Investigation on Microstructure and Properties of Duplex Stainless Steel Welds by Underwater Laser Welding with Different Shielding Gas
Abstract
:1. Introduction
2. Experimental Procedure
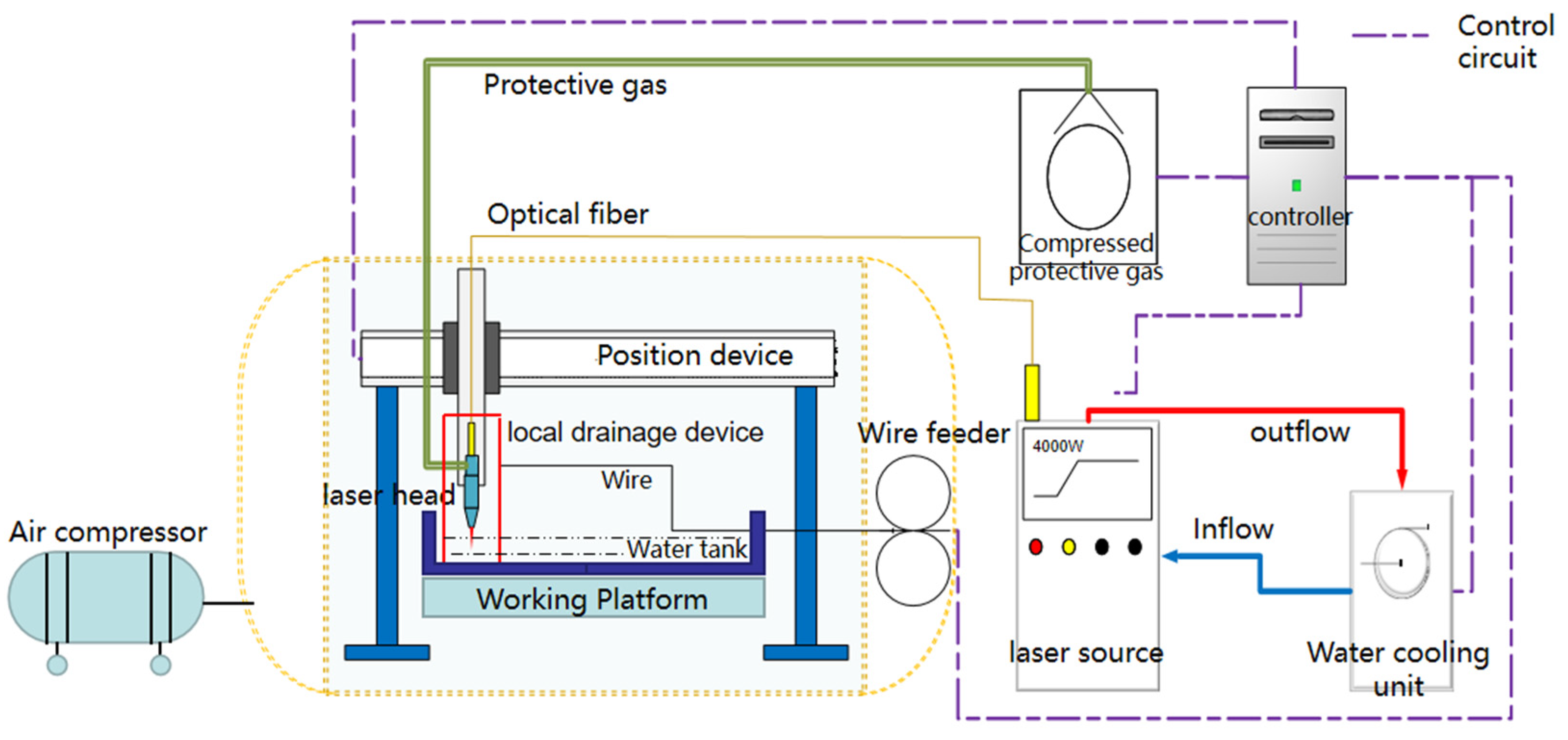
2.1. Equipment and Materials
2.2. Welding Process
2.3. Electrochemical Corrosion Tests
3. Results and Discussion
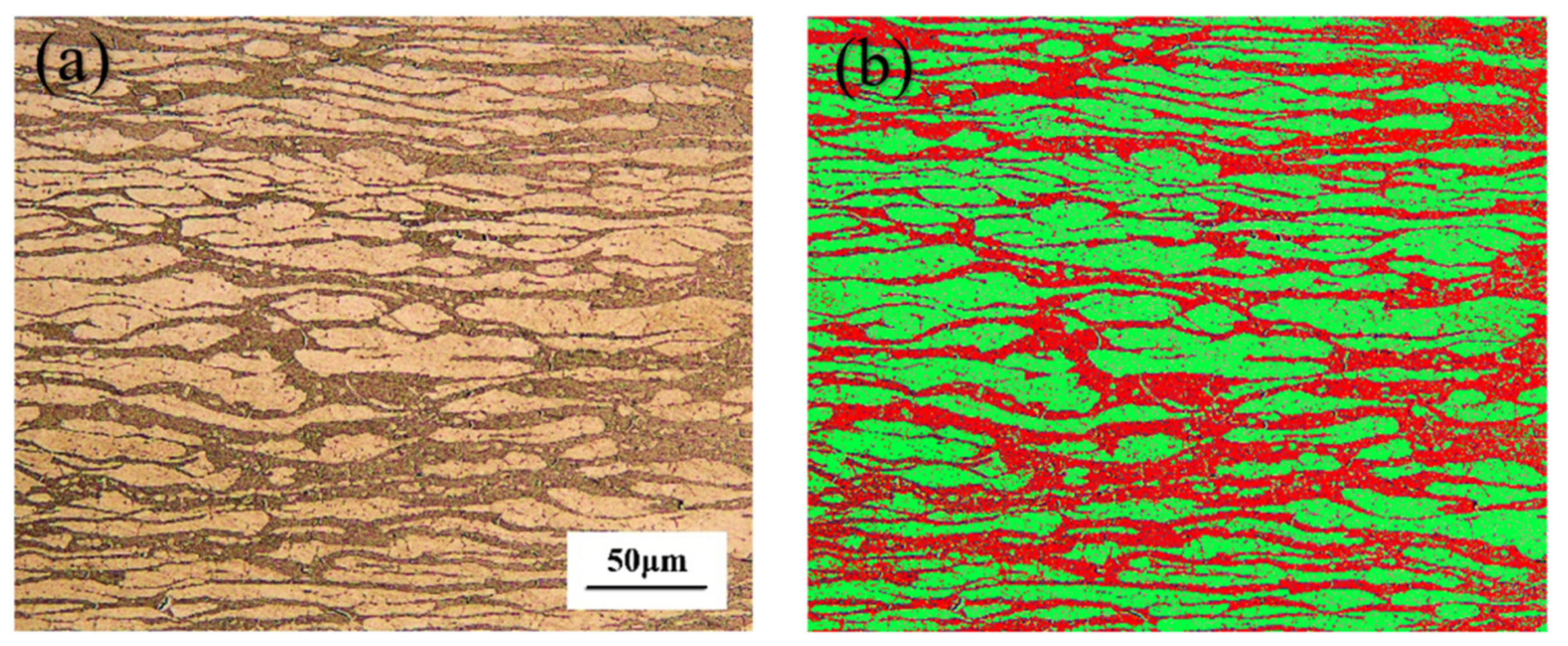
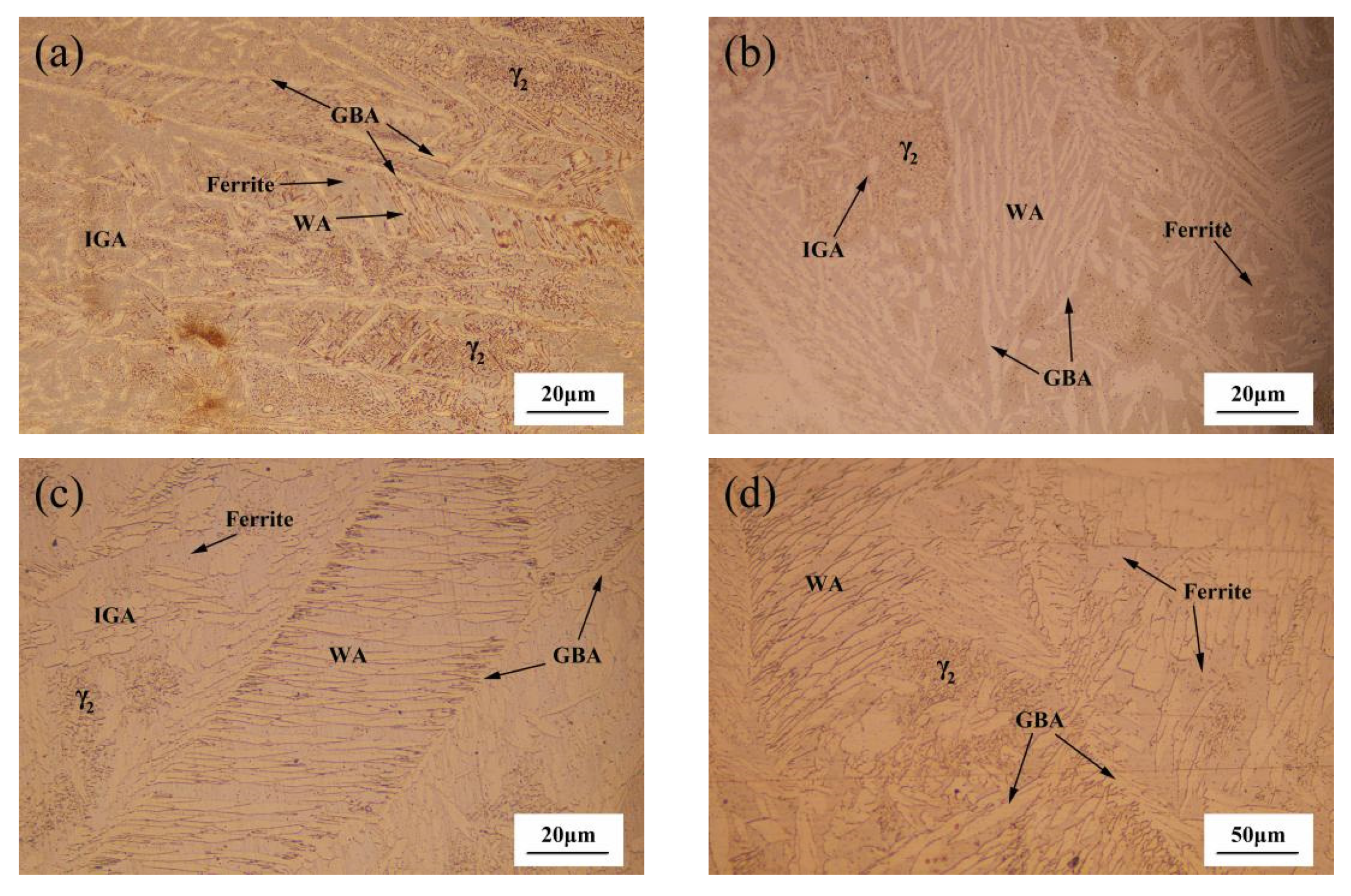
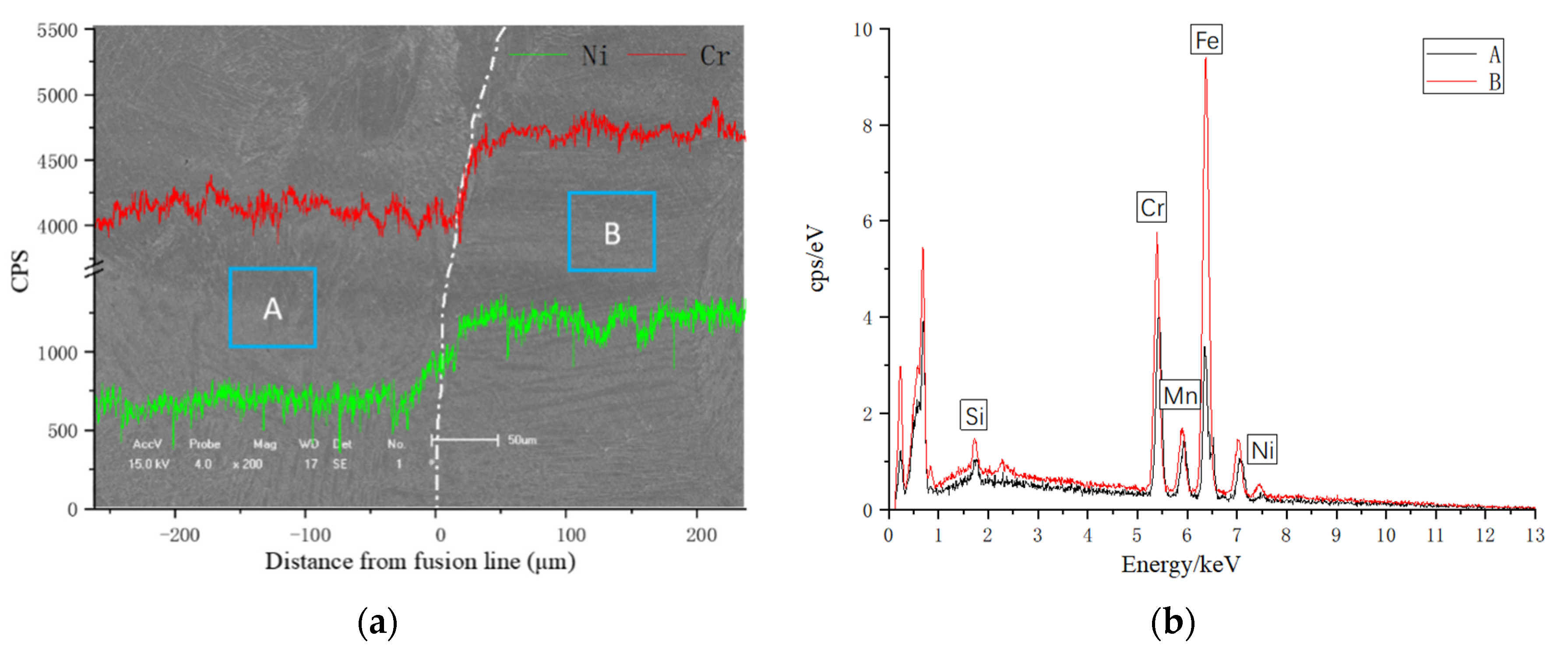
