Analysis of Post-Deposition Recrystallization Processing via Indium Bromide of Cu(In,Ga)Se2 Thin Films
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Post-Deposition Recrystallization at 400 °C
3.2. Post-Deposition Recrystallization at 500 °C
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kato, T. Cu(In,Ga)(Se,S)2 solar cell research in Solar Frontier: Progress and current status. Jpn. J. Appl. Phys. 2017, 56, 04CA02. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Kou, X.L.; Cai, Y.A.; Liu, W.; Yu, T.; Pang, J.; Li, C.J.; Sun, Y. Effect of substrate temperature on the structural and electrical properties of CIGS films based on the one-stage co-evaporation process. Semicond. Sci. Technol. 2010, 25, 055007. [Google Scholar] [CrossRef]
- Reinders, A.; Verlinden, P.; Van Sark, W.; Freundlich, A. Photovoltaic Solar Energy: From Fundamentals to Applications; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Ferekides, C.; Balasubramanian, U.; Mamazza, R.; Viswanathan, V.; Zhao, H.; Morel, D. CdTe thin film solar cells: Device and technology issues. Sol. Energy 2004, 77, 823–830. [Google Scholar] [CrossRef]
- McCandless, B.; Moulton, L.; Birkmire, R. Recrystallization and sulfur diffusion in CdCl2-treated CdTe/CdS thin films. Prog. Photovolt. 1997, 5, 249–260. [Google Scholar] [CrossRef]
- Rodriguez-Alvarez, H.; Barreau, N.; Kaufmann, C.; Weber, A.; Klaus, M.; Painchaud, T.; Schock, H.-W.; Mainz, R. Recrystallization of Cu(In,Ga)Se2 thin films studied by X-ray diffraction. Acta Mater. 2013, 61, 4347–4353. [Google Scholar] [CrossRef]
- Poudel, D.; Karki, S.; Belfore, B.; Rajan, G.; Atluri, S.S.; Soltanmohammad, S.; Rockett, A.; Marsillac, S. Degradation Mechanism Due to Water Ingress Effect on the Top Contact of Cu(In,Ga)Se2 Solar Cells. Energies 2020, 13, 4545. [Google Scholar] [CrossRef]
- Grzeta-Plenkovic, B.; Popovic, S.; Celustka, B.; Santic, B. Crystal data for AgGaxIn1−xSe2 and CuGaxIn1−xSe2. J. Appl. Crystallogr. 1980, 13, 311–315. [Google Scholar] [CrossRef]
- Shafarman, W.N.; Klenk, R.; McCandless, B.E. Device and material characterization of Cu (InGa)Se2 solar cells with increasing band gap. J. Appl. Phys. 1996, 79, 7324–7328. [Google Scholar] [CrossRef]
- Belfore, B.; Ayala, O.; Ashrafee, T.; Rajan, G.; Karki, S.; Marsillac, S. Modeling Diffusion of Impurities in Molybdenum Thin Films as a Function of Substrate Temperature. IEEE J. Photovolt. 2018, 9, 339–343. [Google Scholar] [CrossRef]
