Influence of W Addition on Microstructure and Resistance to Brittle Cracking of TiB2 Coatings Deposited by DCMS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coating Deposition
2.2. Microstructure Examinations
3. Results and Discussion
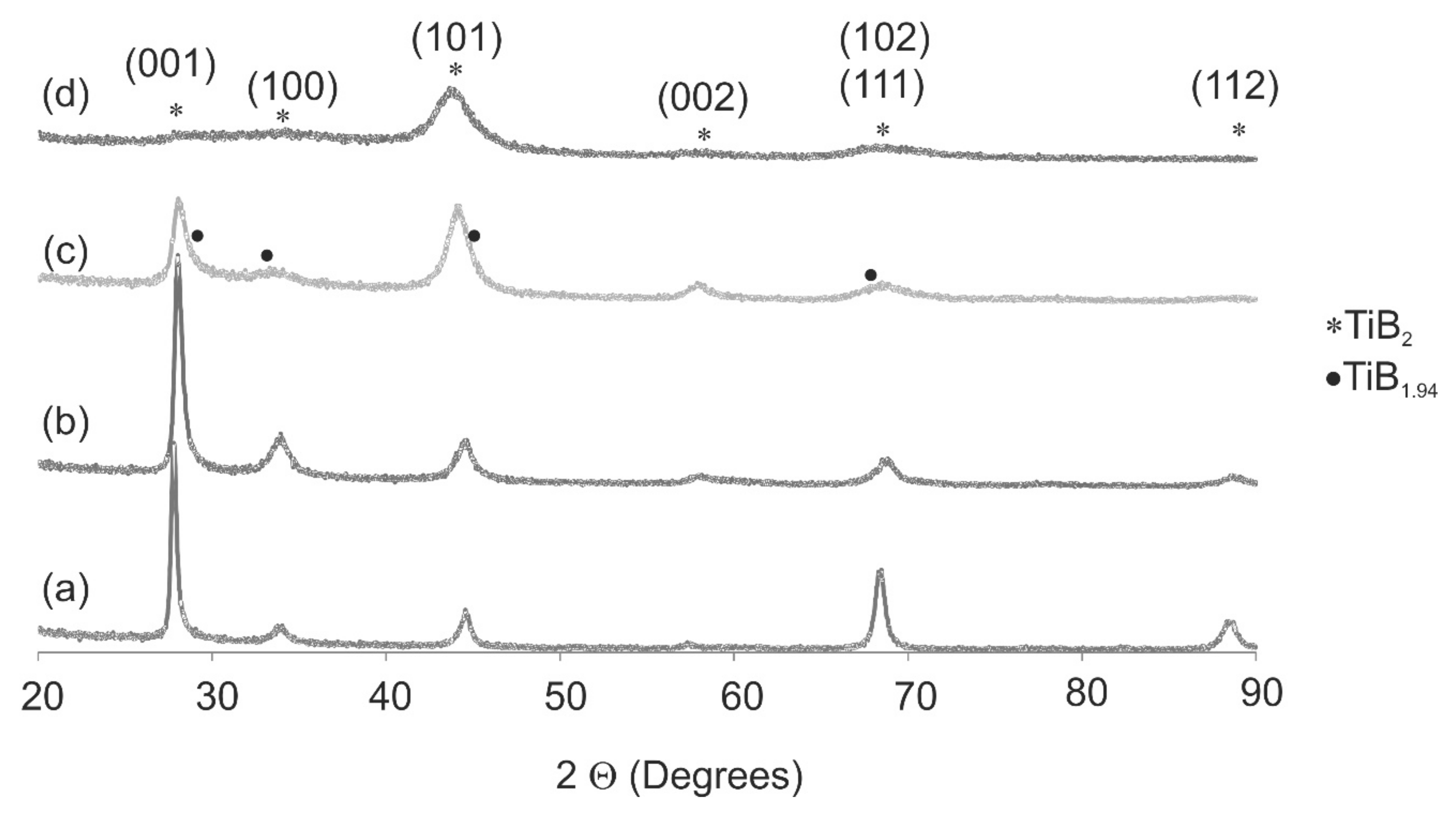
3.1. X-ray Diffraction (XRD)
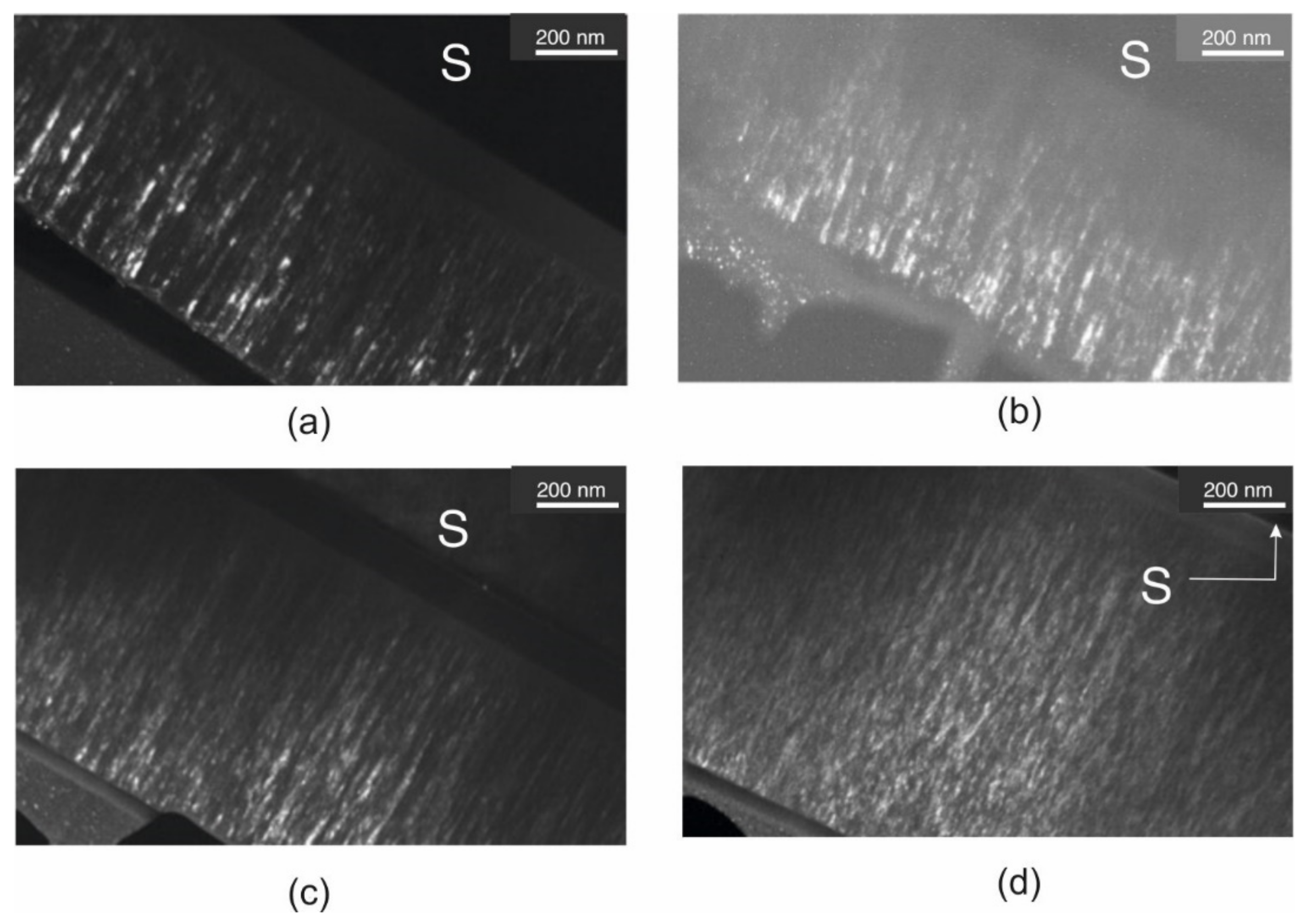
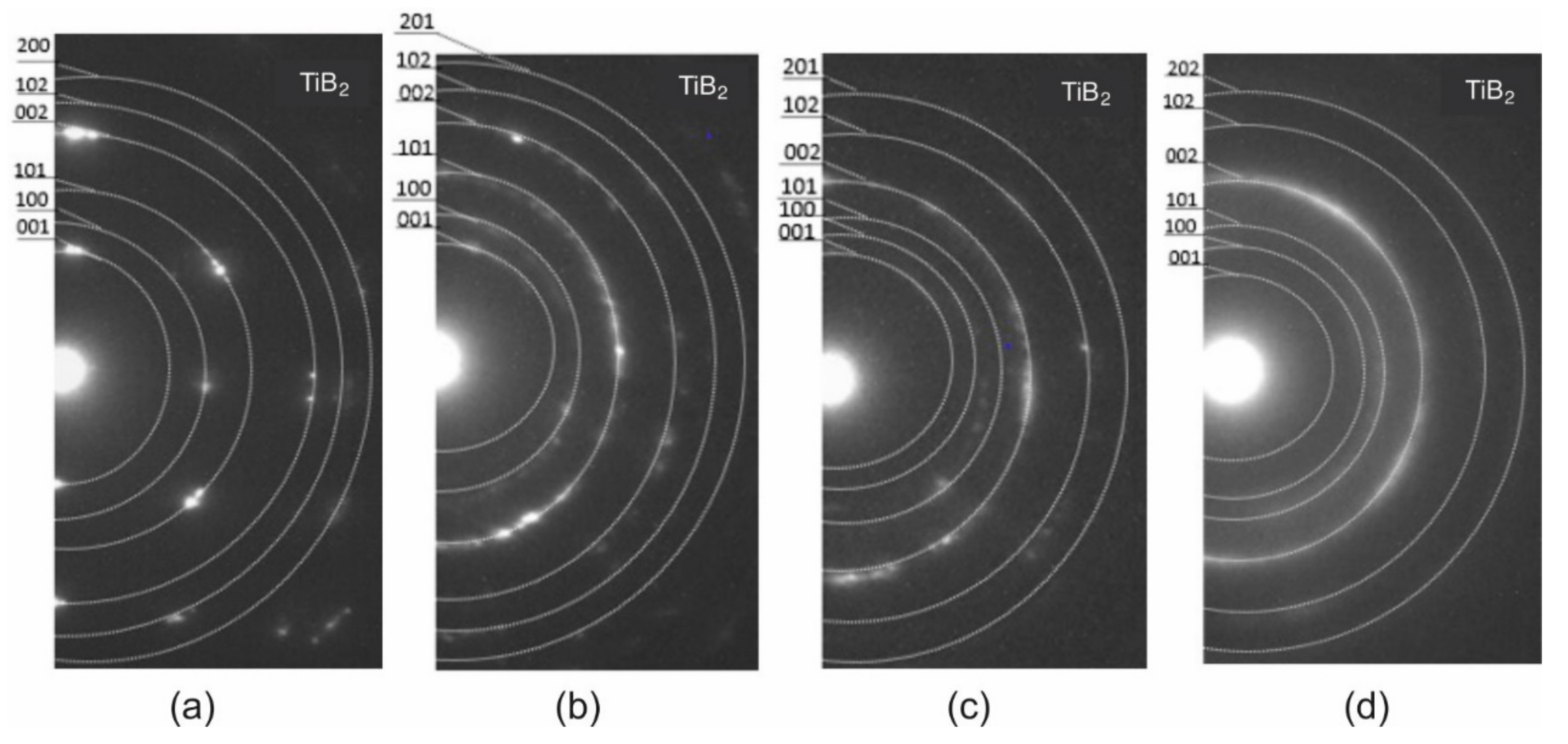
3.2. Transmission Electron Microscopy (TEM)
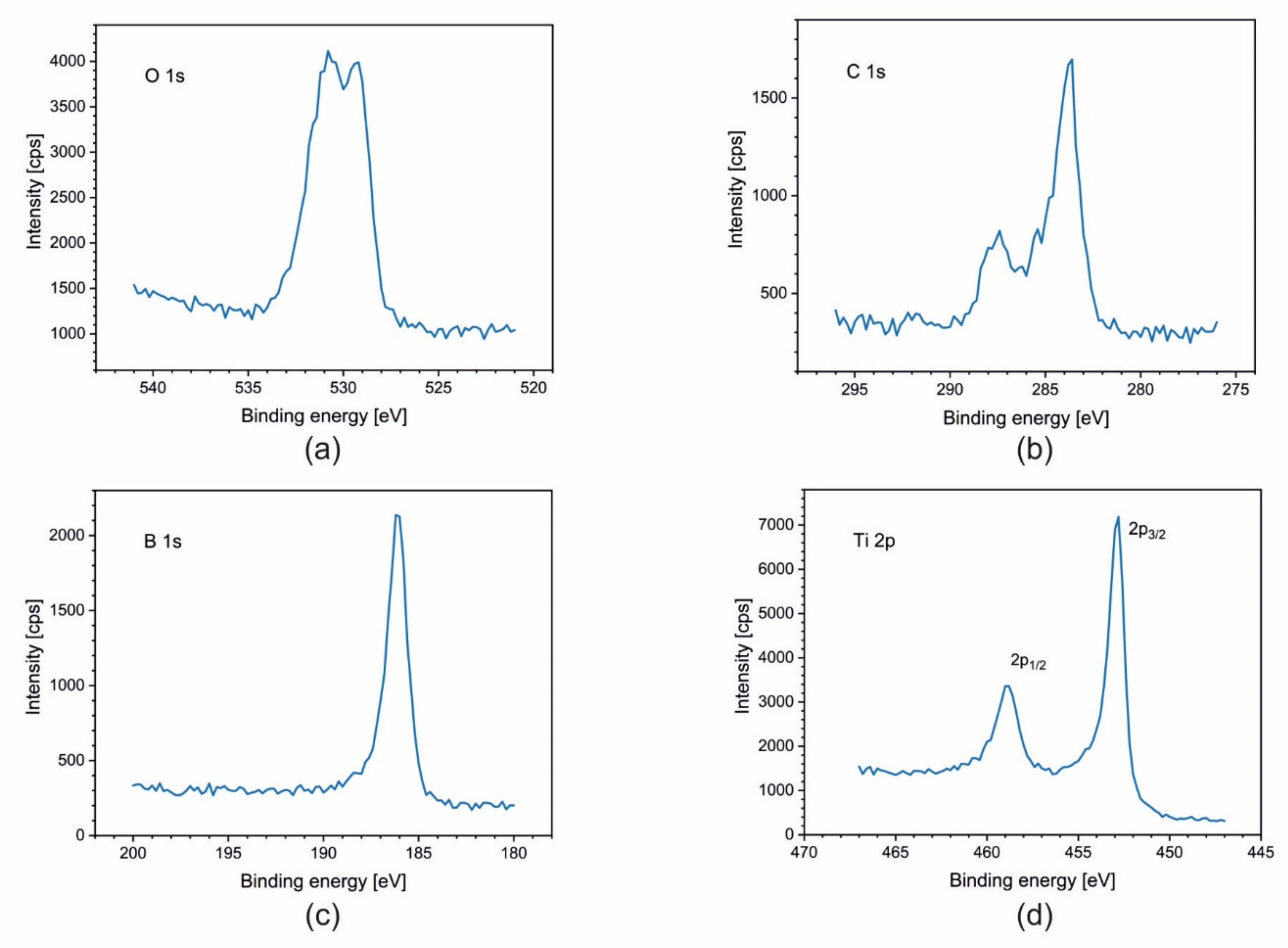
3.3. X-ray Photoelectron Spectroscopy (XPS)
3.4. Atomic Force Microscopy (AFM) and Brittle Cracking Resistance (KIC)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cernuschi, F.; Bison, P.; Mack, D.E.; Merlini, M.; Boldrini, S.; Marchionna, S.; Capelli, S.; Concari, S.; Famengo, A.; Moscatelli, A.; et al. Thermophysical properties of as deposited and aged thermal barrier coatings (TBC) for gas turbines: State-of-the art and advanced TBCs. J. Eur. Ceram. Soc. 2018, 38, 3945–3961. [Google Scholar] [CrossRef]