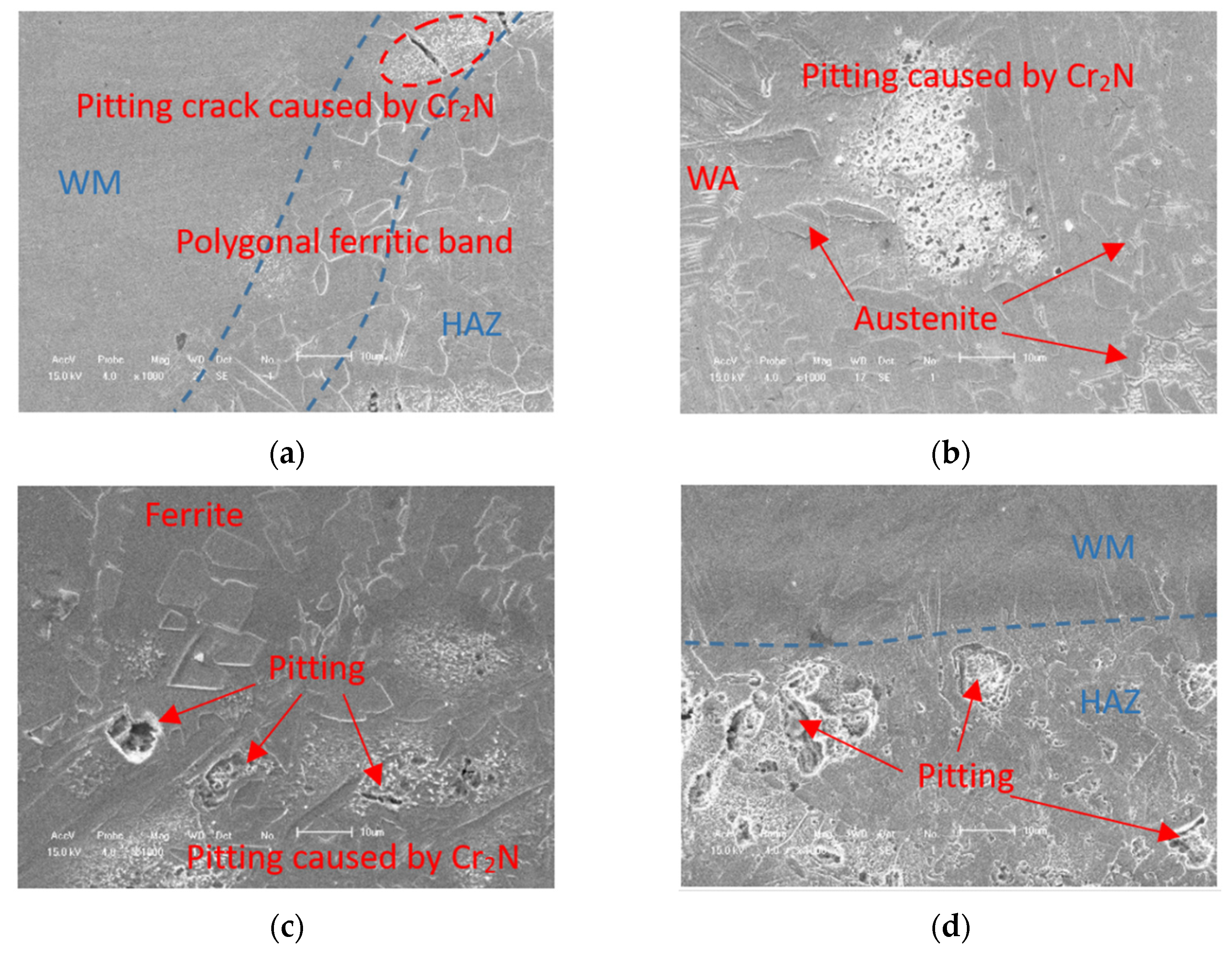
3.1. Microstructural Characterization
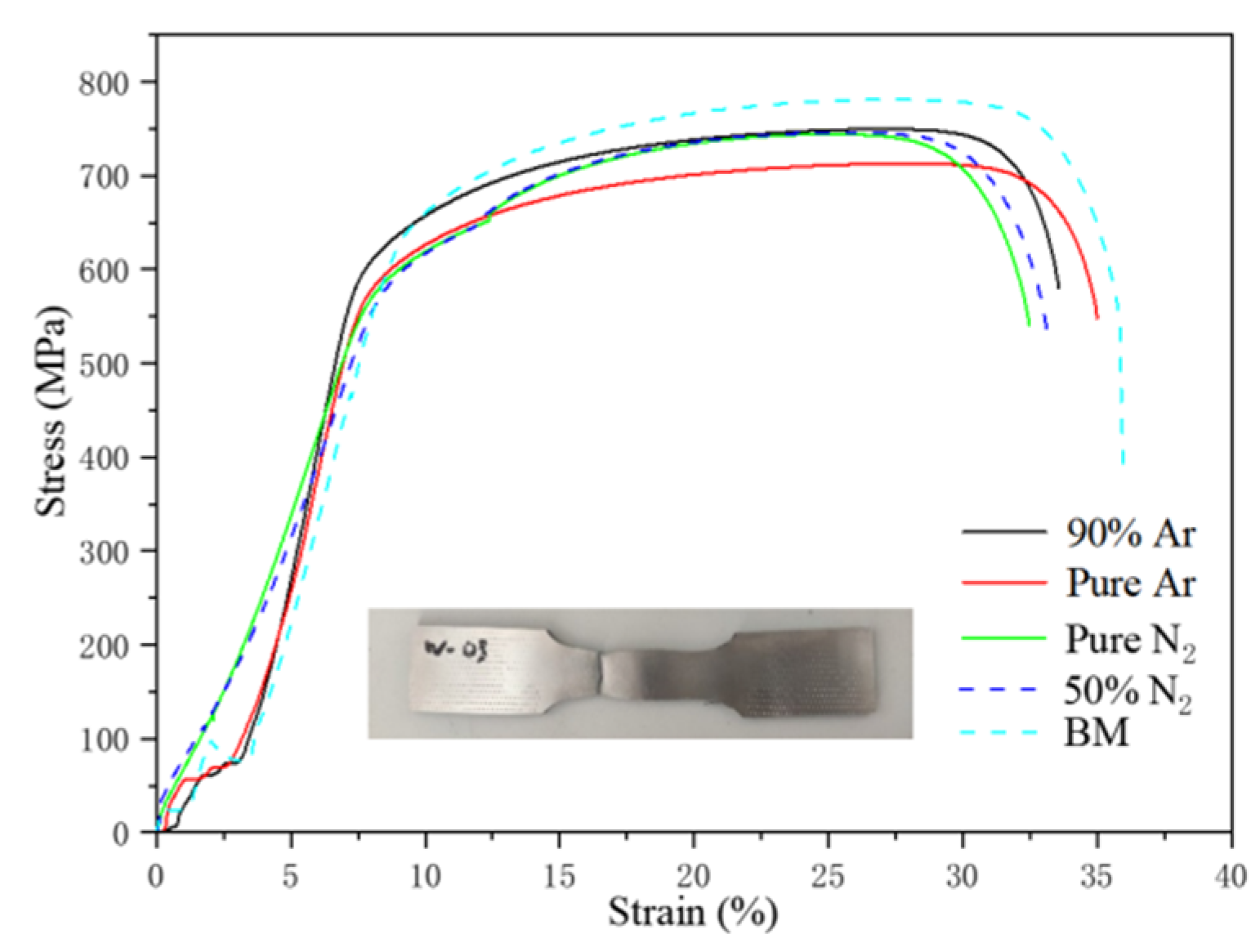
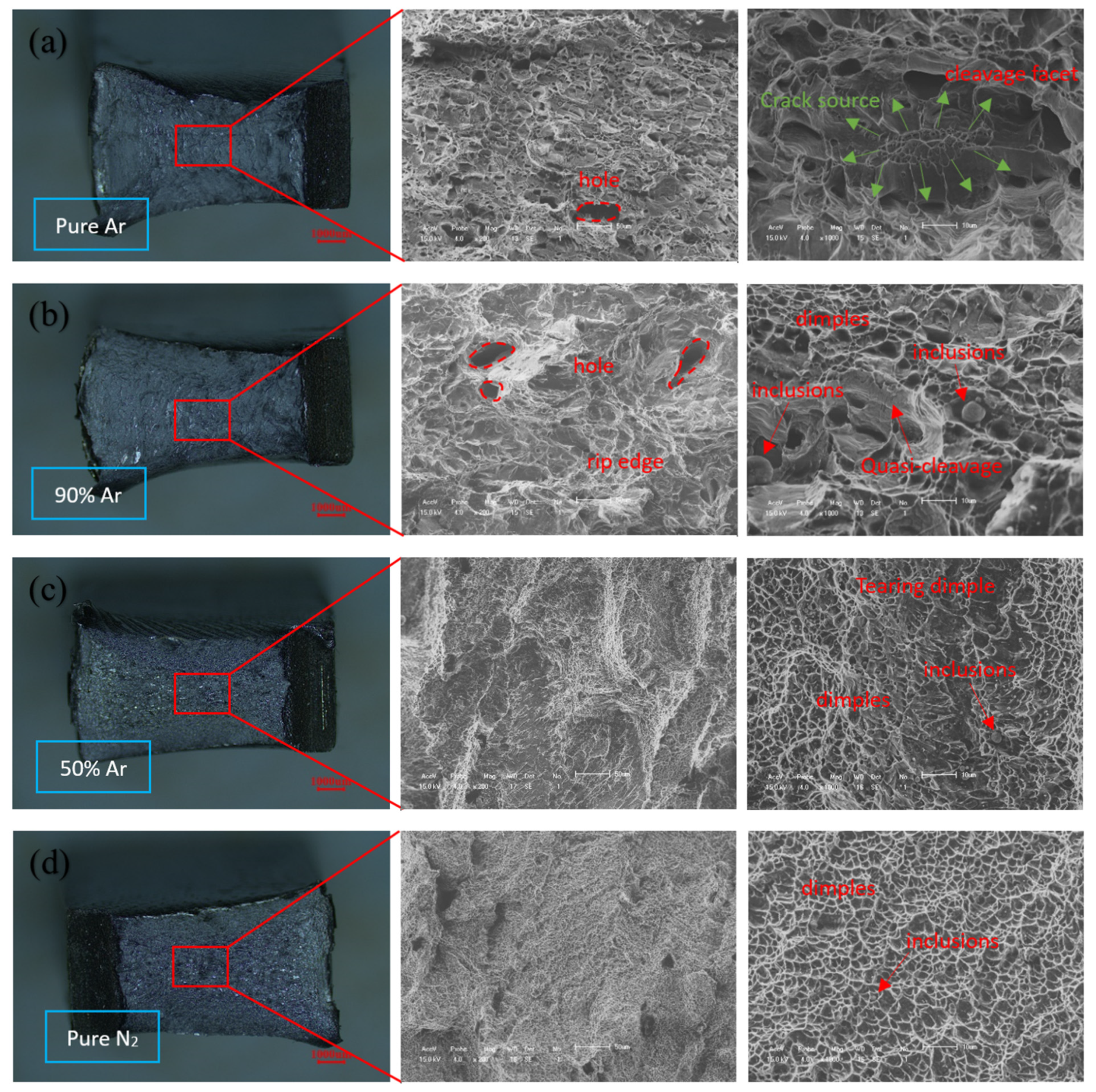
3.2. Mechanical Performance Testing
- The ferrite and austenite in the BM are banded along the rolling direction, whereas the ferrite and austenite in the WM and HAZ are interlaced with different directions, and the grain boundaries are increased, which can lock the dislocation and strengthen the joint;
- The atoms of Cr, Mo and Ni in the weld metal can be remelted at a high temperature, which can replace Fe atoms in the lattice and disturb the original lattice arrangement, and can also make dislocation movement difficult and strengthen the joint. As in HSLA steel, the addition of Mn and other alloying elements, such as copper (Cu), titanium (TI) and vanadium (V), both provide strengthening and an obtain ideal microstructure [26,27];
- The nitrogen atoms in the shielding gas are intercalated into the lattice in the form of an interstitial solid solution. The strengthening effect of the interstitial solid solution is more obvious than that of the replacement solid solution. The nitrogen atoms are mainly concentrated in the austenite phase and directly strengthen the austenite. Therefore, the strength performance of WM is better than that of BM.