- Rudmann, D.; Bilger, G.; Kaelin, M.; Haug, F.-J.; Zogg, H.; Tiwari, A. Effects of NaF coevaporation on structural properties of Cu(In,Ga)Se2 thin films. Thin Solid Film. 2003, 431–432, 37–40. [Google Scholar] [CrossRef]
- Chirilă, A.; Reinhard, P.; Pianezzi, F.; Bloesch, P.; Uhl, A.R.; Fella, C.; Kranz, L.; Keller, D.; Gretener, C.; Hagendorfer, H.; et al. Potassium-induced surface modification of Cu(In,Ga)Se2 thin films for high-efficiency solar cells. Nat. Mater. 2013, 12, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Powalla, M.; Paetel, S.; Ahlswede, E.; Wuerz, R.; Wessendorf, C.D.; Friedlmeier, T.M. Thin-film solar cells exceeding 22% solar cell efficiency: An overview on CdTe-, Cu(In,Ga)Se2-, and perovskite-based materials. Appl. Phys. Rev. 2018, 5, 041602. [Google Scholar] [CrossRef]
- Rajan, G.; Belfore, B.; Karki, S.; Poudel, D.; Kahoui, H.; Lanham, N.; Palmiotti, E.; Soltanmohammad, S.; Rockett, A.; Marsillac, S. Impact of Post-Deposition Recrystallization by Alkali Fluorides on Cu(In,Ga)Se2 Thin-Film Materials and Solar Cells. Thin Solid Film. 2019, 690, 137526. [Google Scholar] [CrossRef]