- Solecka, M.; Kopia, A.; Petrzak, P.; Radziszewska, A. Microstructure, chemical and phase composition of clad layers of inconel 625 and inconel 686. Arch. Metall. Mater. 2018, 63, 513–518. [Google Scholar]
- Koszela, W.; Pawlus, P.; Reizer, R.; Liskiewicz, T. The combined effect of surface texturing and DLC coating on the functional properties of internal combustion engines. Tribol. Int. 2018, 127, 470–477. [Google Scholar] [CrossRef]
- Holleck, H.; Lahres, M. Two-phase TiC/TiB2 hard coatings. Mater. Sci. Eng. 1991, 140, 609–615. [Google Scholar] [CrossRef]
- Vereschaka, A.; Aksenenko, A.; Sitnikov, N.; Migranov, M.; Shevchenko, S.; Sotova, C.; Batako, A.; Andreev, N. Effect of adhesion and tribological properties of modified composite nano-structured multi-layer nitride coatings on WC-Co tools life. Tribol. Int. 2018, 128, 313–327. [Google Scholar] [CrossRef]
- Rydzewski, M.; Kacprzyńska-Gołacka, J.; Osuch-Słomka, E.; Kamińska, M.; Bilewska, K.; Słomka, Z.; Smolik, J.; Mazurkiewicz, A. The Impact of Negative Bias Substrate to Fracture Toughness and Hardness of TiB2 Sputtering Coatings. In Proceedings of the 26th International Conference on Metallurgy and Materials, Brno, Czech, 24–26 May 2017; pp. 1438–1443. [Google Scholar]
