Protective Effects of GIC and S-PRG Filler Restoratives on Demineralization of Bovine Enamel in Lactic Acid Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Bovine Enamel Blocks
2.2. Preparation of Restorative Discs and Arrangement of Specimens into Test Groups
2.3. pH Measurements, Enamel Demineralization, and SEM Observations
2.4. Fluoride Ion Release Measurements
2.5. Analysis of Released Ions
2.6. Statistical Analysis
3. Results
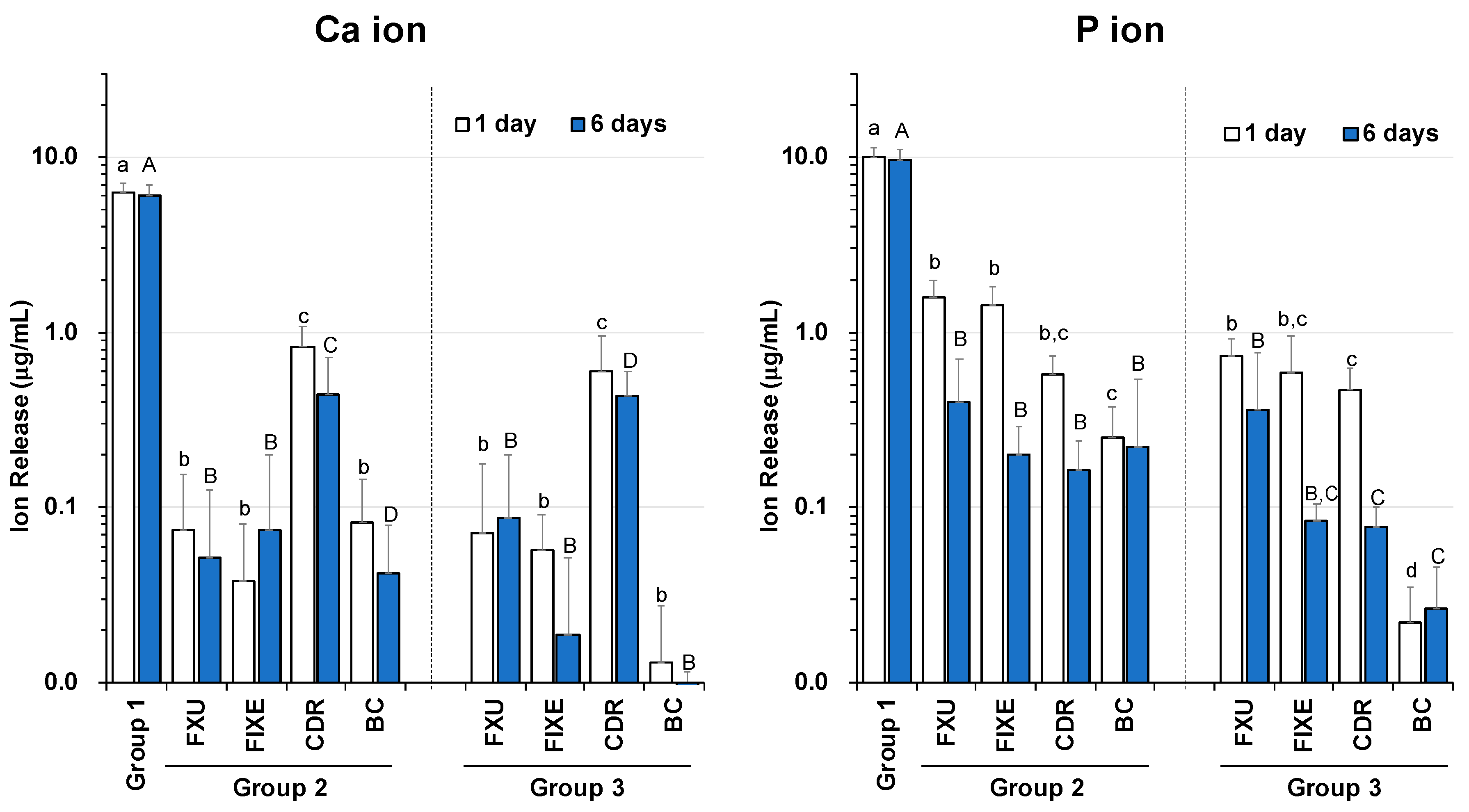
3.1. Release Profiles of Ca and P Ions
3.2. Release Profiles of Al, B, Na, Si, Sr, and Zn Ions
3.3. Fluoride Ion Release
3.4. pH Variations
3.5. SEM Observations
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chan, D.; Hu, W.; Chung, K.-H.; Larsen, R.; Jensen, S.; Cao, D.; Gaviria, L.; Ong, J.L.; Whang, K.; Eiampongpaiboon, T. Reactions: Antibacterial and bioactive dental restorative materials: Do they really work? Am. J. Dent. 2018, 31, 32B–36B. [Google Scholar] [PubMed]
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef]
- Takahashi, N.; Nyvad, B. Ecological Hypothesis of Dentin and Root Caries. Caries Res. 2016, 50, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Hojo, S.; Komatsu, M.; Okuda, R.; Takahashi, N.; Yamada, T. Acid Profiles and pH of Carious Dentin in Active and Arrested Lesions. J. Dent. Res. 1994, 73, 1853–1857. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C.N.; Chung, A.K.H.; Paranjpe, A. Antibacterial and bioactive dental restorative materials: Do they really work? Am. J. Dent. 2018, 31, 3B–5B. [Google Scholar] [PubMed]
- Tyas, M.; Anusavice, K.J.; Mount, G.J.; Frencken, J.E. Minimal intervention dentistry — a review*. Int. Dent. J. 2000, 50, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Federation, F.W.D. FDI policy statement on Minimal Intervention Dentistry (MID) for managing dental caries. Int. Dent. J. 2017, 67, 6–7. [Google Scholar] [CrossRef]
- Hafshejani, T.M.; Zamanian, A.; Venugopal, J.R.; Rezvani, Z.; Sefat, F.; Saeb, M.R.; Vahabi, H.; Zarrintaj, P.; Mozafari, M. Antibacterial glass-ionomer cement restorative materials: A critical review on the current status of extended release formulations. J. Control. Release 2017, 262, 317–328. [Google Scholar] [CrossRef]
- Imataki, R.; Shinonaga, Y.; Nishimura, T.; Abe, Y.; Arita, K. Mechanical and Functional Properties of a Novel Apatite-Ionomer Cement for Prevention and Remineralization of Dental Caries. Materials 2019, 12, 3998. [Google Scholar] [CrossRef]
- Sidhu, S.K.; Nicholson, J.W. A Review of Glass-Ionomer Cements for Clinical Dentistry. J. Funct. Biomater. 2016, 7, 16. [Google Scholar] [CrossRef]
- Wren, A.W.; Coughlan, A.; Hall, M.M.; German, M.; Towler, M.R. Comparison of a SiO2–CaO–ZnO–SrO glass polyalkenoate cement to commercial dental materials: Ion release, biocompatibility and antibacterial properties. J. Mater. Sci. Mater. Electron. 2013, 24, 2255–2264. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka, B.; Nicholson, J.W. Ion release by resin-modified glass-ionomer cements into water and lactic acid solutions. J. Dent. 2006, 34, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Krämer, N.; Schmidt, M.; Lücker, S.; Domann, E.; Frankenberger, R. Glass ionomer cement inhibits secondary caries in an in vitro biofilm model. Clin. Oral Investig. 