- Ranjan, V.; Begou, T.; Little, S.; Collins, R.W.; Marsillac, S. Non-destructive optical analysis of band gap profile, crystalline phase, and grain size for Cu(In,Ga)Se2 solar cells deposited by 1-stage, 2-stage, and 3-stage co-evaporation. Prog. Photovolt Res. Appl. 2014, 22, 77–82. [Google Scholar] [CrossRef]
- Reinhard, P.; Pianezzi, F.; Kranz, L.; Nishiwaki, S.; Chirilă, A.; Buecheler, S.; Tiwari, A.N. Flexible Cu(In,Ga)Se2 solar cells with reduced absorber thickness. Prog. Photovolt. Res. Appl. 2015, 23, 281–289. [Google Scholar] [CrossRef]
- Chirilă, A.; Buecheler, S.; Pianezzi, F.; Bloesch, P.; Gretener, C.; Uhl, A.R.; Fella, C.; Kranz, L.; Perrenoud, J.; Seyrling, S.; et al. Highly efficient Cu(In,Ga)Se2 solar cells grown on flexible polymer films. Nat. Mater. 2011, 10, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, B.; Palchetti, C.; Piacente, V. Sublimation enthalpy of indium tri-bromide. J. Mater. Sci. Lett. 1997, 16, 1395–1397. [Google Scholar] [CrossRef]
- Brunetti, B.; Piacente, V.; Scardala, P. A Study on the Sublimation of Gallium Tribromide. J. Chem. Eng. Data 2009, 54, 2273–2276. [Google Scholar] [CrossRef]
- Sun, Y.; Lin, S.; Li, W.; Cheng, S.; Zhang, Y.; Liu, Y. Review on Alkali Element Doping in Cu(In,Ga)Se2 Thin Films and Solar Cells. Engineering 2017, 3, 452–459. [Google Scholar] [CrossRef]
