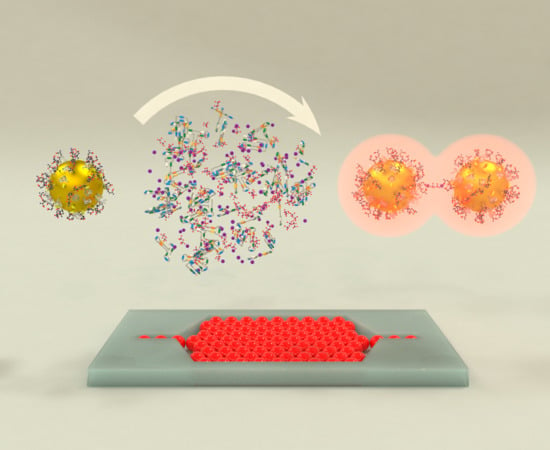
Multifuntional Gold Nanoparticles for the SERS Detection of Pathogens Combined with a LAMP–in–Microdroplets Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacterial Culture
2.3. Synthesis and Functionalization of Gold Nanoparticles (AuNPs)
2.4. Characterization
2.5. Food Sample Analysis
2.6. LAMP
2.7. Microfluidic Devices Fabrication
2.8. Microdroplets for LAMP-on-a-Chip
2.9. SERS Measurements
3. Results
3.1. Preparation and Characterization of Multifunctional Gold Nanoparticles (AuNPs)
3.1.1. Optimization of Multifunctional AuNPs
3.1.2. Bioinspired Indirect SERS Detection
3.1.3. SERS Monitoring of LAMP with 1NAT:GSH:TPEG-AuNPs
3.2. LAMP on-a-Chip SERS Detection
3.2.1. LAMP on-a-Chip Setup
3.2.2. LAMP on-a-Chip for DNA Extracted from L. monocytogenes Culture
3.2.3. LAMP on-a-Chip for DNA Extracted from Food Products
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ros, A. Manabu Tokeshi (Ed.): Applications of microfluidic systems in biology and medicine. Anal. Bioanal. Chem. 2020, 412, 2389–2390. [Google Scholar] [CrossRef]
- Aryasomayajula, A.; Bayat, P.; Rezai, P.; Selvaganapathy, P.R. Microfluidic Devices and Their Applications. In Springer Handbook of Nanotechnology; Springer Science and Business Media LLC: Berlin, Germany, 2017; pp. 487–536. [Google Scholar]
- Watson, C.; Senyo, S.E. All-in-one automated microfluidics control system. HardwareX 2019, 5, e00063. [Google Scholar] [CrossRef]
- Franke, T.A.; Wixforth, A. Microfluidics for Miniaturized Laboratories on a Chip. ChemPhysChem 2008, 9, 2140–2156. [Google Scholar] [CrossRef]
- Ceuppens, S.; Li, D.; Uyttendaele, M.; Renault, P.; Ross, P.; Van Ranst, M.; Cocolin, L.; Donaghy, J.; Ross, P. Molecular Methods in Food Safety Microbiology: Interpretation and Implications of Nucleic Acid Detection. Compr. Rev. Food Sci. Food Saf. 2014, 13, 551–577. [Google Scholar] [CrossRef] [Green Version]
- Suea-Ngam, A.; Howes, P.D.; Srisa-Art, M.; Demello, A.J. Droplet microfluidics: From proof-of-concept to real-world utility? Chem. Commun. 2019, 55, 9895–9903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Howes, P.D.; Demello, A.J. Recent Advances in Droplet Microfluidics. Anal. Chem. 2019, 92, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Huebner, A.; Sharma, S.; Srisa-Art, M.; Hollfelder, F.; Edel, J.B.; Demello, A.J.; Sharma, S. Microdroplets: A sea of applications? Lab Chip 2008, 8, 1244. [Google Scholar] [CrossRef]