2017, 22, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, Y.; Iwasa, M.; Murayama, R.; Miyazaki, M.; Nagafuji, A.; Nakatsuka, T. Detection of ions released from S-PRG fillers and their modulation effect. Dent. Mater. J. 2010, 29, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Nomura, R.; Morita, Y.; Matayoshi, S.; Nakano, K. Inhibitory effect of surface pre-reacted glass-ionomer (S-PRG) eluate against adhesion and colonization by Streptococcus mutans. Sci. Rep. 2018, 8, 5056. [Google Scholar] [CrossRef] [PubMed]
- Kaga, N.; Toshima, H.; Nagano-Takebe, F.; Hashimoto, M.; Nezu, T.; Yokoyama, A.; Endo, K.; Kaga, M. Inhibition of enamel demineralization by an ion-releasing tooth-coating material. Am. J. Dent. 2019, 32, 27–30. [Google Scholar]
- Kawasaki, K.; Kambara, M. Effects of Ion-Releasing Tooth-Coating Material on Demineralization of Bovine Tooth Enamel. Int. J. Dent. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Kaga, M.; Kakuda, S.; Ida, Y.; Toshima, H.; Hashimoto, M.; Endo, K.; Sano, H. Inhibition of enamel demineralization by buffering effect of S-PRG filler-containing dental sealant. Eur. J. Oral Sci. 2013, 122, 78–83. [Google Scholar] [CrossRef]
- Miyaji, H.; Mayumi, K.; Miyata, S.; Nishida, E.; Shitomi, K.; Hamamoto, A.; Tanaka, S.; Akasaka, T. Comparative biological assessments of endodontic root canal sealer containing surface pre-reacted glass-ionomer (S-PRG) filler or silica filler. Dent. Mater. J. 2020, 39, 287–294. [Google Scholar] [CrossRef]
- Davidson, C.L.; Mjör, I.A. (Eds.) Advances in Glass-Ionomer Cements; Quintessence Publishing Co. Inc.: Carol Stream, IL, USA, 1999; pp. 15–24. ISBN 978-0867153606. [Google Scholar]
- Shahid, S.; Hassan, U.; Billington, R.; Hill, R.; Anderson, P. Glass ionomer cements: Effect of strontium substitution on esthetics, radiopacity and fluoride release. Dent. Mater. 2014, 30, 308–313. [Google Scholar] [CrossRef]
- Shiozawa, M.; Takahashi, H.; Iwasaki, N.; Wada, T.; Uo, M. Effect of immersion time of restorative glass ionomer cements and immersion duration in calcium chloride solution on surface hardness. Dent. Mater. 2014, 30, e377–e383. [Google Scholar] [CrossRef] [PubMed]
- Billington, R.; Williams, J.; Pearson, G. Ion processes in glass ionomer cements. J. Dent. 2006, 34, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Jima, Y.; Koulourides, T. Fluoride Incorporation into and Retention in Remineralized Enamel. J. Dent. Res. 1989, 68, 1289–1292. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, J. Dental caries: A dynamic disease process. Aust. Dent. J. 2008, 53, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Dedhiya, M.G.; Young, F.; Higuchi, W.I. Mechanism for the Retardation of the Acid Dissolution Rate of Hydroxyapatite by Strontium. J. Dent. Res. 1973, 52, 1097–1109. [Google Scholar] [CrossRef] [PubMed]
- Thuy, T.T.; Nakagaki, H.; Kato, K.; Hung, P.A.; Inukai, J.; Tsuboi, S.; Nakagaki, H.; Hirose, M.N.; Igarashi, S.; Robinson, C. Effect of strontium in combination with fluoride on enamel remineralisation in vitro. Arch. Oral Boil. 2008, 53, 1017–1022. [Google Scholar] [CrossRef]
