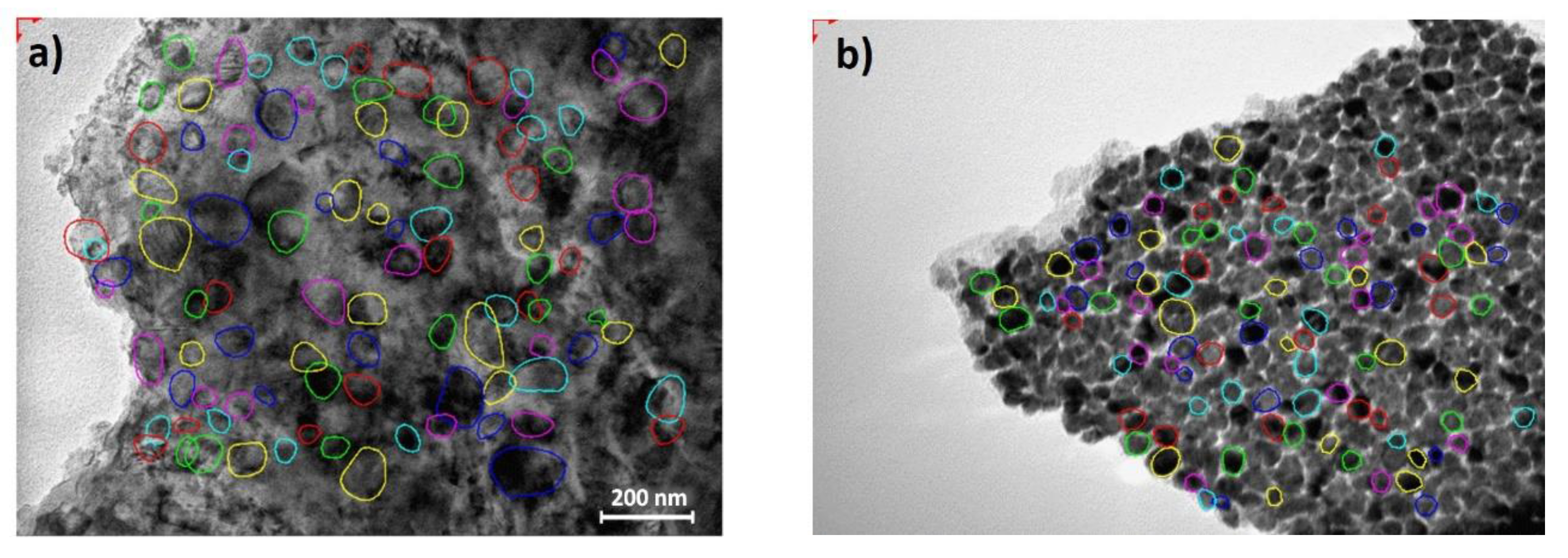
3.6. Transmission Electron Microscopy Investigation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, B.-H.; Huanga, H.J.; Huang, S.-H.; Hsiao, C.-N. Platinum thin films with good thermal and chemical stability fabricated by inductively coupled plasma-enhanced atomic layer deposition at low temperatures. Thin Solid Films 2014, 566, 93–98. [Google Scholar] [CrossRef]
- Beck, G.; Bachmann, C.; Bretzler, R.; Kmeth, R. Thermal stability of platinum, palladium and silver films on yttrium-stabilised zirconia. Thin Solid Films 2014, 573, 164–175. [Google Scholar] [CrossRef]
- Mizuhashi, S.; Cordonier, C.E.J.; Matsui, H.; Honma, H.; Takai, O. Comparative study on physical and electrochemical characteristics of thin films deposited from electroless platinum plating baths. Thin Solid Films 2016, 619, 328–335. [Google Scholar] [CrossRef]
- Mamun, M.A.; Gu, D.; Baumgart, H.; Elmustafa, A.A. Nanomechanical properties of platinum thin films synthesized by atomic layer deposition. Surf. Coat. Technol. 2015, 265, 185–190. [Google Scholar] [CrossRef]
- Jung, W.; Kim, J.J.; Tuller, H.L. Investigation of nanoporous platinum thin films fabricated by reactive sputtering: Application as micro-SOFC electrode. J. Power Sources 2015, 275, 860–865. [Google Scholar] [CrossRef] [Green Version]
- Schmid, P.; Zarfl, C.; Triendl, F.; Maier, F.J.; Schwarz, S.; Schneider, M.; Schmid, U. Impact of adhesion promoters and sputter parameters on the electro-mechanical properties of Pt thin films at high temperatures. Sens. Actuators A Phys. 2019, 285, 149–157. [Google Scholar] [CrossRef]
- Schmid, P.; Triendl, F.; Zarfl, C.; Schwarz, S.; Artner, W.; Schneider, M.; Schmid, U. Electro-mechanical properties of multilayered aluminum nitride and platinum thin films at high temperatures. Sens. Actuators A Phys. 2019, 293, 128–135. [Google Scholar] [CrossRef]
- Polosan, S.; Secu, M. X-ray excited luminescence and photoluminescence of Bi4 (GeO4) 3 glass-ceramics. Radiat. Meas. 2010, 45, 409–411. [Google Scholar] [CrossRef]