- Salmon, A.R.; Esteban, R.; Taylor, R.W.; Hugall, J.; Smith, C.; Whyte, G.; Scherman, O.A.; Aizpurua, J.; Abell, C.; Baumberg, J.J. Monitoring Early-Stage Nanoparticle Assembly in Microdroplets by Optical Spectroscopy and SERS. Small 2016, 12, 1788–1796. [Google Scholar] [CrossRef] [Green Version]
- Abalde-Cela, S.; Auguié, B.; Fischlechner, M.; Huck, W.T.S.; Alvarez-Puebla, R.A.; Liz-Marzán, L.M.; Abell, C. Microdroplet fabrication of silver–agarose nanocomposite beads for SERS optical accumulation. Soft Matter 2011, 7, 1321–1325. [Google Scholar] [CrossRef] [Green Version]
- Gao, R.; Cheng, Z.; Wang, X.; Yu, L.; Guo, Z.; Zhao, G.; Choo, J. Simultaneous immunoassays of dual prostate cancer markers using a SERS-based microdroplet channel. Biosens. Bioelectron. 2018, 119, 126–133. [Google Scholar] [CrossRef]
- Choi, N.; Lee, J.; Ko, J.; Jeon, J.H.; Rhie, G.-E.; Demello, A.; Choo, J. Integrated SERS-Based Microdroplet Platform for the Automated Immunoassay of F1 Antigens in Yersinia pestis. Anal. Chem. 2017, 89, 8413–8420. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.Q.; Huang, W.; Chin, L.K.; Lei, L.; Lin, Z.P.; Ser, W.; Chen, H.; Ayi, T.C.; Yap, P.H.; Chen, C.H.; et al. Droplet optofluidic imaging for λ-bacteriophage detection via co-culture with host cell Escherichia coli. Lab Chip 2014, 14, 3519–3524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Dong, T. A Microfluidic Device for Continuous Sensing of Systemic Acute Toxicants in Drinking Water. Int. J. Environ. Res. Public Health 2013, 10, 6748–6763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Best, R.; Abalde-Cela, S.; Abell, C.; Smith, A.G. Applications of Microdroplet Technology for Algal Biotechnology. Curr. Biotechnol. 2016, 5, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Bowman, E.K.; Alper, H.S. Microdroplet-Assisted Screening of Biomolecule Production for Metabolic Engineering Applications. Trends Biotechnol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Hümmer, D.; Kurth, F.; Naredi-Rainer, N.; Dittrich, P.S. Single cells in confined volumes: Microchambers and microdroplets. Lab Chip 2016, 16, 447–458. [Google Scholar] [CrossRef]
- Fakruddin, M.; Bin Mannan, K.S.; Chowdhury, A.; Mazumdar, R.M.; Hossain, N.; Islam, S.; Chowdhury, A. Nucleic acid amplification: Alternative methods of polymerase chain reaction. J. Pharm. Bioallied Sci. 2013, 5, 245–252. [Google Scholar] [CrossRef]