- Berger, M.; Coronel, E.; Olsson, E. Microstructure of d.c. magnetron sputter TiB2 coatings. Surf. Coat. Technol. 2004, 185, 240–244. [Google Scholar] [CrossRef]
- Kullmer, R.; Lugmair, C.; Figueras, A.; Bassas, J.; Stoiber, M.; Mitterer, C. Microstructure, mechanical and tribological properties of PACVD Ti(B,N) and TiB2 coatings. Surf. Coat. Technol. 2003, 1229, 174–175. [Google Scholar] [CrossRef]
- Bose, S.; Pathak, L.C.; Singh, R. Response of boride coating on the Ti-6Al-4V alloy to corrosion and fretting corrosion behavior in Ringer’s solution for bio-implant application. Appl. Surf. Sci. 2018, 433, 1158–1174. [Google Scholar] [CrossRef]
- Lubas, L. Assessment and application of TiB2 coating in sliding pair under lubrication conditions. Wear 2012, 296, 504–509. [Google Scholar] [CrossRef]
- Rydzewski, M.; Kacprzyńska-Gołacka, J.; Słomka, Z.; Mazurkiewicz, A.; Smolik, J. The impact of magnetron source power on mechanical properties and phase composition of TiB2 coatings. Probl. Ekspl. 2016, 4, 53–61. [Google Scholar]
