Low-Temperature Deposition of Transparent Conducting Films Applied to Flexible Electrochromic Devices
Abstract
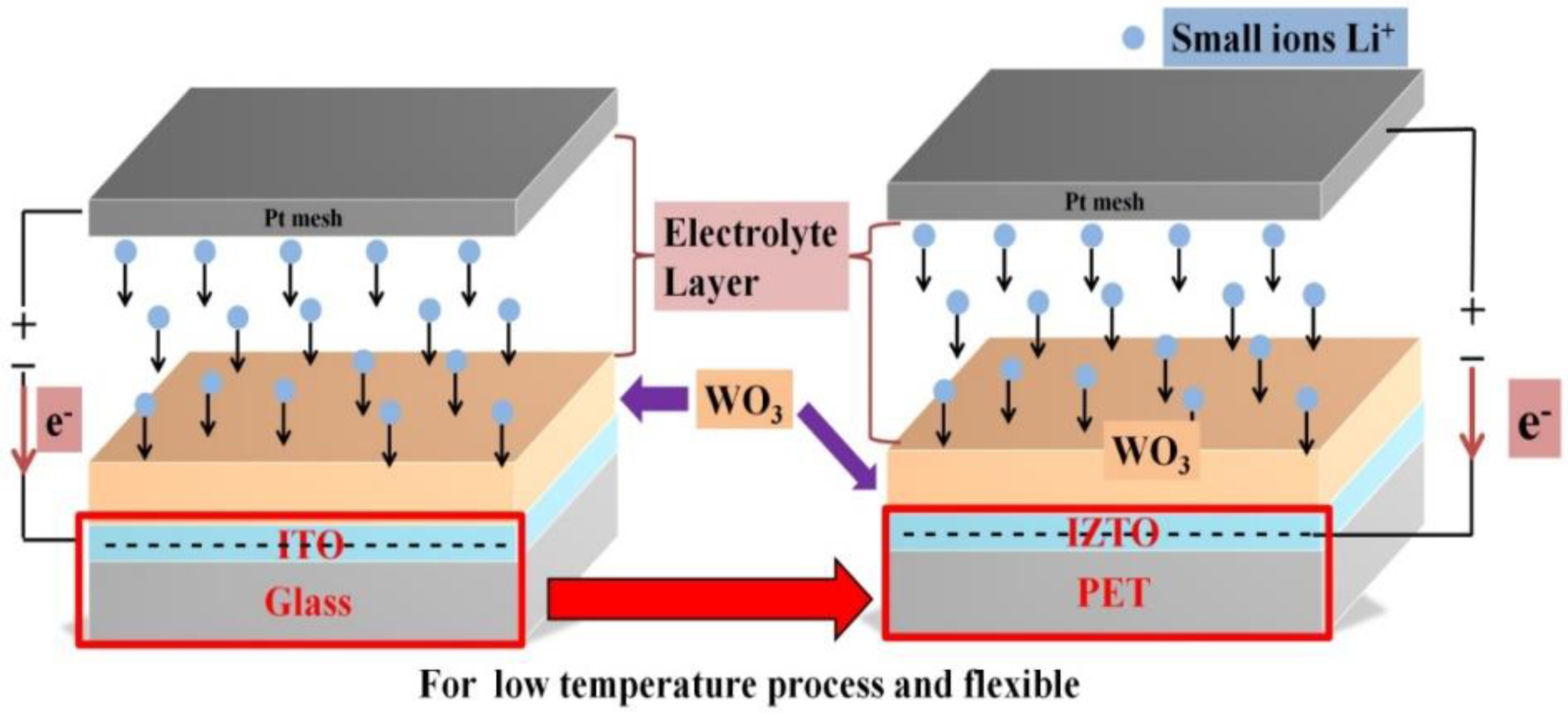
:1. Introduction
2. Experimental Methods
2.1. Methods
2.2. Characterization
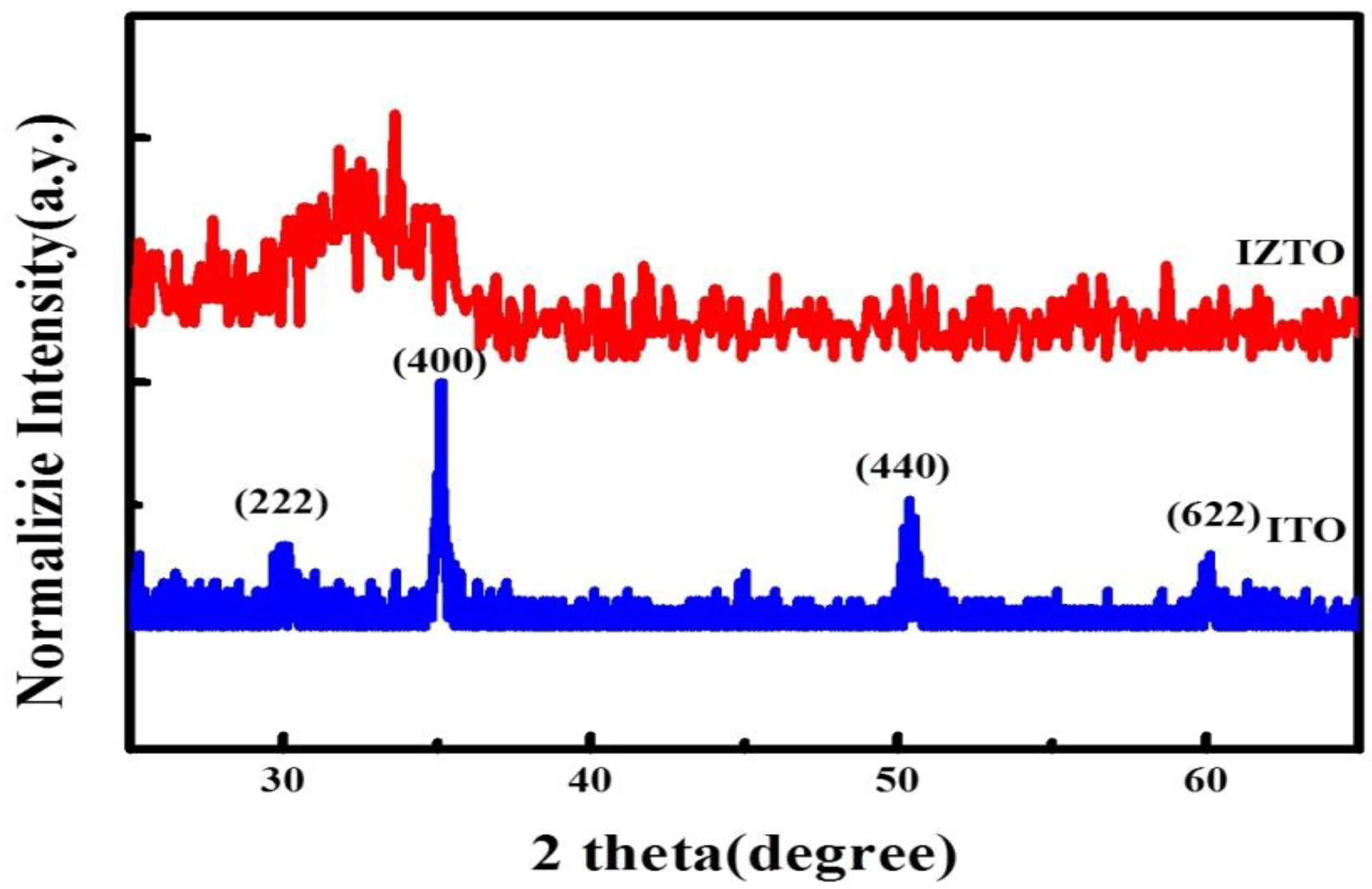
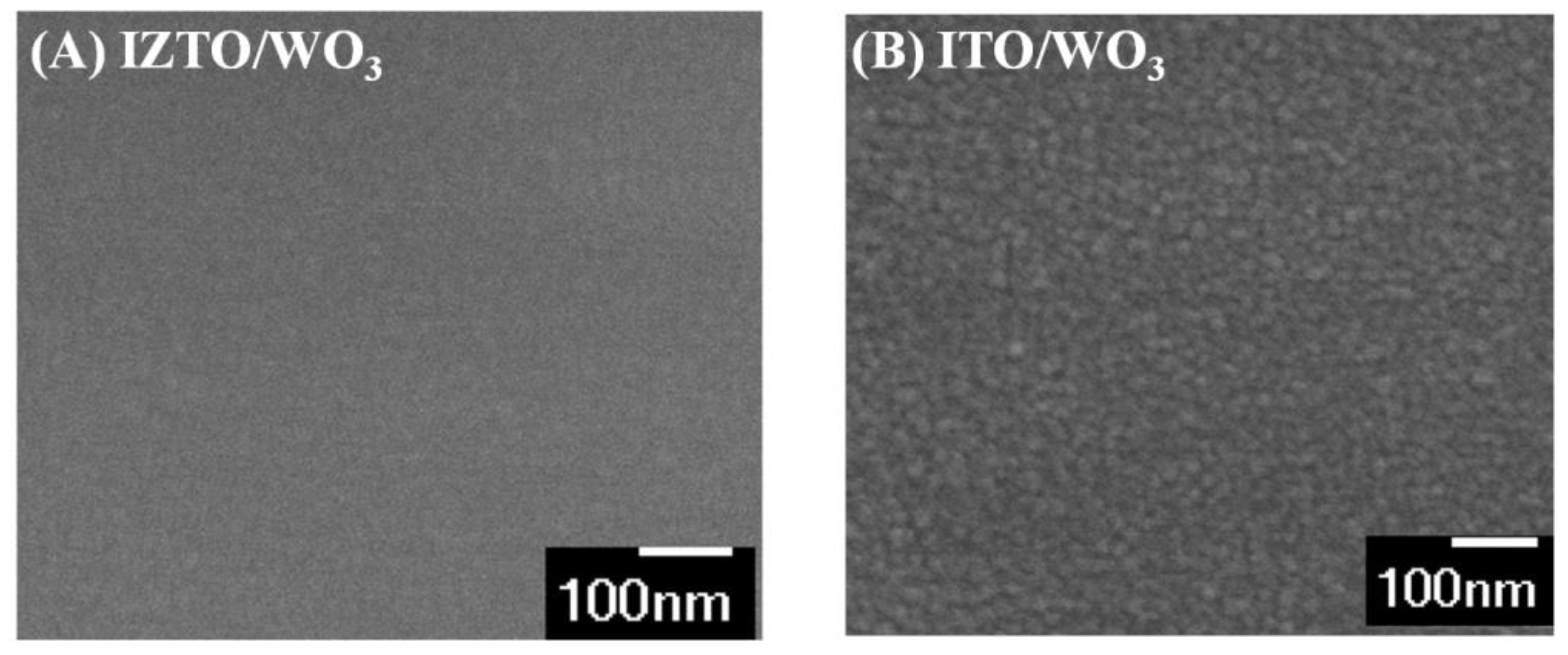
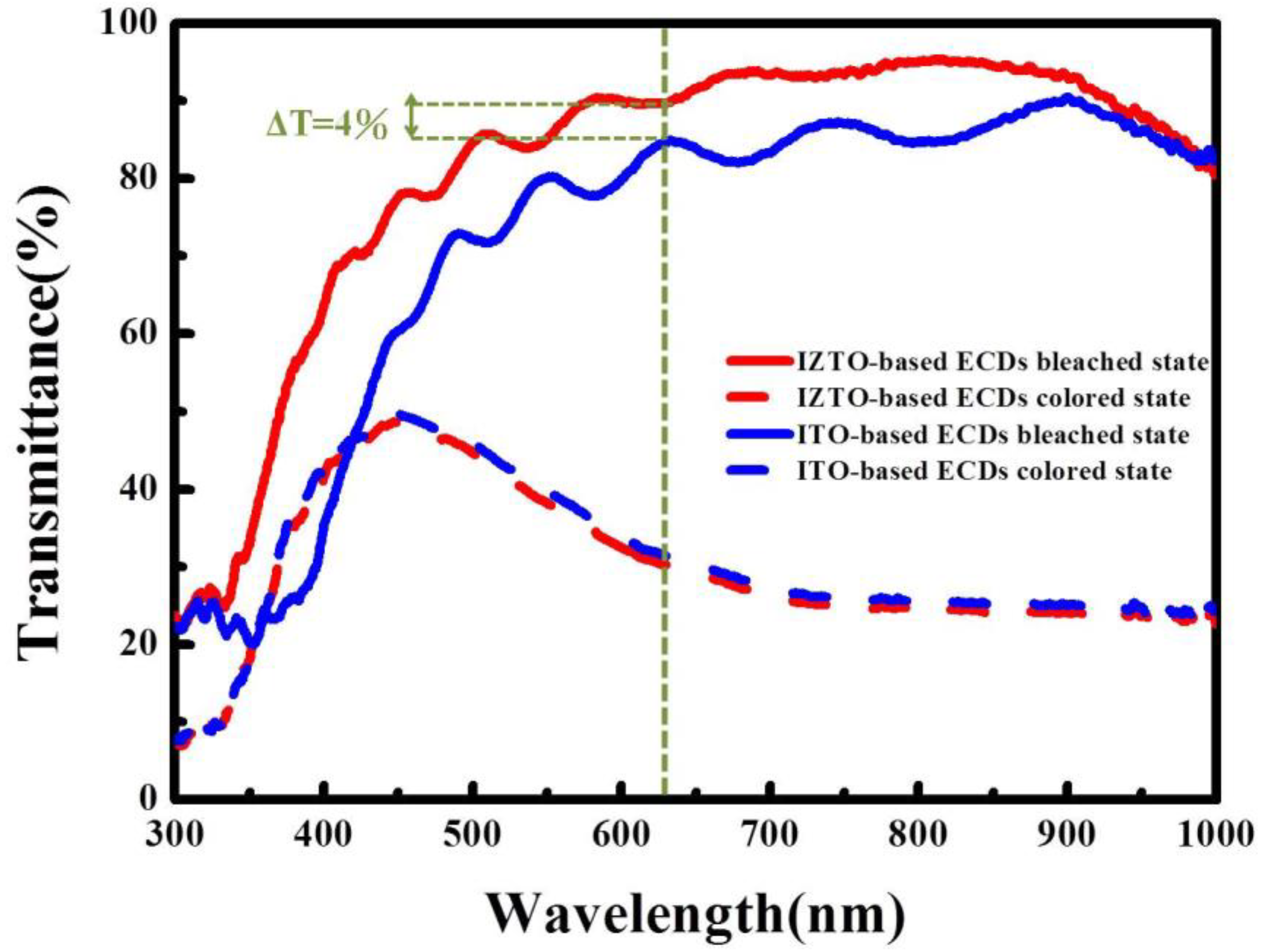
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Granqvist, C.G.; Arvizu, M.A.; Pehlivan, I.B.; Qu, H.Y.; Wen, R.T.; Niklasson, G.A. Electrochromic materials and devices for energy efficiency and human comfort in buildings: A critical review. Electrochim. Acta 2018, 259, 1170. [Google Scholar] [CrossRef] [Green Version]
- Sami, O.; Cedric, G.G.; Christophe, D.; Cedric, D.; Raphael, S. All inorganic thin film electrochromic device using LiPON as the ion conductor. Sol. Energy Mater. Sol. Cells 2016, 145, 2. [Google Scholar]
- Corrente, G.A.; Cospito, S.; Capodilupo, A.L.; Beneduci, A. Mixed-Valence Compounds as a New Route for Electrochromic Devices with High Coloration Efficiency in the Whole Vis-NIR Region. Appl. Sci. 2020, 10, 8372. [Google Scholar] [CrossRef]
- Vidotti, M.; Cordoba de Torresi, S.I. Electrostatic layer-by-layer and electrophoretic depositions as methods for electrochromic nanoparticle immobilization. Electrochim. Acta 2009, 54, 2800–2804. [Google Scholar] [CrossRef]