- Garibyan, L.; Avashia, N. Polymerase chain reaction. J. Investig. Dermatol. 2013, 133, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Hindson, B.J.; Ness, K.D.; Masquelier, D.A.; Belgrader, P.; Heredia, N.J.; Makarewicz, A.J.; Bright, I.J.; Lucero, M.Y.; Hiddessen, A.L.; Legler, T.C.; et al. High-Throughput Droplet Digital PCR System for Absolute Quantitation of DNA Copy Number. Anal. Chem. 2011, 83, 8604–8610. [Google Scholar] [CrossRef]
- Notomi, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, 63. [Google Scholar] [CrossRef] [Green Version]
- Arunrut, N.; Kampeera, J.; Sirithammajak, S.; Sanguanrut, P.; Proespraiwong, P.; Suebsing, R.; Kiatpathomchai, W. Sensitive Visual Detection of AHPND Bacteria Using Loop-Mediated Isothermal Amplification Combined with DNA-Functionalized Gold Nanoparticles as Probes. PLoS ONE 2016, 11, e0151769. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Nagamine, K.; Tomita, N.; Notomi, T. Detection of Loop-Mediated Isothermal Amplification Reaction by Turbidity Derived from Magnesium Pyrophosphate Formation. Biochem. Biophys. Res. Commun. 2001, 289, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, J.; Xander, N.C.; Frohme, M.; Glökler, J. Shining a light on LAMP assays— A comparison of LAMP visualization methods including the novel use of berberine. Biotechniques 2015, 58, 189–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, J.K.F.; Yip, S.P.; Lee, T.M.H. Ultrasensitive and Closed-Tube Colorimetric Loop-Mediated Isothermal Amplification Assay Using Carboxyl-Modified Gold Nanoparticles. Small 2014, 10, 1495–1499. [Google Scholar] [CrossRef]
- Qin, A.; Fu, L.T.; Wong, J.K.F.; Chau, L.Y.; Yip, S.P.; Lee, T.M.H. Precipitation of PEG/Carboxyl-Modified Gold Nanoparticles with Magnesium Pyrophosphate: A New Platform for Real-Time Monitoring of Loop-Mediated Isothermal Amplification. ACS Appl. Mater. Interfaces 2017, 9, 10472–10480. [Google Scholar] [CrossRef]
- Garrido-Maestu, A.; Azinheiro, S.; Carvalho, J.; Abalde-Cela, S.; Carbó-Argibay, E.; Diéguez, L.; Piotrowski, M.; Kolen’Ko, Y.V.; Prado, M. Combination of Microfluidic Loop-Mediated Isothermal Amplification with Gold Nanoparticles for Rapid Detection of Salmonella spp. in Food Samples. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, F.; Li, Q.; Wang, L.; Fan, C. Isothermal Amplification of Nucleic Acids. Chem. Rev. 2015, 115, 12491–12545. [Google Scholar] [CrossRef]
- Yuan, H.; Chao, Y.; Shum, H.C. Droplet and Microchamber-Based Digital Loop-Mediated Isothermal Amplification (dLAMP). Small 2020, 16, e1904469. [Google Scholar] [CrossRef]
- Ma, Y.-D.; Luo, K.; Chang, W.-H.; Lee, G.-B. A microfluidic chip capable of generating and trapping emulsion droplets for digital loop-mediated isothermal amplification analysis. Lab Chip 2018, 18, 296–303. [Google Scholar] [CrossRef] [Green Version]