- Munro, R.G. Material properties of titanium diboride. J. Res. Natl. Inst. Stand. Technol. 2000, 105, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Mikula, M.; Grančič, B.; Buršíková, V.; Csuba, A.; Držík, M.; Kavecký, Š.; Plecenik, A.; Kúš, P. Mechanical properties of superhard TiB2 coatings prepared by DC magnetron sputtering. Vacuum 2007, 82, 278–281. [Google Scholar] [CrossRef]
- Wang, H.; Wang, B.; Li, S.; Xue, Q.; Huang, F. Toughening magnetron sputtered TiB2 coatings by Ni addition. Surf. Coat. Technol. 2013, 232, 767–774. [Google Scholar] [CrossRef]
- Contreras, E.; Galindez, Y.; Gómez, M.A. Microstructure, mechanical and tribological properties of TiBC coatings by DC magnetron sputtering onto AISI M2 steel using independent TiB2 and graphite targets. Surf. Coat. Technol. 2018, 350, 298–306. [Google Scholar] [CrossRef]
- Xiaoqing, L.; Meng, X.; Min, L.; Zhaoguo, Q.; Dechang, Z. Preparation and enhanced wear resistance of HVAF-sprayed Fe-TiB2 cermet coating reinforced by carbon nanotubes. Surf. Coat. Technol. 2021, 408, 126860. [Google Scholar]