- Bouessay, I.; Rougier, A.; Poizot, P.; Moscovici, J.; Michalowicz, A.; Tarascon, J.-M. Electrochromic degradation in nickel oxide thin film: A self-discharge and dissolution phenomenon. Electrochim. Acta 2005, 50, 3737–3745. [Google Scholar] [CrossRef]
- Watanabe, Y.; Imaizumi, K.; Nakamura, K.; Kobayashi, N. Effect of counter electrode reaction on coloration properties of phthalate-based electrochromic cell. Sol. Energy Mater. Sol. Cells 2011, 99, 88–94. [Google Scholar] [CrossRef]
- Kobayashi, T.; Yoneyama, H.; Tamura, H. Polyaniline film-coated electrodes as electrochromic display devices. J. Electroanal. Chem. 1984, 161, 419–423. [Google Scholar] [CrossRef]
- Silva, A.J.C.; Ferreira, S.M.; Santos, D.D.P.; Navarro, M.; Tonholo, J.; Ribeiro, A.S. A multielectrochromic copolymer based on pyrrole and thiophene derivatives. Sol. Energy Mater. Sol. Cells 2012, 103, 108–113. [Google Scholar] [CrossRef]
- Augusto, T.; Neto, É.T.; Neto, Â.A.T.; Vichessi, R.; Vidotti, M.; de Torresi, S.I.C. Electrophoretic deposition of Au@PEDOT nanoparticles towards the construction of high-performance electrochromic electrodes. Sol. Energy Mater. Sol. Cells 2013, 118, 72–80. [Google Scholar] [CrossRef]
- Granqvist, C.G. Electrochromics for smart windows: Oxide-based thin films and devices. Thin Solid Films 2014, 564, 1. [Google Scholar] [CrossRef]