- Kant, K.; Abalde-Cela, S. Surface-Enhanced Raman Scattering Spectroscopy and Microfluidics: Towards Ultrasensitive Label-Free Sensing. Biosensors 2018, 8, 62. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Lorenzo, L.; Garrido-Maestu, A.; Bhunia, A.K.; Espiña, B.; Prado, M.; Diéguez, L.; Abalde-Cela, S. Gold Nanostars for the Detection of Foodborne Pathogens via Surface-Enhanced Raman Scattering Combined with Microfluidics. ACS Appl. Nano Mater. 2019, 2, 6081–6086. [Google Scholar] [CrossRef]
- Pyrak, E.; Krajczewski, J.; Kowalik, A.; Kudelski, A.; Jaworska, A. Surface Enhanced Raman Spectroscopy for DNA Biosensors—How Far Are We? Molecules 2019, 24, 4423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Draz, M.S.; Lu, X. Development of a Loop Mediated Isothermal Amplification (LAMP) - Surface Enhanced Raman spectroscopy (SERS) Assay for the Detection of Salmonella Enterica Serotype Enteritidis. Theranostics 2016, 6, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Allerberger, F.; Wagner, M. Listeriosis: A resurgent foodborne infection. Clin. Microbiol. Infect. 2010, 16, 16–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17. [CrossRef] [Green Version]
- Ràfols, C.P.; Serrano, N.; Díaz-Cruz, J.M.; Ariño, C.; Esteban, M. A Chemically-Bound Glutathione Sensor Bioinspired by the Defense of Organisms against Heavy Metal Contamination: Optimization of the Immobilization Conditions. Chemosensors 2017, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Valko, M.; Jomová, K.; Rhodes, C.J.; Kuca, K.; Musilek, K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2015, 90, 1–37. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55. [Google Scholar] [CrossRef]
- Garrido-Maestu, A.; Chapela, M.-J.; Román, B.; Fajardo, P.; Lago, J.; Vieites, J.M.; Cabado, A.G.; Chapela, M.-J.; Rom?n, B. A new multiplex real-time PCR developed method for Salmonella spp. and Listeria monocytogenes detection in food and environmental samples. Food Control. 2013, 30, 76–85. [Google Scholar] [CrossRef]
- Garrido-Maestu, A.; Azinheiro, S.; Carvalho, J.; Fuciños, P.; Prado, M. Development and evaluation of loop-mediated isothermal amplification, and Recombinase Polymerase Amplification methodologies, for the detection of Listeria monocytogenes in ready-to-eat food samples. Food Control. 2018, 86, 27–34. [Google Scholar] [CrossRef]