- Fu, Y.; Du, H.; Huang, W.; Zhang, S.; Hu, M. TiNi-based thin films in MEMS applications: A review. Sens. Actuators A Phys. 2004, 112, 395–408. [Google Scholar] [CrossRef]
- Secu, C.E.; Bartha, C.; Polosan, S.; Secu, M. Thermally activated conversion of a silicate gel to an oxyfluoride glass ceramic: Optical study using Eu3+ probe ion. J. Lumin. 2014, 146, 539–543. [Google Scholar] [CrossRef]
- Sakaliūnienė, J.; Abakevičienė, B.; Šlapikas, K.; Tamulevičius, S. Influence of magnetron sputtering deposition conditions and thermal treatment on properties of platinum thin films for positive electrode–electrolyte–negative electrode structure. Thin Solid Films 2015, 594, 101–108. [Google Scholar] [CrossRef]
- Rodríguez, A.; Morant-Minana, M.C.; Dias-Ponte, A.; Martínez-Calderón, M.; Gómez-Aranzadi, M.; Olaizola, S.M. Femtosecond laser induced periodic surface nanostructuring of sputtered platinum thin films. Appl. Surf. Sci. 2015, 351, 135–139. [Google Scholar] [CrossRef]
- Beck, G.; Bachmann, C.; Bretzler, R.; Kmeth, R. Epitaxial and non-epitaxial platinum, palladium and silver films on yttrium-stabilised zirconia. Mater. Chem. Phys. 2015, 158, 107–114. [Google Scholar] [CrossRef]
- Petrăşescu, L.; Ciupină, V.; Tutun, Ş.G.; Vlădoiu, R.; Prodan, G.; Poroşnicu, C.; Vasile, E.; Prioteasa, I.; Manu, R. Carbon—Platinum nanostructured catalysts for hydrogen fuel cells. J. Optoelectron. Adv. Matter 2015, 17, 1464–1470. [Google Scholar]
- Avril, L.; Bourgeois, S.; Simon, P.; Domenichini, B.; Zanfoni, N.; Herbst, F.; Imhoff, L. Nanostructured Pt-TiO2 composite thin films obtained by direct liquid injection metal organic chemical vapor deposition: Control of chemical state by X-ray photoelectron spectroscopy. Thin Solid Films 2015, 591, 237–244. [Google Scholar] [CrossRef]
- Grubera, W.; Baehtz, C.; Horisberger, M.; Ratschinskid, I.; Schmidta, H. Microstructure and strain relaxation in thin nanocrystalline platinum films produced via different sputtering techniques. Appl. Surf. Sci. 2016, 368, 341–347. [Google Scholar] [CrossRef]
- Şennik, E.; Ürdem, Ş.; Erkovan, M.; Kılınç, N. Sputtered platinum thin films for resistive hydrogen sensor application. Mater. Lett. 2016, 177, 104–107. [Google Scholar] [CrossRef]