- Phan, G.T.; Pham, D.V.; Patil, R.A.; Tsai, C.H.; Lai, C.C.; Yeh, W.C.; Liou, Y.; Ma, Y.R. Fast-switching electrochromic smart windows based on NiO-nanorods counter electrode. Sol. Energy Mater. Sol. Cells 2021, 231, 111306. [Google Scholar] [CrossRef]
- Zhang, W.; Li, H.; Yu, W.W.; Elezzabi, A.Y. Excitonic complexes and optical gain in two-dimensional molybdenum ditelluride well below the Mott transition. Light Sci. Appl. 2020, 9, 121. [Google Scholar] [CrossRef]
- Lee, C.; Oh, Y.; Yoon, I.S.; Kim, S.H.; Ju, B.K.; Hong, J.M. Flash-induced nanowelding of silver nanowire networks for transparent stretchable electrochromic devices. Sci. Rep. 2018, 8, 2763. [Google Scholar] [CrossRef] [Green Version]
- Cai, G.; Darmawan, P.; Cui, M.; Chen, J.; Wang, X.; Eh, A.L.S.; Magdassi, S.; Lee, P.S. Inkjet-printed all solid-state electrochromic devices based on NiO/WO3 nanoparticle complementary electrodes. Nanoscale 2016, 8, 348. [Google Scholar] [CrossRef]
- Teissier, A.; Dubon, J.P.; Aubert, P.H.; Vidal, F.; Remaury, S.; Crouzet, J.; Chevrot, C. Feasibility of conducting semi-IPN with variable electro-emissivity: A promising way for spacecraft thermal control. Sol. Energy Mater. Sol. Cells 2012, 99, 116. [Google Scholar] [CrossRef]
- Yan, C.; Kang, W.; Wang, J.; Cui, M.; Wang, X.; Foo, C.Y.; Chee, K.J.; Lee, P.S. Stretchable and wearable electrochromic devices. ACS Nano 2014, 8, 316. [Google Scholar] [CrossRef]