- Kawasaki, S.; Horikoshi, N.; Okada, Y.; Takeshita, K.; Sameshima, T.; Kawamoto, S. Multiplex PCR for Simultaneous Detection of Salmonella spp., Listeria monocytogenes, and Escherichia coli O157:H7 in Meat Samples. J. Food Prot. 2005, 68, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M.; Stroock, A.D. Flexible Methods for Microfluidics. Phys. Today 2001, 54, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid Prototyping of Microfluidic Systems in Poly(dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef] [PubMed]
- Khlebtsov, N.G.; Mel’Nikov, A.G.; Bogatyrev, V.A.; Alekseeva, A.V.; Khlebtsov, B.N. Depolarization of light scattered by gold nanospheres and nanorods. Opt. Spectrosc. 2006, 100, 448–455. [Google Scholar] [CrossRef]
- Göeken, K.L.; Subramaniam, V.; Gill, R. Enhancing spectral shifts of plasmon-coupled noble metal nanoparticles for sensing applications. Phys. Chem. Chem. Phys. 2015, 17, 422–427. [Google Scholar] [CrossRef] [Green Version]
- Acres, R.; Feyer, V.; Tsud, N.; Carlino, E.; Prince, K. Mechanisms of Aggregation of Cysteine Functionalized Gold Nanoparticles. J. Phys. Chem. C 2014, 118, 10481–10487. [Google Scholar] [CrossRef]
- Arifuzzaman, M.D.; Zhao, Y. Water-Soluble Molecularly Imprinted Nanoparticle Receptors with Hydrogen-Bond-Assisted Hydrophobic Binding. J. Org. Chem. 2016, 81, 7518–7526. [Google Scholar] [CrossRef]
- Moaseri, E.; Bollinger, J.A.; Changalvaie, B.; Johnson, L.; Schroer, J.; Johnston, K.P.; Truskett, T.M. Reversible Self-Assembly of Glutathione-Coated Gold Nanoparticle Clusters via pH-Tunable Interactions. Langmuir 2017, 33, 12244–12253. [Google Scholar] [CrossRef]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef]
- Alvarez-Puebla, R.A.; Dos Santos Júnior, D.S.; Aroca, R.F. Surface-enhanced Raman scattering for ultrasensitive chemical analysis of 1 and 2-naphthalenethiols. Analyst 2004, 129, 1251. [Google Scholar] [CrossRef]
- Ma, S.; Huang, Q. A SERS study of oxidation of glutathione under plasma irradiation. RSC Adv. 2015, 5, 57847–57852. [Google Scholar] [CrossRef]
- Jenkins, R.H.; Tuma, R.; Juuti, J.T.; Bamford, D.H.; Thomas, G.J. Raman spectral studies of nucleic acids series. part LXIX - A novel Raman spectrophotometric method for quantitative measurement of nucleoside triphosphate hydrolysis. Biospectroscopy 1999, 5, 3–8. [Google Scholar] [CrossRef]
- D’Amico, F.; Addobbati, R.; Rizzardi, C.; Rossi, B.; Cammisuli, F.; Gessini, A.; Masciovecchio, C.; Pascolo, L. Oxidative damage in DNA bases revealed by UV resonant Raman spectroscopy. Analyst 2015, 140, 1477–1485. [Google Scholar] [CrossRef]