3.3. Charpy V-Notch Impact Tests
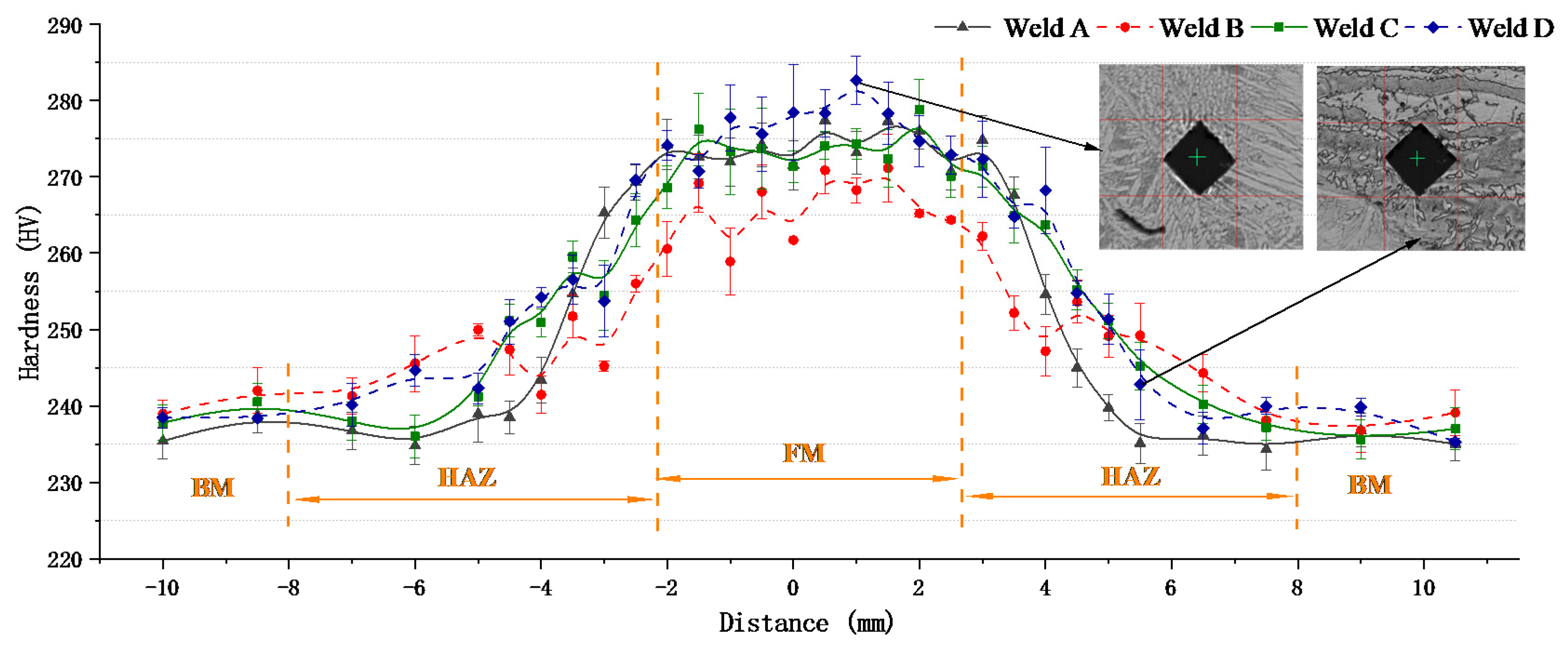
3.4. Hardness Tests
3.5. Potentiodynamic Polarization
4. Conclusions
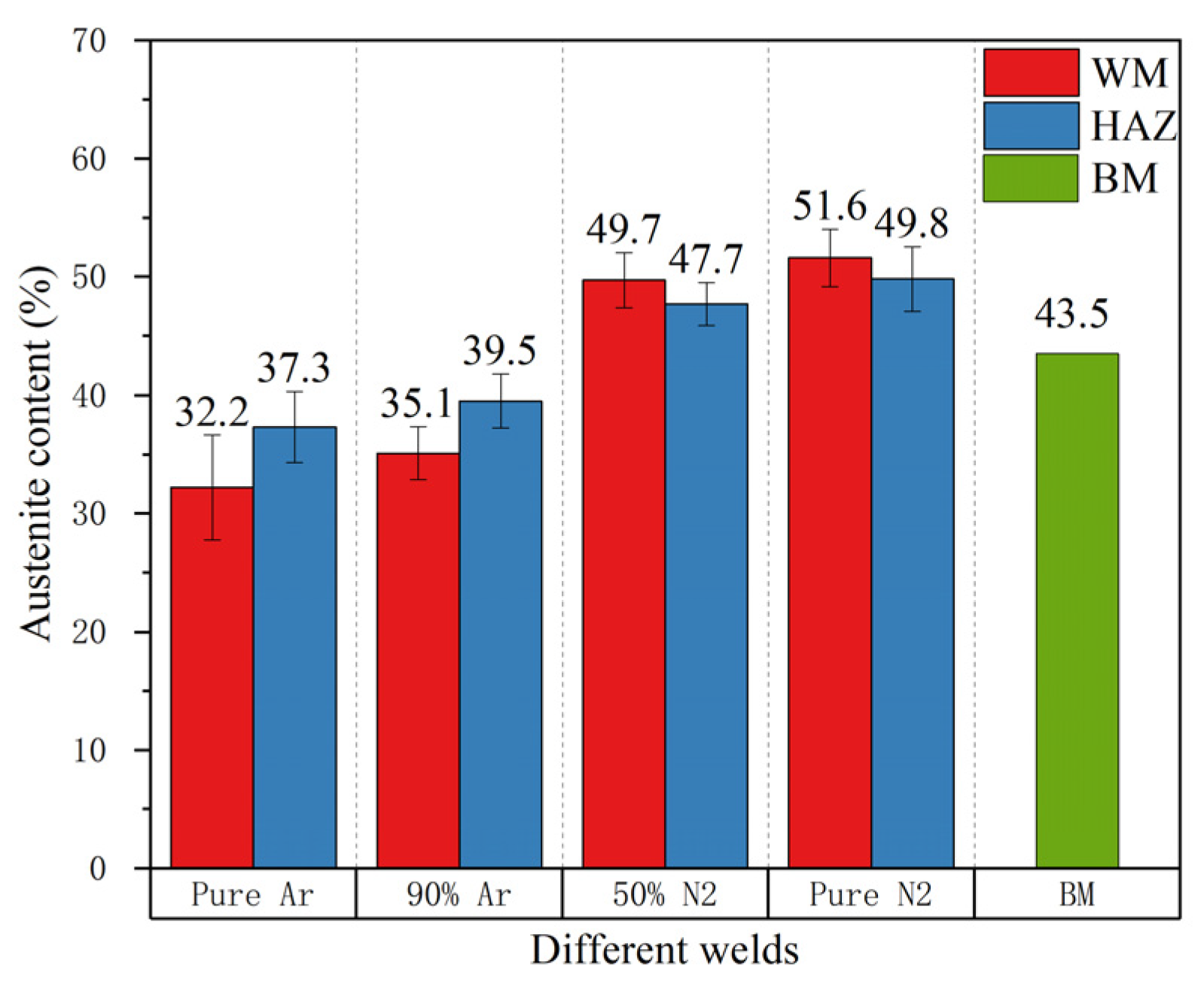
- The addition of nitrogen in the shielding gas can increase the austenite content in the weld zone to approximately 51.6% higher than that of pure argon (the nitrogen shielded is 51.6%, the pure argon shielded is 32.2%), but the transformation type of the ferrite–austenite is not affected significantly;
- The increase in nitrogen content in the shielding gas does not affect the strength of the joint, and the base metal is still the weakest part of the joint;
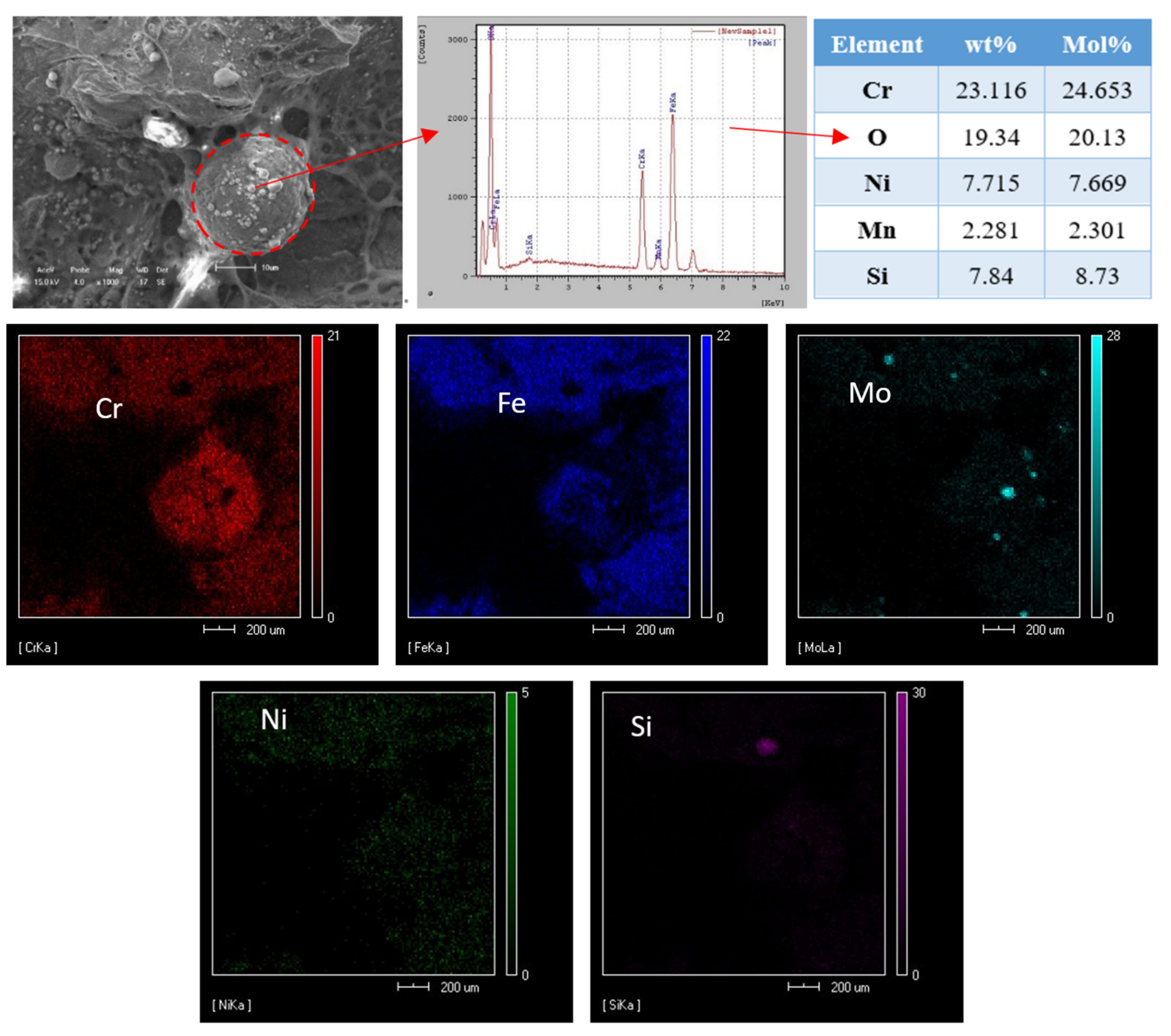
- The evaporation loss of nitrogen in the weld pool means that it is easy to form harmful phases, such as an Fe-Cr intermetallic compound in the weld, which is not conducive to the impact toughness of the weld;
- The addition of nitrogen in the shielding gas is beneficial to austenite regeneration during solidification. The pitting corrosion resistance of the four kinds of welds is not as good as that of base metal; The heat affected zone has poor corrosion resistance. The weld with pure nitrogen protection has the highest Et value and the best corrosion resistance;
- There is a carbon-poor ferrite band near the fusion line in the HAZ. Due to the fact that the solubility of nitrogen in ferrite decreases rapidly with the decrease in temperature, the supersaturated nitrogen combines with chromium to form chromium nitride precipitation. The precipitation of Cr2N results in partial Cr depletion, which is the main reason for the weak pitting resistance of HAZ.