- Smolik, J.; Mazurkiewicz, A.; Garbacz, H.; Kopia, A. Tungsten doped TiB2 coatings obtained by magnetron sputtering. J. Mach. Constr. Maint. 2018, 4, 27–32. [Google Scholar]
- Burton, A.W.; Ong, K.; Rea, T.; Chan, I. On the estimation of average crystallite size of zeolites from the Scherrer equation: A critical evaluation of its application to zeolites with one-dimensional pore systems. Mater. Lett. 2009, 117, 75–90. [Google Scholar] [CrossRef]
- Smolik, J.; Kacprzyńska-Gołacka, J.; Sowa, S.; Piasek, A. The Analysis of Resistance to Brittle Cracking of Tungsten Doped TiB2 Coatings Obtained by Magnetron Sputtering. J. Coat. Technol. Res. 2020, 10, 807. [Google Scholar]
- Kalfagiannis, N.; Volonakis, G.; Tsetseris, L.; Logothetidis, S. Excess of boron in TiB2 superhard thin films: A combined experimental and ab initio study. J. Phys. D Appl. Phys. 2011, 44, 385402. [Google Scholar] [CrossRef]
- Ding, J.; Lee, D.; Mei, H.; Zhang, T.; Kang, M.; Wang, Q.; Kim, K. Influence of Si addition on structure and properties of TiB2-Si nanocomposite coatings deposited by high-power impulse magnetron sputtering. Ceram. Int. 2019, 45, 6363–6372. [Google Scholar] [CrossRef]
- Chlupa, Z.; Bača, L.; Halasová, M.; Neubauer, E.; Hadraba, H.; Stelzer, N.; Roupcová, P. Effect of metallic dopants on the microstructure and mechanical properties of TiB2. J. Eur. Ceram. Soc. 2015, 35, 2745–2754. [Google Scholar] [CrossRef]
- Euchner, H.; Mayrhofer, P.H.; Riedl, H.; Klimashin, F.F.; Limbeck, A.; Polcik, P.; Kolozsvari, S. Solid solution hardening of vacancy stabilized TixW1-xB. Acta Mater. 2015, 101, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Newirkowez, A.; Cappi, B.; Telle, R.; Schmidt, H. (Ti, W, Cr) B2 coatings produced by dc magnetron sputtering. Thin. Solid. Films. 2012, 520, 1775–1778. [Google Scholar] [CrossRef]
- Neidhardt, J.; Mraz, S.; Schneider, J.M.; Strub, E.; Bohne, W.; Liedke, B.; Moller, W.; Mitterer, C.J. Experiment and simulation of the compositional evolution of Ti–B thin films deposited by sputtering of a compound target. Appl. Phys. 2008, 104, 063304. [Google Scholar] [CrossRef]