- Vladoiu, R.; Tichy, M.; Mandes, A.; Dinca-Balan, V.; Kudrna, P. Thermionic Vacuum Arc—A versatile technology for thin film deposition and its applications. Coatings 2020, 10, 211. [Google Scholar] [CrossRef] [Green Version]
- Musa, G.; Mustata, I.; Blideran, M.; Ciupina, V.; Vladoiu, R.; Prodan, G.; Vasile, E.; Ehrich, H. Thermionic vacuum arc- new technique for high purity carbon thin film deposition. Acta Phys. Slovaca 2005, 55, 417–421. [Google Scholar]
- Lungu, C.P.; Mustata, I.; Musa, G.; Lungu, A.M.; Brinza, O.; Moldovan, C.; Rotaru, C.; Iosub, R.; Sava, F.; Popescu, M.; et al. Unstressed carbon-metal films deposited by thermionic vacuum arc method. J. Optoelectron. Adv. Mater. 2006, 8, 74–77. [Google Scholar]
- Ciupina, V.; Vladoiu, R.; Lungu, C.P.; Dinca, V.; Contulov, M.; Mandes, A.; Popov, P.; Prodan, G. Investigation of the SiC thin films synthetized by Thermionic Vacuum Arc method (TVA). Eur. Phys. J. D 2012, 66, 99. [Google Scholar] [CrossRef]
- Vladoiu, R.; Dinca, V.; Musa, G. Surface energy evaluation of unhydrogenated DLC thin film deposited by thermionic vacuum arc (TVA) method. Eur. Phys. J. D 2009, 54, 433–437. [Google Scholar] [CrossRef]
- Musa, G.; Vladoiu, R.; Ciupina, V.; Janick, J. Raman spectra of carbon thin films. J. Optoelectron. Adv. Mater. 2006, 8, 621–623. [Google Scholar]
- Vladoiu, R.; Ciupina, V.; Lungu, C.P.; Bursikova, V.; Musa, G. Thermionic vacuum arc (TVA) deposited tungsten thin film characterization. J. Optoelectron. Adv. Mater. 2006, 8, 71–73. [Google Scholar]
- Musa, G.; Vladoiu, R.; Ciupina, V.; Lungu, C.; Mustata, I.; Pat, S.; Akan, T.; Ekem, N. Characteristics of boron thin films obtained by TVA technology. J. Optoelectron. Adv. Mater. 2006, 8, 617–620. [Google Scholar]
- Vladoiu, R.; Ciupina, V.; Mandes, A.; Dinca, V.; Prodan, M.; Musa, G. Growth and characteristics of tantalum oxide thin films deposited using thermionic vacuum arc technology. J. Appl. Phys. 2010, 108, 093301. [Google Scholar] [CrossRef]