Author Contributions
Funding
Conflicts of Interest
References
- Westin, E.M.; Johansson, M.M.; Bylund, L.-Å.; Pettersson, R.F.A. Effect on microstructure and properties of super duplex stainless steel welds when using backing gas containing nitrogen and hydrogen. Weld. World 2014, 58, 347–354. [Google Scholar] [CrossRef]
- Chen, W. The study on microstructure and corrosion resistance properties of duplex stainless steel. Mater. Sci. 2019, 9, 632–638. [Google Scholar]
- Fan, Y.; Liu, T.G.; Xin, L.; Han, Y.M.; Lu, Y.H.; Shoji, T. Thermal aging behaviors of duplex stainless steels used in nuclear power plant: A review—Sciencedirect. J. Nucl. Mater. 2020, 544, 152693. [Google Scholar] [CrossRef]
- Zhu, J.; Jiao, X.; Zhou, C.; Gao, H. Applications of Underwater Laser Peening in Nuclear Power Plant Maintenance. Energy Procedia 2012, 16, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Jiao, X.; Chen, M.X.; Zhou, C.; Hui, G. Research of chamber local dry underwater welding system and drainage properties. China Weld. 2013, 16, 27–29. [Google Scholar]
- Zhou, C.; Jiao, X.; Xue, L.; Chen, J. Study on automatic hyperbaric welding applied in sub-sea pipelines repair. In Proceedings of the International Offshore and Polar Engineering Conference 2010, Beijing, China, 20–25 June 2010. [Google Scholar]
- Zhou, C.F.; Jiao, X.D.; Zhu, J.L.; Gao, H.; Shen, Q.P.; Yu, Y.; Zhang, J.B. Study on Local Dry Welding of 304 Stainless Steel in Nuclear Power Stations Repair. Adv. Mater. Res. 2012, 460, 415–419. [Google Scholar] [CrossRef]
- Zhang, X.; Ashida, E.; Shono, S.; Matsuda, F. Effect of shielding conditions of local dry cavity on weld quality in underwater Nd:YAG laser welding. J. Mater. Process. Tech. 2006, 174, 34–41. [Google Scholar] [CrossRef]
- Feng, X.; Cui, X.; Zheng, W.; Lu, B.; Dong, M.; Wen, X.; Zhao, Y.; Jin, G. Effect of the protective materials and water on the repairing quality of nickel aluminum bronze during underwater wet laser repairing. Opt. Laser Technol. 2019, 114, 140–145. [Google Scholar] [CrossRef]
- Guo, N.; Xing, X.; Zhao, H.; Tan, C.; Feng, J.; Deng, Z. Effect of water depth on weld quality and welding process in underwater fiber laser welding. Mater. Des. 2017, 115, 112–120. [Google Scholar] [CrossRef]
- Guo, N.; Cheng, Q.; Zhang, X.; Fu, Y.; Huang, L. Microstructure and Mechanical Properties of Underwater Laser Welding of Titanium Alloy. Materials 2019, 12, 2703. [Google Scholar] [CrossRef] [Green Version]
- Tomków, J.; Janeczek, A.; Rogalski, G.; Wolski, A. Underwater Local Cavity Welding of S460N Steel. Materials 2020, 13, 5535. [Google Scholar] [CrossRef]
- Gunn, R. Duplex Stainless Steels: Microstructure, Properties and Applications; Anti-Corrosion Methods and Materials; Woodhead Publishing: Cambridge, UK, 2003; p. 45. [Google Scholar]
- Kiser, S.D. Welding Metallurgy and Weldability of Stainless Steels; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Park, Y.H.; Lee, Z.H. Effect of nitrogen and heat treatment on the microstructure and tensile properties of 25Cr-7Ni-1.5Mo-3WxN duplex stainless steel castings. Mater. Sci. Eng. A 2001, 297, 78–84. [Google Scholar] [CrossRef]
- Hosseini, V.A.; Wessman, S.; Hurtig, K.; Karlsson, L. Nitrogen loss and effects on microstructure in multipass TIG welding of a super duplex stainless steel. Mater. Des. 2016, 98, 88–97. [Google Scholar] [CrossRef]
- Hosseini, V.A.; Karlsson, L. Physical and kinetic simulation of nitrogen loss in high temperature heat affected zone of duplex stainless steels. Materials 2019, 6, 100325. [Google Scholar] [CrossRef]
- Nowacki, J.; Łukojc, A. Microstructural transformations of heat affected zones in duplex steel welded joints. Mater. Charact. 2006, 56, 436–441. [Google Scholar] [CrossRef]
- Chen, T.; Yang, J.-R. Microstructural characterization of simulated heat affected zone in a nitrogen-containing 2205 duplex stainless steel. Mater. Sci. Eng. A 2002, 338, 166–181. [Google Scholar] [CrossRef]
- Sakai, Y.; Aida, G.; Suga, T.; Nakano, T. Development of various flux-cored wires and their application in Japan. In IIW/IISS Doc. XII-1131-89; Welding Company: Tokyo, Japan, 1989. [Google Scholar]
- Miura, M.; Ogawa, K. Hydrogen embrittlement cracking in duplex stainless steel weld metal. In IIW Doc., IX-1461-87; Welding Company: Tokyo, Japan, 2000. [Google Scholar]
- Zhang, Z.; Jing, H.; Xu, L.; Han, Y.; Zhao, L. Investigation on microstructure evolution and properties of duplex stainless steel joint multi-pass welded by using different methods. Mater. Des. 2016, 109, 670–685. [Google Scholar] [CrossRef]
- Keskitalo, M.; Mäntyjärvi, K.; Sundqvist, J.; Powell, J.; Kaplan, A. Laser welding of duplex stainless steel with nitrogen as shielding gas. J. Mater. Process. Technol. 2015, 216, 381–384. [Google Scholar] [CrossRef]
- Ornig, H.; Richter, M. WRC-1992-Diagramm löst DeLong-Diagramm ab. Schweiss. Schneid. 1997, 49, 467–470. [Google Scholar]
- Baghdadchi, A.; Hosseini, V.A.; Hurtig, K.; Karlsson, L. Promoting austenite formation in laser welding of duplex stainless steel-impact of shielding gas and laser reheating. Weld. World 2020, 65, 499–511. [Google Scholar] [CrossRef]