- Hellgren, N.; Thörnberg, J.; Zhirkov, I.; Sortica, M.A.; Petrov, I.; Greene, J.E.; Hult-man, L.; Rosen, J. High-power impulse magnetron sputter deposition of TiBx thin films: Effects of pressure and growth temperature. Vacuum 2019, 169, 108884. [Google Scholar] [CrossRef]
- Thornberg, J.; Palisaitis, J.; Hellgren, N.; Klimashin, F.; Ghafoor, N.; Zhirkov, I.; Azina, C.; Battaglia, J.; Kusiak, A.; Sortica, M.; et al. Microstructure and materials properties of understoichiometric TiBx thin films grown by HiPIMS. Surf. Coat. Technol. 2020, 404, 126537. [Google Scholar] [CrossRef]

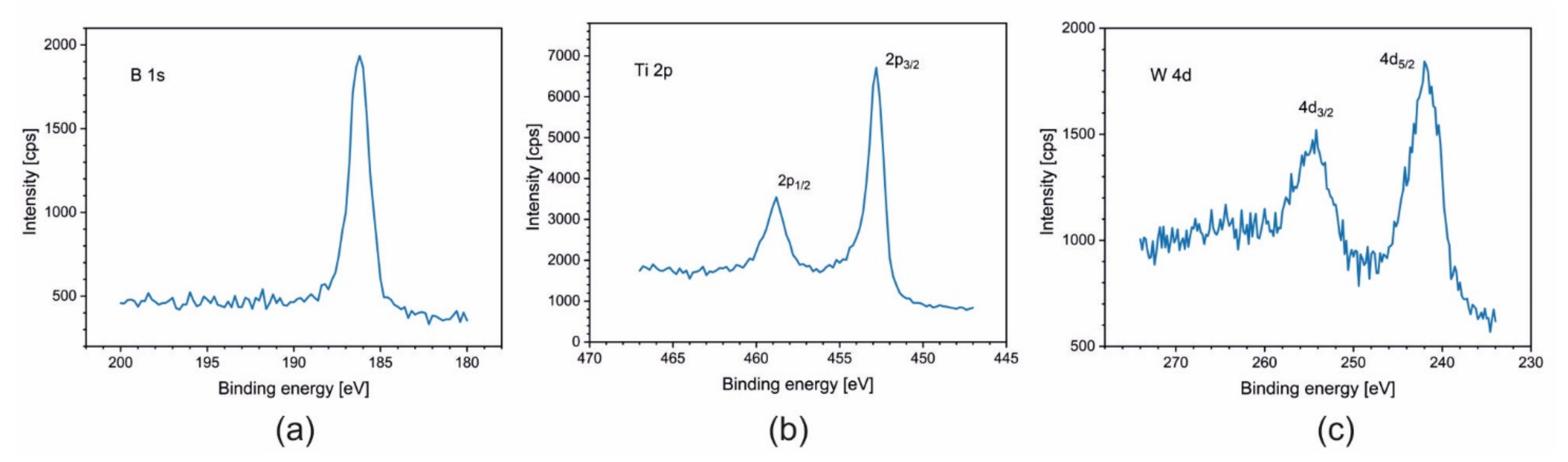
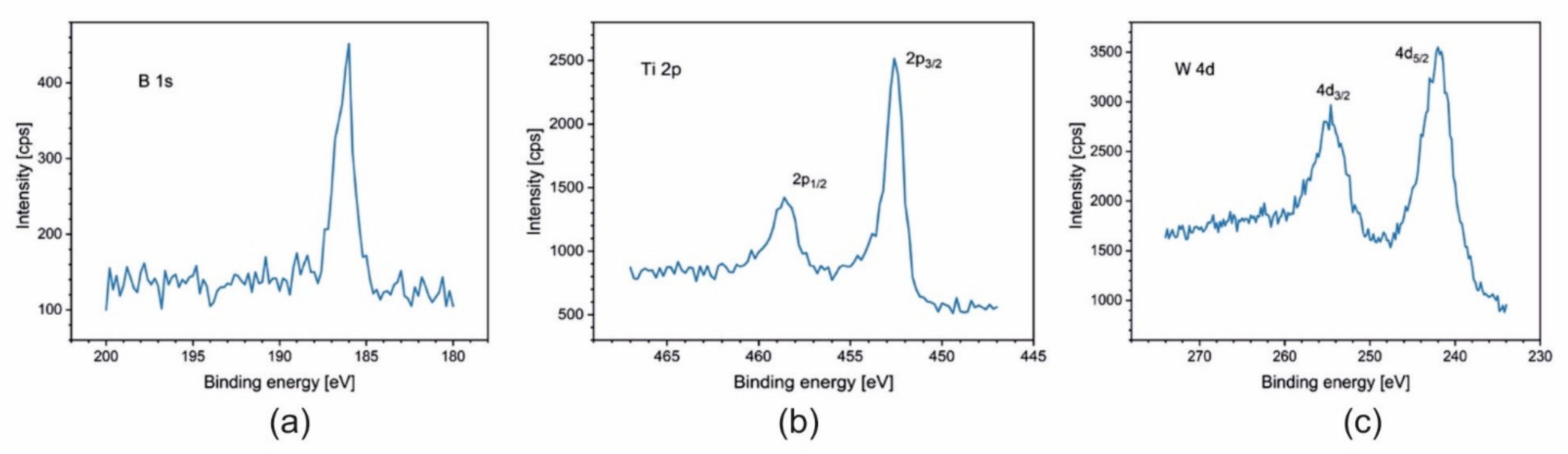



| Sample | Ti [at.%] | W [at.%] | B [at.%] | B/[Ti + W] |
|---|---|---|---|---|
| TiB2 | 26 | 0 | 74 | 2.8 |
| Ti0.78W0.22B2 | 25 | 7 | 68 | 2.1 |
| Ti0.58W0.42B2 | 21 | 15 | 64 | 1.8 |
| Ti0.5W0.5B2 | 20 | 20 | 60 | 1.5 |
| Sample | Ra [nm] | Rmax [nm] | Rq [nm] | KIC |
|---|---|---|---|---|
| TiB2 | 1.58 ± 0.15 | 11.1 ± 1.1 | 1.99 ± 1.1 | 1.8 |