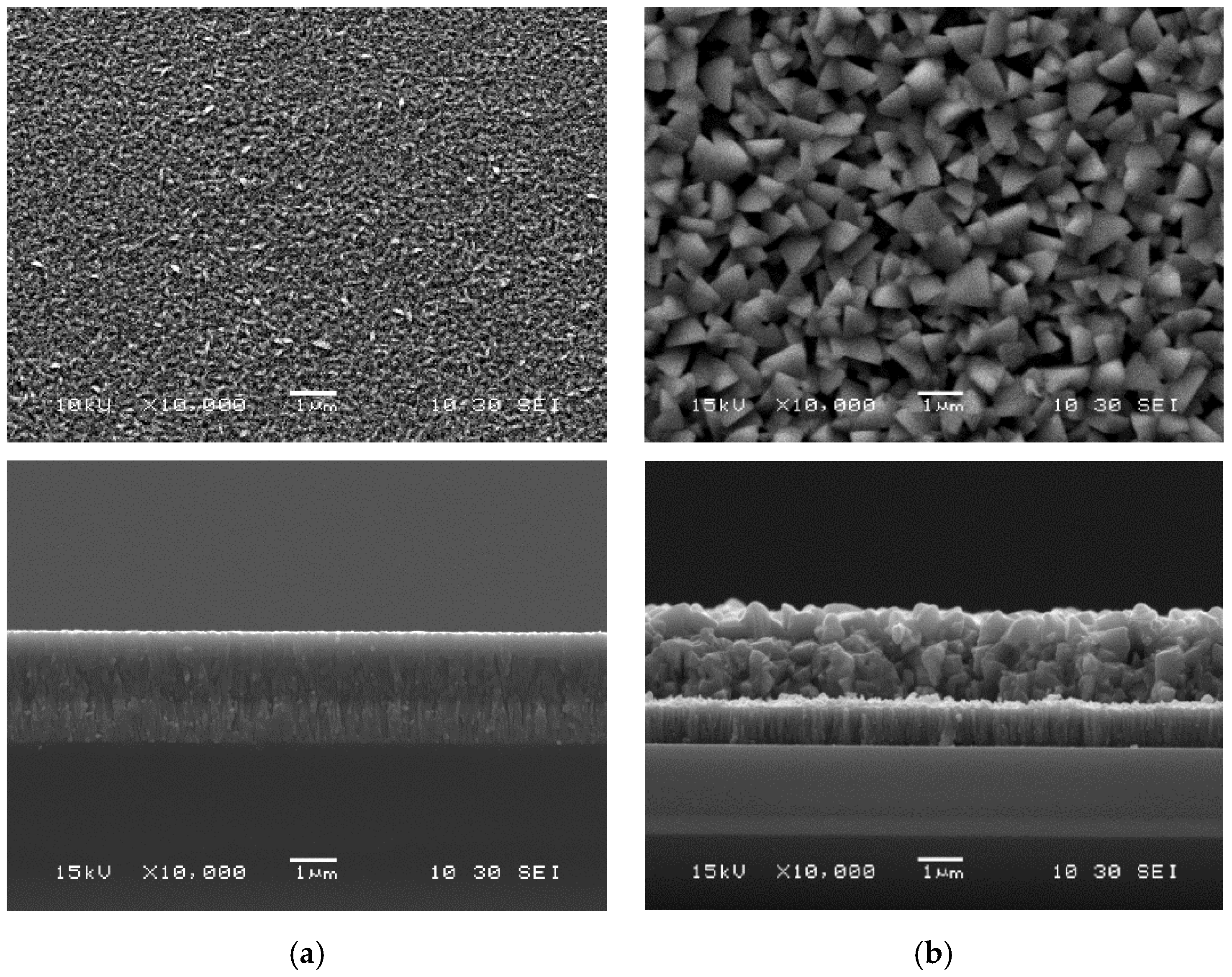

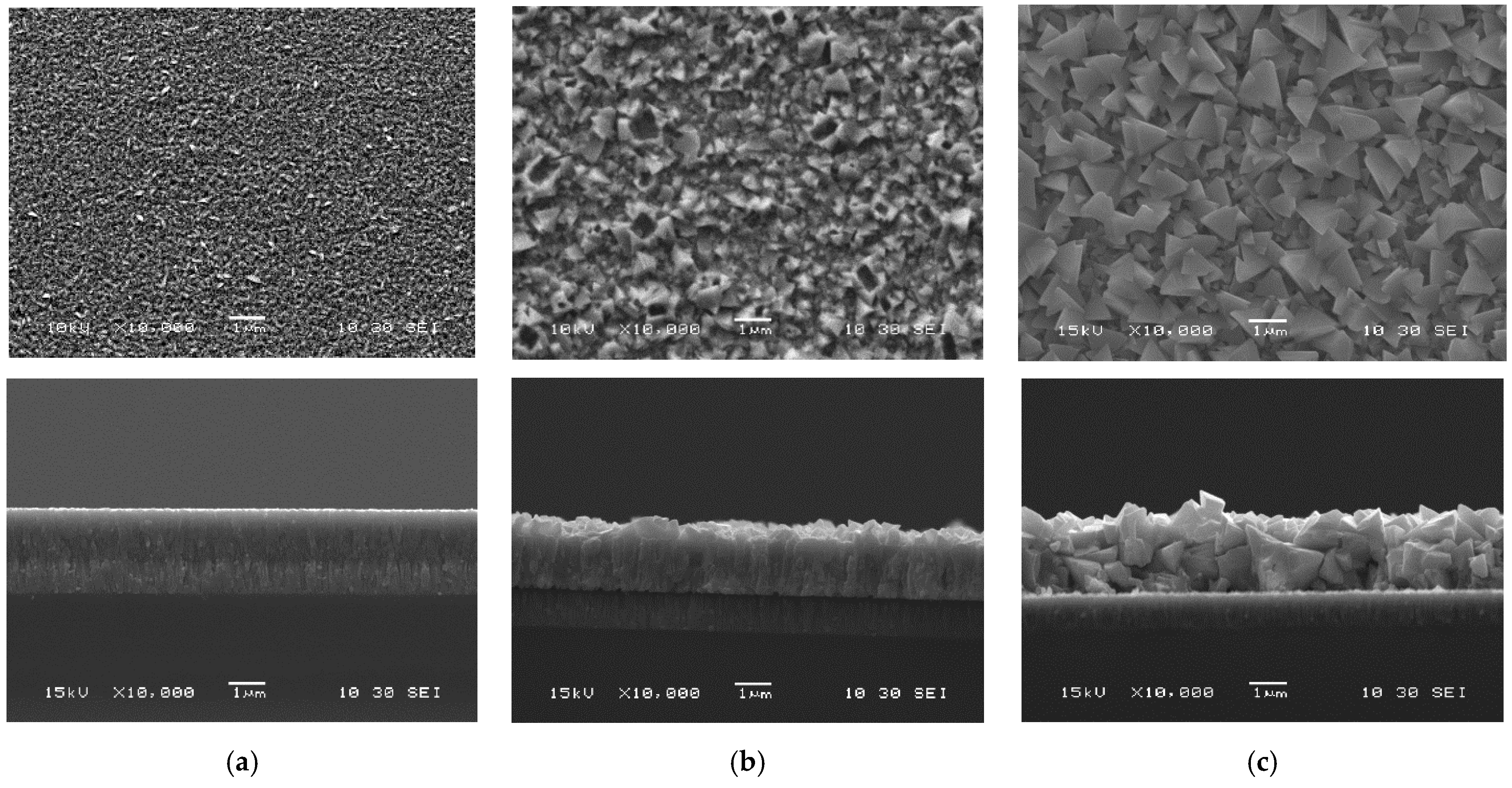
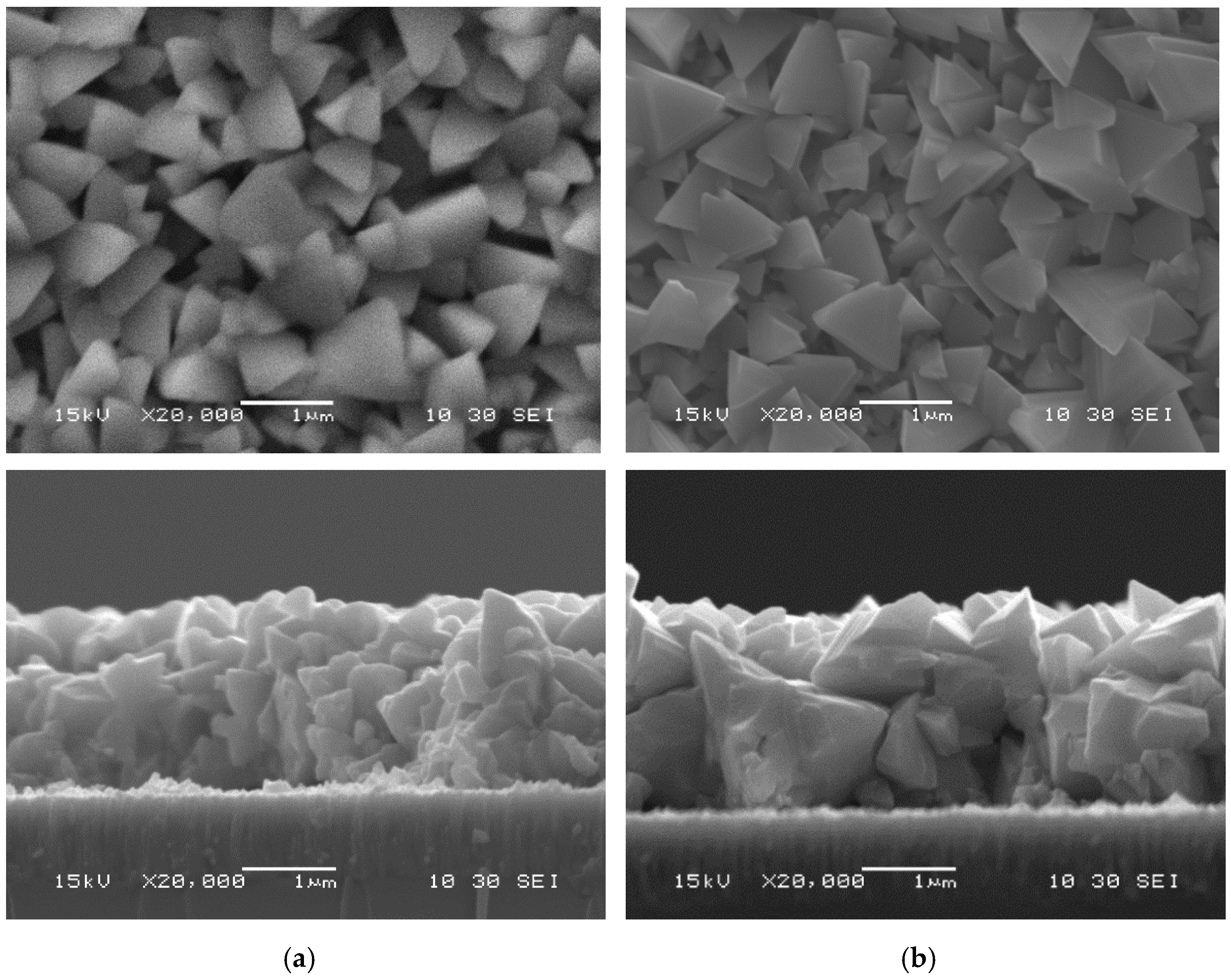
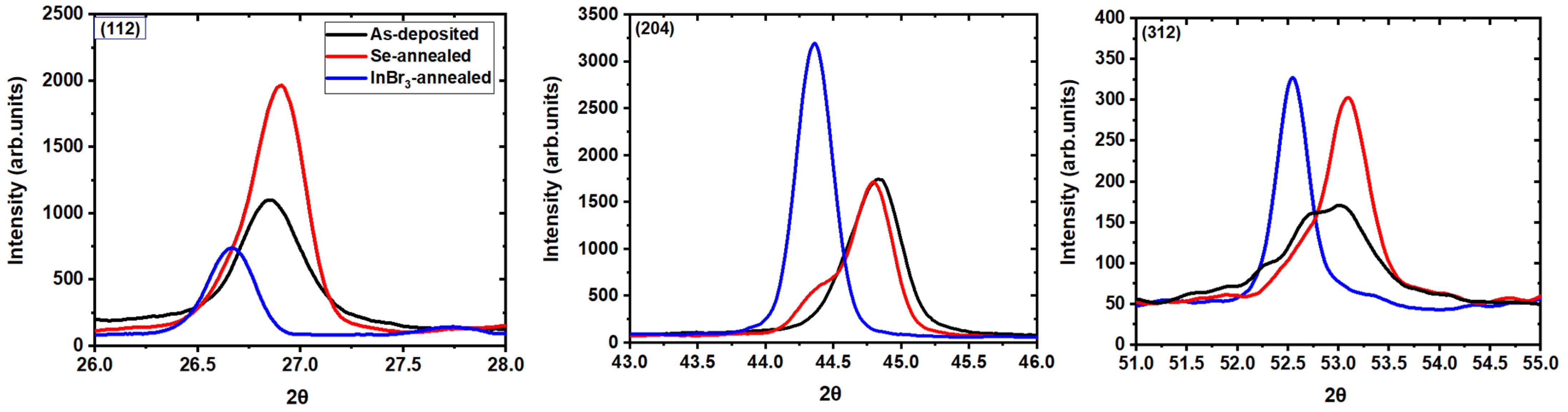

| Characterizations | Parameters | As-Deposited | InBr3-Annealed | ||||
|---|---|---|---|---|---|---|---|
| XRD | Peaks | (112) | (220)/(204) | (312) | (112) | (220)/(204) | (312) |
| Angle (°) | 26.8 | 44.8 | 52.9 | 26.6 | 44.3 | 52.5 | |
| Intensity (counts) | 1092 | 1714 | 170 | 890 | 3900 | 227 | |
| FWHM (°) | 0.29 | 0.39 | 0.48 | 0.17 | 0.19 | 0.29 | |
| Ga/III | 0.24 | 0.07 | |||||
| XRF | Ga/III | 0.24 | 0.05 | ||||
| Cu/III | 0.85 | 0.72 | |||||
| Hall effect | Carrier Concentration (NA) (1016 cm−3) | 0.5 | 5.2 | ||||
| Mobility (µ) (cm2/s) | 2.4 | 4.3 | |||||
| Conductivity (10−3 ohm−1·cm−1) | 1.9 | 35.7 | |||||
| Characterization | Parameters | As Deposited | Se-Annealed | InBr3-Annealed | |||
|---|---|---|---|---|---|---|---|
| XRD | Peaks | (112) | (220)/(204) | (112) | (220)/(204) | (112) | (220)/(204) |
| Angle (°) | 26.8 | 44.8 | 26.8 | 44.3 | 26.6 | 44.3 | |
| 44.7 | |||||||
| Intensity (counts) | 1092 | 1714 | 1875 | 512 | 719 | 3221 | |
| 1688 | |||||||
| FWHM (°) | 0.29 | 0.39 | 0.19 | 0.40 | 0.19 | 0.22 | |