- Vladoiu, R.; Mandes, A.; Dinca-Balan, V.; Prodan, G.; Kudrna, P.; Tichý, M. Magnesium plasma diagnostics by heated probe and characterization of the Mg thin films deposited by thermionic vacuum arc technology. Plasma Sources Sci. Technol. 2015, 24, 035008. [Google Scholar] [CrossRef]
- Mandes, A.; Vladoiu, R.; Dinca, V.; Prodan, G. Binary C-Ag Plasma Breakdown and Structural Characterization of the deposited thin films by Thermionic Vacuum Arc (TVA) method. IEEE Trans. Plasma Sci. 2014, 42, 2806–2807. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Intermittent Contact Mode. Available online: https://www.ntmdt-si.com/resources/spm-principles/atomic-force-microscopy/amplitude-modulation-afm/intermittent-contact-mode (accessed on 28 March 2020).
- Pelegri, A.A.; Huang, X. Nanoindentation on soft film/hard substrate and hard film/soft substrate material systems with finite element analysis. Compos. Sci. Technol. 2008, 68, 147–155. [Google Scholar] [CrossRef]
- Vladoiu, R.; Mandes, A.; Dinca-Balan, V.; Bursikova, V. Structural and mechanical properties of nanostructured C-Ag thin films synthesized by Thermionic Vacuum Arc method. J. Nanomater. 2018, 2018, 9632041. [Google Scholar] [CrossRef] [Green Version]
- Bursikova, V.; Sťahel, P.; Navratil, Z.; Bursik, J.; Janca, J. Surface Energy Evaluation of Plasma Treated Materials by Contact Angle Measurement; Masaryk University: Brno, Czech Republic, 2004; ISBN 80-210-3563-3. [Google Scholar]
- Wenzel, R. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Teodorescu, V.S.; Blanchin, M.-G. Fast and Simple Specimen Preparation for TEM Studies of Oxide Films Deposited on Silicon Wafers. Microsc. Microanal. 2009, 15, 15–19. [Google Scholar] [CrossRef]
- Langford, J.W. Scherrer Scherrer after sixty years: A survey and some new results in the determination of crystallite size. J. Appl. Cryst. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Nelson, J.B.; Riley, D.P. An Experimental Investigation of Extrapolation Methods in the Derivation of Accurate Unit-Cell Dimensions of Crystals. Proc. Phys. Soc. 1945, 57, 160–176. [Google Scholar] [CrossRef]