- Karaballi, R.A.; Nel, A.; Krishnan, S.; Blackburn, J.M.; Brosseau, C.L. Development of an electrochemical surface-enhanced Raman spectroscopy (EC-SERS) aptasensor for direct detection of DNA hybridization. Phys. Chem. Chem. Phys. 2015, 17, 21356–21363. [Google Scholar] [CrossRef] [PubMed]
- Cruje, C.; Chithrani, D. Polyethylene Glycol Functionalized Nanoparticles for Improved Cancer Treatment. Rev. Nanosci. Nanotechnol. 2014, 3, 20–30. [Google Scholar] [CrossRef]
- Teh, S.-Y.; Lin, R.; Hung, L.-H.; Lee, A.P. Droplet microfluidics. Lab Chip 2008, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Lee, H.; Lin, Z.; Granick, S. Chemical Imaging in a Surface Forces Apparatus: Confocal Raman Spectroscopy of Confined Poly(dimethylsiloxane). Langmuir 2005, 21, 5685–5688. [Google Scholar] [CrossRef] [PubMed]
- Matovic, V.; Bulat, Z.; Djukić-Cosić, D.; Soldatović, D. Antagonism between cadmium and magnesium: A possible role of magnesium in therapy of cadmium intoxication. Magnes. Res. 2010, 23, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Kaabi, R.; Abderrabba, M.; Gómez-Ruiz, S.; Del Hierro, I. Bioinspired materials based on glutathione-functionalized SBA-15 for electrochemical Cd(II) detection. Microporous Mesoporous Mater. 2016, 234, 336–346. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J. Bioinspired thiol functionalized carbon dots for rapid detection of lead (II) ions in human serum. Opt. Mater. 2020, 99, 109514. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Cowie, A.C. Emerging Point-of-care Technologies for Food Safety Analysis. Sensors 2019, 19, 817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Q.; Gao, Y.; Yu, B.; Ren, H.; Qiu, L.; Han, S.; Jin, W.; Jin, Q.; Mu, Y. Self-priming compartmentalization digital LAMP for point-of-care. Lab Chip 2012, 12, 4755–4763. [Google Scholar] [CrossRef] [PubMed]
- Gansen, A.; Herrick, A.M.; Dimov, I.K.; Lee, L.P.; Chiu, D.T. Digital LAMP in a sample self-digitization (SD) chip. Lab Chip 2012, 12, 2247–2254. [Google Scholar] [CrossRef] [PubMed]
- Selck, D.A.; Karymov, M.A.; Sun, B.; Ismagilov, R.F. Increased Robustness of Single-Molecule Counting with Microfluidics, Digital Isothermal Amplification, and a Mobile Phone versus Real-Time Kinetic Measurements. Anal. Chem. 2013, 85, 11129–11136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, B.; Shen, F.; McCalla, S.; Kreutz, J.E.; Karymov, M.A.; Ismagilov, R.F. Mechanistic Evaluation of the Pros and Cons of Digital RT-LAMP for HIV-1 Viral Load Quantification on a Microfluidic Device and Improved Efficiency via a Two-Step Digital Protocol. Anal. Chem. 2013, 85, 1540–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]