| 0.22 | |||||||
| Ga/III | 0.24 | 0.09 | 0.08 | ||||
| 0.30 | |||||||
| XRF | Ga/III | 0.24 | 0.24 | 0.04 | |||
| Cu/III | 0.85 | 0.84 | 0.70 | ||||
| Hall Effect | Carrier Concentration (NA) (1016 cm−3) | 0.5 | 1.8 | 7.1 | |||
| Mobility (µ) (cm2/s) | 2.4 | 3.5 | 6.2 | ||||
| Conductivity (10−3 ohm−1·cm−1) | 1.9 | 10.1 | 70.5 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poudel, D.; Belfore, B.; Ashrafee, T.; Karki, S.; Rajan, G.; Rockett, A.; Marsillac, S. Analysis of Post-Deposition Recrystallization Processing via Indium Bromide of Cu(In,Ga)Se2 Thin Films. Materials 2021, 14, 3596. https://doi.org/10.3390/ma14133596
Poudel D, Belfore B, Ashrafee T, Karki S, Rajan G, Rockett A, Marsillac S. Analysis of Post-Deposition Recrystallization Processing via Indium Bromide of Cu(In,Ga)Se2 Thin Films. Materials. 2021; 14(13):3596. https://doi.org/10.3390/ma14133596
Chicago/Turabian StylePoudel, Deewakar, Benjamin Belfore, Tasnuva Ashrafee, Shankar Karki, Grace Rajan, Angus Rockett, and Sylvain Marsillac. 2021. "Analysis of Post-Deposition Recrystallization Processing via Indium Bromide of Cu(In,Ga)Se2 Thin Films" Materials 14, no. 13: 3596. https://doi.org/10.3390/ma14133596