- Klinger, M.; Jäger, A. Crystallographic Tool Box (CrysTBox). J. Appl. Crystallogr. 2015, 48, 2012–2018. [Google Scholar] [CrossRef] [Green Version]

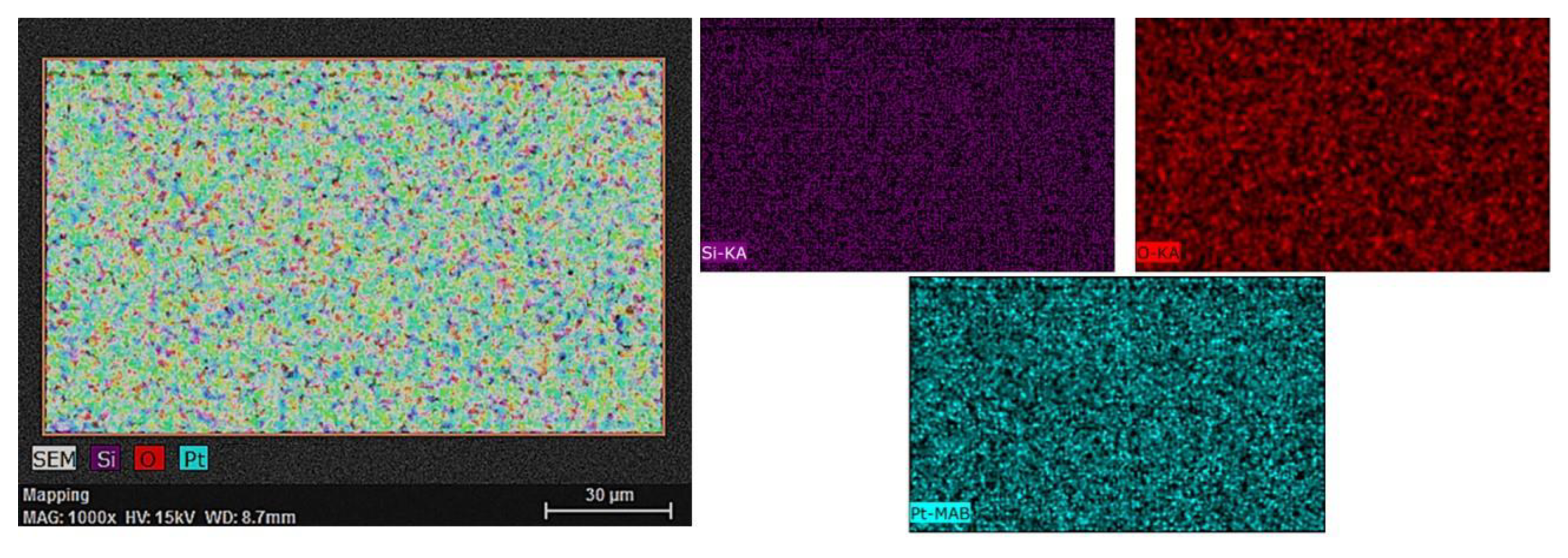
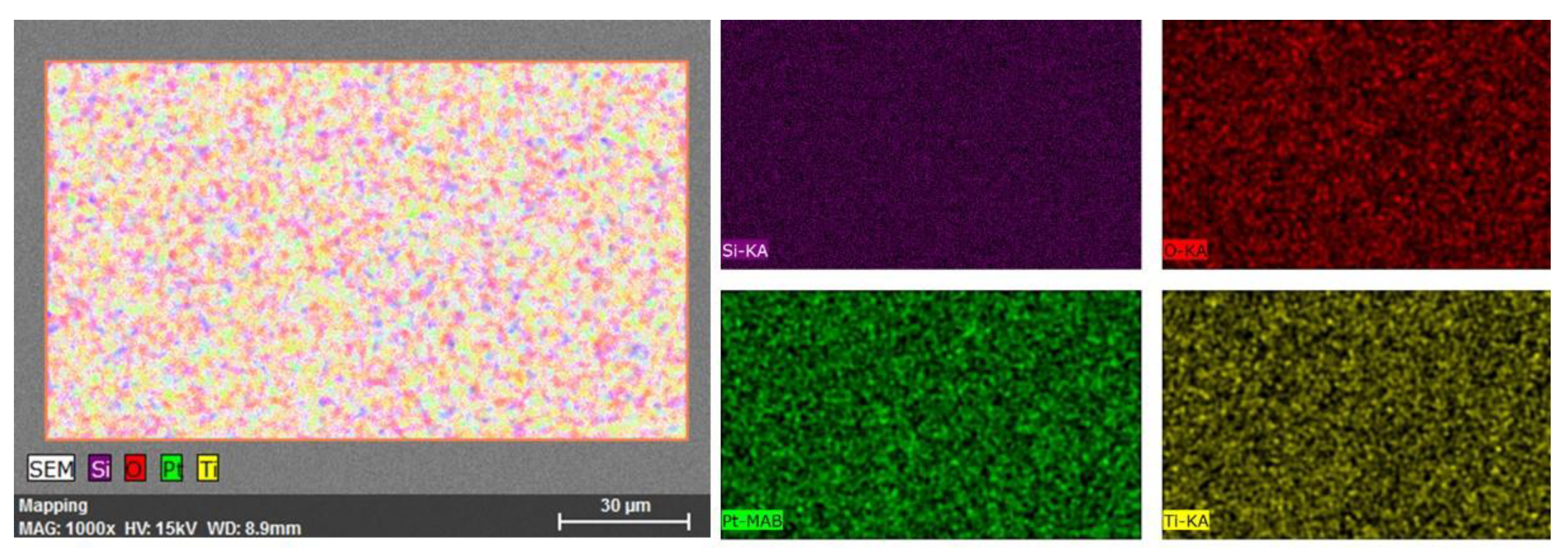



| Element | Z | Edge | (wt.%) | (Norm. wt.%) | (Norm. at.%) | Error in % |
|---|---|---|---|---|---|---|
| Silicon | 14 | K | 42.89953 | 39.53 | 69.59 | 1.4 |
| Oxygen | 8 | K | 3.74228 | 5.33 | 12.39 | 0.7 |
| Platinum | 78 | M | 53.3582 | 55.14 | 18.02 | 1.9 |
| Element | Z | Edge | (wt.%) | (Norm. wt.%) | (Norm. at.%) | Error in % |
|---|---|---|---|---|---|---|
| Silicon | 14 | K | 32.14497 | 29.95 | 55.40 | 0.50 |
| Oxygen | 8 | K | 3.933436 | 3.47 | 3.15 | 0.50 |
| Platinum | 78 | M | 54.41712 | 57.01 | 34.33 | 0.20 |
| Titanium | 22 | K | 9.504505 | 9.57 | 7.06 | 0.04 |
| Deposited Material | Applied Force (N) | Distance Traveled Before Reaching the Rubstrate (m) | ||
|---|---|---|---|---|
| Pt | 0.5 | 17.5 | 0.3628 | 0.38 |
| 1 | 14.0 | 0.2885 | ||
| 3 | 10.0 | 0.4723 | ||
| PtTi | 0.5 | 19.0 | 0.3553 | 0.35 |
| 1 | 16.5 | 0.2688 | ||
| 3 | 12.5 | 0.4175 | ||
| Sample | Rp (µm) | Rz (µm) | Rt (µm) | Rc (µm) | Ra(µm) | Rq(µm) |
|---|---|---|---|---|---|---|
| Pt/Gl | 0.020 | 0.033 | 0.210 | 0.013 | 0.003 | 0.007 |
| PtTi/Gl | 0.131 | 0.171 | 0.279 | 0.077 | 0.008 | 0.017 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cozma, S.; Vlǎdoiu, R.; Mandes, A.; Dinca, V.; Prodan, G.; Buršíková, V. Characterization of Platinum-Based Thin Films Deposited by Thermionic Vacuum Arc (TVA) Method. Materials 2020, 13, 1796. https://doi.org/10.3390/ma13071796
Cozma S, Vlǎdoiu R, Mandes A, Dinca V, Prodan G, Buršíková V. Characterization of Platinum-Based Thin Films Deposited by Thermionic Vacuum Arc (TVA) Method. Materials. 2020; 13(7):1796. https://doi.org/10.3390/ma13071796
Chicago/Turabian StyleCozma, Sebastian, Rodica Vlǎdoiu, Aurelia Mandes, Virginia Dinca, Gabriel Prodan, and Vilma Buršíková. 2020. "Characterization of Platinum-Based Thin Films Deposited by Thermionic Vacuum Arc (TVA) Method" Materials 13, no. 7: 1796. https://doi.org/10.3390/ma13071796