- Pouraliakbara, H.; Khalajb, G.; Jandaghia, M.R.; Khalajb, M.J. Study on the correlation of toughness with chemical composition and tensile test results in microalloyed API pipeline steels. J. Min. Metall. 2015, 51, 173–178. [Google Scholar] [CrossRef]
- Khalaj, G.; Azimzadegan, T.; Khoeini, M.; Etaat, M. Artificial neural networks application to predict the ultimate tensile strength of X70 pipeline steels. Neural Comput. Appl. 2012, 23, 2301–2308. [Google Scholar] [CrossRef]
- Karlsson, L.; Arcini, H.; Bergquist, E.-L.; Weidow, J.; Börjesson, J. Effects of Alloying Concepts on Ferrite Morphology and Toughness of Lean Duplex Stainless Steel Weld Metals. Weld. World 2010, 54, R350–R359. [Google Scholar] [CrossRef]
- Kurt, B.; Orhan, N.; Ozel, S. Interface structure of diffusion bonded duplex stainless steel and medium carbon steel couple. Sci. Technol. Weld. Join. 2009, 12, 1035–1040. [Google Scholar] [CrossRef]
- Wang, S.; Ma, Q.; Li, Y. Characterization of microstructure, mechanical properties and corrosion resistance of dissimilar welded joint between 2205 duplex stainless steel and 16MnR. Mater. Des. 2011, 32, 831–837. [Google Scholar] [CrossRef]
- Pan, C.; Zhang, Z. Morphologies of the transition region in dissimilar austenitic-ferritic welds. Mater. Charact. 1996, 36, 5–10. [Google Scholar] [CrossRef]
- Celik, A.; Alsaran, A. Mechanical and Structural Properties of Similar and Dissimilar Steel Joints—Influence of weld metal on corrosion. Mater. Charact. 1999, 43, 311–318. [Google Scholar] [CrossRef]
- Korhonen, S. Ruostumattomat Teräkset ja Niiden Soveltuvuus Konepajan Ohutlevytuotantoon; Lutpub: Lahti, Finland, 2010. [Google Scholar]
- Outokumpu. Outokumpu Welding Handbook; Outokumpu: Helsinki, Finland, 2010. [Google Scholar]





| Parameter (Unit) | Value |
|---|---|
| Rated power (W) | 6000 |
| Wavelength (nm) | 1075–1085 |
| Divergence angle (Rad) | <0.1 |
| Operation mode | Continuous wave |
| Fiber core diameter (μm) | 100 |
| Materials | C | Si | Mn | P | S | Cr | Ni | Mo | N | Cu | Co | Nb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Base metal | 0.023 | 0.59 | 4.9 | 0.0199 | 0.001 | 21.5 | 1.62 | 0.26 | 0.21 | 0.24 | 0.025 | - |
| Filler metal | 0.012 | 0.35 | 1.59 | 0.015 | 0.001 | 22.56 | 8.62 | 3.05 | 0.15 | 0.06 | 0.049 | 0.002 |
| Tensile Strength (MPa) | Yield Strength (MPa) | Elongation (%) | Hardness HB | Impact (J) | Ferrite Content (%) | ||
|---|---|---|---|---|---|---|---|
| 25 °C | 130 °C | 25 °C | 130 °C | ||||
| 703 | 602 | 453 | 371 | 49 | 207 | 98 | 56.5 |
| Specimen No. | Layers | Focal Spot Diameter (mm) | Wire Speed (m/min) | Laser Power (kW) | Speed (m/min) | Shielding Gas and Flow Rate (L/min) |
|---|---|---|---|---|---|---|
| A | Root | 5 | 3.2 | 5000 | 0.6 | Pure Ar, 25 |
| Filling/finishing | 5 | 2.6 | 5000 | 0.6 | ||
| B | Root | 5 | 3.2 | 5000 | 0.6 | 90%Ar + 10%N2, 25 |
| Filling/finishing | 5 | 2.6 | 5000 | 0.6 | ||
| C | Root | 5 | 3.2 | 5000 | 0.6 | 50%N2 + 50%Ar, 25 |
| Filling/finishing | 5 | 2.6 | 5000 | 0.6 | ||
| D | Root | 5 | 3.2 | 5000 | 0.6 | Pure N2, 25 |
| Filling/finishing | 5 | 2.6 | 5000 | 0.6 |
| No. | Tensile Strength Rm/MPa | Yield Strength Rp0.2/MPa | Elongation/% | Fracture Location |
|---|---|---|---|---|
| A | 749 | 589 | 28.5 | BM |
| B | 721 | 581 | 30.5 | BM |
| C | 747 | 592 | 26 | BM |
| D | 748 | 586 | 26.5 | BM |
| BM | 766 | 608 | 32.5 | BM |
| Weld | Points | Fe | Cr | Ni | Mn | Mo | Si |
|---|---|---|---|---|---|---|---|
| A | 1 | 65.494 | 22.793 | 7.332 | 2.448 | 1.056 | 0.877 |
| 2 | 67.624 | 21.552 | 4.156 | 3.889 | 0.828 | 1.951 | |
| B | 1 | 66.004 | 22.154 | 7.296 | 3.058 | 0.513 | 0.975 |
| 2 | 67.786 | 21.364 | 4.514 | 3.891 | 1.447 | 0.998 | |
| C | 1 | 64.691 | 23.269 | 6.803 | 3.043 | 0.293 | 1.901 |
| 2 | 66.826 | 22.416 | 3.115 | 4.168 | 1.096 | 2.379 | |
| D | 1 | 64.574 | 23.763 | 6.771 | 2.448 | 0.785 | 1.692 |
| 2 | 67.872 | 22.310 | 3.350 | 4.196 | 1.048 | 1.224 |
| Shielding Gas | Icorr μA/cm2 | Ecorr mVSCE | ip μA/cm2 | Et mVSCE | Et − Ef mV |
|---|---|---|---|---|---|
| A | 151 | 3297 | 52 | 357 | 528 |
| B | 145 | 3056 | 50 | 348 | 521 |
| C | 74 | 2756 | 36 | 799 | 1002 |
| D | 68 | 2731 | 33 | 847 | 1010 |
| BM | 67 | 2689 | 34 | 824 | 919 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Shao, C.; Jiao, X.; Zhu, J.; Cai, Z.; Li, C. Investigation on Microstructure and Properties of Duplex Stainless Steel Welds by Underwater Laser Welding with Different Shielding Gas. Materials 2021, 14, 4774. https://doi.org/10.3390/ma14174774
Wang K, Shao C, Jiao X, Zhu J, Cai Z, Li C. Investigation on Microstructure and Properties of Duplex Stainless Steel Welds by Underwater Laser Welding with Different Shielding Gas. Materials. 2021; 14(17):4774. https://doi.org/10.3390/ma14174774
Chicago/Turabian StyleWang, Kai, Changlei Shao, Xiangdong Jiao, Jialei Zhu, Zhihai Cai, and Congwei Li. 2021. "Investigation on Microstructure and Properties of Duplex Stainless Steel Welds by Underwater Laser Welding with Different Shielding Gas" Materials 14, no. 17: 4774. https://doi.org/10.3390/ma14174774