- Danine, A.; Cojocaru, L.; Faure, C.; Olivier, C.; Toupance, T.; Campet, G.; Rougier, A. Room Temperature UV treated WO3 thin films for electrochromic devices on paper substrate. Electrochim. Acta 2014, 129, 113. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, Y.; Tang, Z.; He, L.; Liu, X. Highly stable and flexible ITO-free electrochromic films with bi-functional stacked MoO3/Ag/MoO3 structures. Electrochim. Acta 2016, 189, 184. [Google Scholar] [CrossRef]
- Macher, S.; Schott, M.; Sassi, M.; Facchinetti, I.; Ruffo, R.; Patriarca, G.; Beverina, L.; Posset, U.; Giffin, G.A.; Löbmann, P. New roll-to-roll processable PEDOT-based polymer with colorless bleached state for flexible electrochromic devices. Adv. Funct. Mater. 2020, 30, 1906254. [Google Scholar] [CrossRef]
- Li, X.; Perera, K.; He, J.; Gumyusenge, A.; Mei, J. Solution-processable electrochromic materials and devices: Roadblocks and strategies towards large-scale applications. J. Mater. Chem. C 2019, 7, 12761. [Google Scholar] [CrossRef]
- Chang, C.C.; Chi, P.W.; Chandan, P.; Lin, C.K. Electrochemistry and rapid electrochromism control of MoO3/V2O5 hybrid nanobilayers. Nat. Mater. 2019, 12, 2475. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.F.; Chiou, B.S. Properties of radio-frequency magnetron sputtered ITO films without in-situ substrate heating and post-deposition annealing. Thin Solid Films 1994, 247, 201. [Google Scholar] [CrossRef]