- Dabsie, F.; Gregoire, G.L.; Sixou, M.; Sharrock, P. Does strontium play a role in the cariostatic activity of glass ionomer? J. Dent. 2009, 37, 554–559. [Google Scholar] [CrossRef]
- Shimazu, K.; Ogata, K.; Karibe, H. Evaluation of the ion-releasing and recharging abilities of a resin-based fissure sealant containing S-PRG filler. Dent. Mater. J. 2011, 30, 923–927. [Google Scholar] [CrossRef]
- Czarnecka, B.; Limanowska-Shaw, H.; Nicholson, J.W. Buffering and ion-release by a glass-ionomer cement under near-neutral and acidic conditions. Biomaterials 2002, 23, 2783–2788. [Google Scholar] [CrossRef]
- Kitagawa, H.; Miki-Oka, S.; Mayanagi, G.; Abiko, Y.; Takahashi, N.; Imazato, S. Inhibitory effect of resin composite containing S-PRG filler on Streptococcus mutans glucose metabolism. J. Dent. 2018, 70, 92–96. [Google Scholar] [CrossRef]






| Material/(Code) | Manufacturer | Composition/Filler | Lot No. |
|---|---|---|---|
| GlasIonomer FX ULTRA | Shofu Inc. | Powder: fluoroaluminosilicate glass, polyacrylic acid | P: 061049 |
| /(FXU) | Liquid: polyacrylic acid, polybasic carboxylic acid, | L: 061034 | |
| distilled water | |||
| Fuji IX GP Extra | GC Corp. | Powder: fluoroaluminosilicate glass, pigments, | P: 1108171 |
| /(FIXE) | fluorescent material | ||
| Liquid: acrylic acid-tricarboxylic acid co-polymer, | L: 1108171 | ||
| tartaric acid, distilled water | |||
| CAREDYNE RESTORE | GC Corp. | Powder: fluoroaluminosilicate glass, | P: 1812121 |
| /(CDR) | fluorozincsilicate glass | ||
| Liquid: acrylic acid-tricarboxylic acid co-polymer, | L: 1812071 | ||
| polyacrylic acid, distilled water | |||
| PRG Barrier Coat | Shofu Inc. | Base: S-PRG glass fillers, distilled water | P: 031503 |
| /(BC) | methacrylic acid monomer, others | ||
| Active: Phosphoric acid monomer, | L: 111711 | ||
| bis-MPEPP, carboxylic acid monomer, TEGDMA, | |||
| photoinitiator, methacrylic acid monomer, | |||
| polybasic carboxylic acid, others |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaga, N.; Nagano-Takebe, F.; Nezu, T.; Matsuura, T.; Endo, K.; Kaga, M. Protective Effects of GIC and S-PRG Filler Restoratives on Demineralization of Bovine Enamel in Lactic Acid Solution. Materials 2020, 13, 2140. https://doi.org/10.3390/ma13092140
Kaga N, Nagano-Takebe F, Nezu T, Matsuura T, Endo K, Kaga M. Protective Effects of GIC and S-PRG Filler Restoratives on Demineralization of Bovine Enamel in Lactic Acid Solution. Materials. 2020; 13(9):2140. https://doi.org/10.3390/ma13092140
Chicago/Turabian StyleKaga, Naoyuki, Futami Nagano-Takebe, Takashi Nezu, Takashi Matsuura, Kazuhiko Endo, and Masayuki Kaga. 2020. "Protective Effects of GIC and S-PRG Filler Restoratives on Demineralization of Bovine Enamel in Lactic Acid Solution" Materials 13, no. 9: 2140. https://doi.org/10.3390/ma13092140
APA StyleKaga, N., Nagano-Takebe, F., Nezu, T., Matsuura, T., Endo, K., & Kaga, M. (2020). Protective Effects of GIC and S-PRG Filler Restoratives on Demineralization of Bovine Enamel in Lactic Acid Solution. Materials, 13(9), 2140. https://doi.org/10.3390/ma13092140