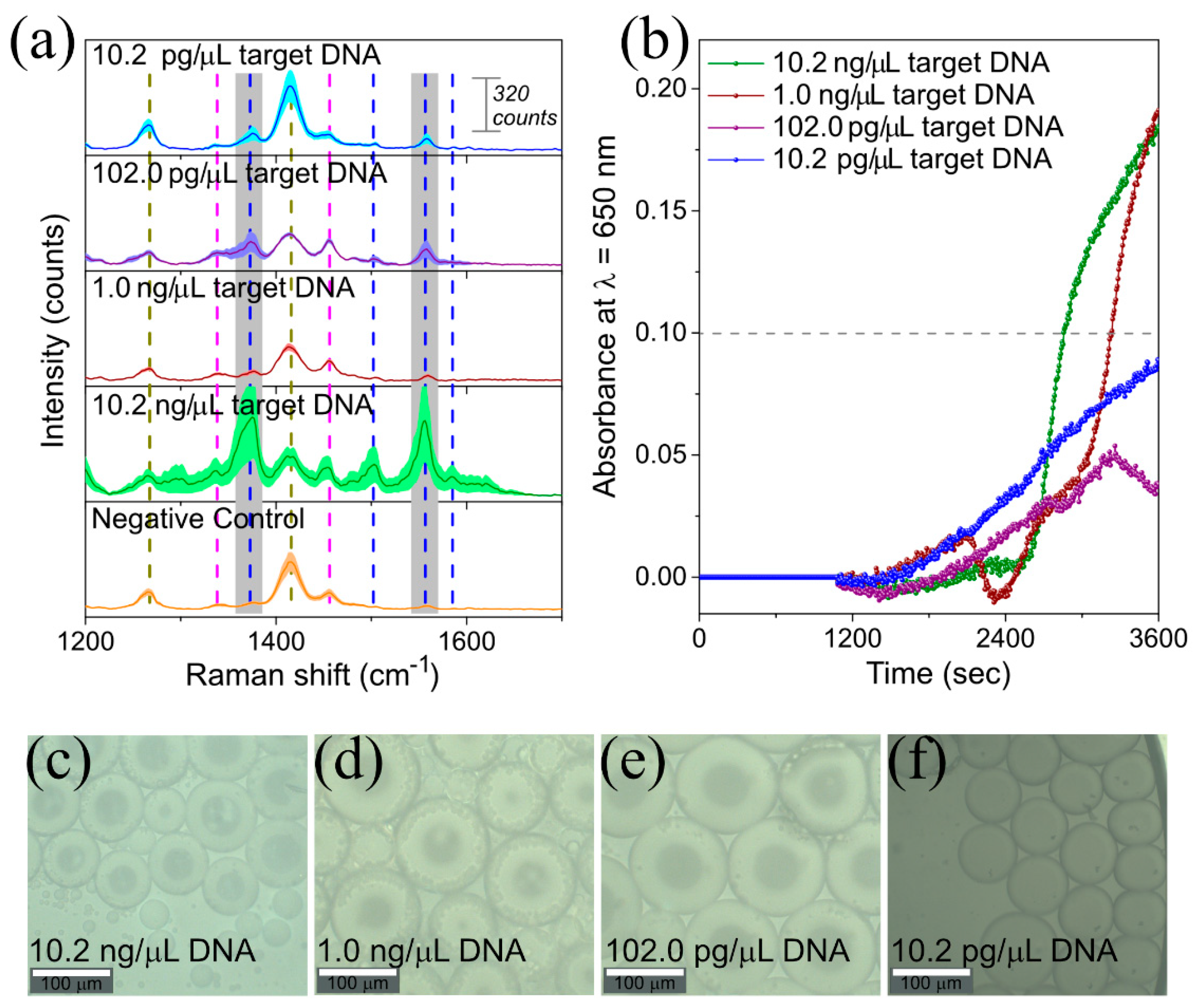
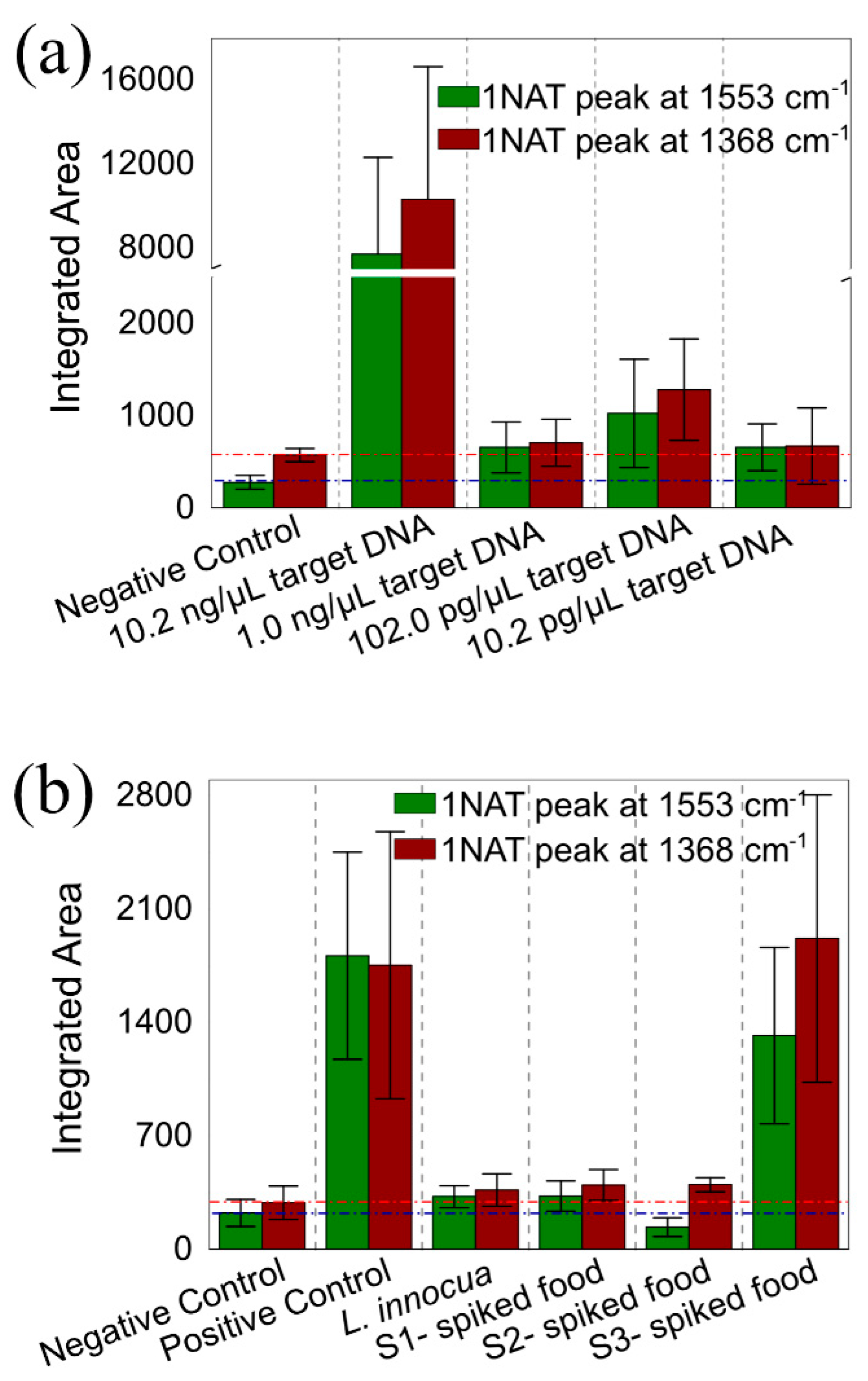

| Sample Label | Description |
|---|---|
| Negative Control | Water added instead of target DNA in the LAMP reaction |
| Positive Control | 10.2 ng/µL target DNA from L. monocytogenes in the LAMP reaction |
| 10.2 ng/µL target DNA | 10.2 ng/µL target DNA from L. monocytogenes in the LAMP reaction |
| 1.0 ng/µL target DNA | 1.0 ng/µL target DNA from L. monocytogenes in the LAMP reaction |
| 102.0 pg/µL target DNA | 102.0 pg/µL target DNA from L. monocytogenes in the LAMP reaction |
| 10.2 pg/µL target DNA | 10.2 pg/µL target DNA from L. monocytogenes in the LAMP reaction |
| L. innocua | 13.4 ng/µL DNA from L. innocua in the LAMP reaction |
| S1 – spiked food | DNA extracted from 25 mL UHT milk inoculated with 10 CFU of L. monocytogenes |
| S2 – spiked food | DNA extracted from 25 mL UHT milk inoculated with 90 CFU of L. monocytogenes |
| S3 – spiked food | DNA extracted from 25 mL UHT milk inoculated with 90 × 102 CFU of L. monocytogenes |
| Citrate-AuNPs | 1NAT:GSH-AuNPs | 1NAT:GSH:lowTPEG-AuNPs | 1NAT:GSH:highTPEG-AuNPs | |
|---|---|---|---|---|
| Hydrodynamic diameter 1 (nm) | 20.2 ± 0.1 | 714.6 ± 75.9 | 92.6 ± 8.5 | 37.3 ± 2.2 |
| PDI 2 (%) | 33 ± 2 | 106 ± 15 | 42 ± 2 | 35 ± 16 |
| Z potential 3 (mV) | 69.1 ± 1.8 | 20.8 ± 1.9 | 15.3 ± 4.8 | 30.6 ± 1.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, A.; Paris, J.L.; Roumani, F.; Diéguez, L.; Prado, M.; Espiña, B.; Abalde-Cela, S.; Garrido-Maestu, A.; Rodriguez-Lorenzo, L. Multifuntional Gold Nanoparticles for the SERS Detection of Pathogens Combined with a LAMP–in–Microdroplets Approach. Materials 2020, 13, 1934. https://doi.org/10.3390/ma13081934
Teixeira A, Paris JL, Roumani F, Diéguez L, Prado M, Espiña B, Abalde-Cela S, Garrido-Maestu A, Rodriguez-Lorenzo L. Multifuntional Gold Nanoparticles for the SERS Detection of Pathogens Combined with a LAMP–in–Microdroplets Approach. Materials. 2020; 13(8):1934. https://doi.org/10.3390/ma13081934
Chicago/Turabian StyleTeixeira, Alexandra, Juan L. Paris, Foteini Roumani, Lorena Diéguez, Marta Prado, Begoña Espiña, Sara Abalde-Cela, Alejandro Garrido-Maestu, and Laura Rodriguez-Lorenzo. 2020. "Multifuntional Gold Nanoparticles for the SERS Detection of Pathogens Combined with a LAMP–in–Microdroplets Approach" Materials 13, no. 8: 1934. https://doi.org/10.3390/ma13081934