- Yu, W.; Shen, L.; Meng, F.; Long, Y.; Ruan, S.; Chen, W. Effects of the optical microcavity on the performance of ITO-free polymer solar cells with WO3/Ag/WO3 transparent electrode. Sol. Energy Mater. Sol. Cells 2012, 100, 226. [Google Scholar] [CrossRef]
- Seo, S.J.; Choi, C.G.; Hwang, Y.H.; Bae, B.S. High performance solution-processed amorphous zinc tin oxide thin film transistor. J. Phys. D Appl. Phys. 2009, 42, 035106. [Google Scholar] [CrossRef]
- Arai, T. Oxide-TFT technologies for next-generation AMOLED displays. J. Soc. Inf. Disp. 2012, 20, 156. [Google Scholar] [CrossRef]
- Ko, Y.D.; Kim, Y.S. Room temperature deposition of IZTO transparent anode films for organic light-emitting diodes. Mater. Res. Bull. 2012, 47, 2800. [Google Scholar] [CrossRef]
- Choi, K.H.; Jeong, J.A.; Kim, H.K. Dependence of electrical, optical, and structural properties on the thickness of IZTO thin films grown by linear facing target sputtering for organic solar cells. Sol. Energy Mater. Sol. Cells 2010, 94, 1822. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, G.; Xiao, Y.; Gao, F.; Wang, M.; Wang, Q.; Wang, S.; Zuo, H.; Diao, X. An all-thin-film inorganic electrochromic device monolithically fabricated on flexible PET/ITO substrate by magnetron sputtering. Mater. Lett. 2015, 142, 232. [Google Scholar] [CrossRef]
- Grosfil, P.; Lutsko, J.F. Impact of Surface Roughness on Crystal Nucleation. Crystals 2021, 11, 4. [Google Scholar] [CrossRef]