| Ti0.78W0.22B2 | 1.46 ± 0.15 | 12.9 ± 1.2 | 1.81 ± 1.1 | 2.8 |
| Ti0.58W0.42B2 | 1.03 ± 0.1 | 8.10 ± 0.8 | 1.25 ± 1.1 | 8.5 |
| Ti0.5W0.5B2 | 1.34 ± 0.13 | 8.9 ± 0.9 | 1.63 ± 1.1 | 11.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chudzik-Poliszak, E.; Cieniek, Ł.; Moskalewicz, T.; Kowalski, K.; Kopia, A.; Smolik, J. Influence of W Addition on Microstructure and Resistance to Brittle Cracking of TiB2 Coatings Deposited by DCMS. Materials 2021, 14, 4664. https://doi.org/10.3390/ma14164664
Chudzik-Poliszak E, Cieniek Ł, Moskalewicz T, Kowalski K, Kopia A, Smolik J. Influence of W Addition on Microstructure and Resistance to Brittle Cracking of TiB2 Coatings Deposited by DCMS. Materials. 2021; 14(16):4664. https://doi.org/10.3390/ma14164664
Chicago/Turabian StyleChudzik-Poliszak, Edyta, Łukasz Cieniek, Tomasz Moskalewicz, Kazimierz Kowalski, Agnieszka Kopia, and Jerzy Smolik. 2021. "Influence of W Addition on Microstructure and Resistance to Brittle Cracking of TiB2 Coatings Deposited by DCMS" Materials 14, no. 16: 4664. https://doi.org/10.3390/ma14164664