- Bae, J.H.; Moon, J.M.; Jeong, S.W.; Kim, J.J.; Kang, J.W.; Kim, D.G.; Kim, J.K.; Park, J.W.; Kim, H.K. Transparent conducting indium zinc tin oxide anode for highly efficient phosphorescent organic light emitting diodes. J. Electrochem. Soc. 2008, 155, J1. [Google Scholar] [CrossRef]
- Chen, P.W.; Chang, C.T.; Ko, T.F.; Hsu, S.C.; Li, K.D.; Wu, J.Y. Fast response of complementary electrochromic device based on WO 3 /NiO electrodes. Sci. Rep. 2020, 10, 8430. [Google Scholar] [CrossRef]
- Li, K.D.; Chen, P.W.; Chang, K.S.; Hsu, S.C.; Jan, D.J. Indium-Zinc-Tin-Oxide Film Prepared by Reactive Magnetron Sputtering for Electrochromic Applications. Materials 2018, 11, 2221. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Barnabé, A.; Thimont, Y.; Wang, J.; He, Y.; Liu, Q.; Zhong, X.; Dong, G.; Yang, J.; Diao, X. Optimized properties of innovative electrochromic device using ITO/Ag/ITO electrodes. Electrochim. Acta 2019, 301, 200. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Cheong, H.M.; Tracy, C.E.; Mascarenhas, A. Alternating current impedance and Raman spectroscopic study on electrochromic a-WO3 Films. Appl. Phys. Lett. 2000, 76, 3908–3910. [Google Scholar] [CrossRef]
- Lee, S.H.; Deshpande, R.; Parilla, P.; Jones, K.; To, B.; Mahan, A.; Dillon, A.C. Crystalline WO3 Nanoparticles for Highly Improved Electrochromic Applications. Adv. Mater. 2006, 18, 763–766. [Google Scholar] [CrossRef]
- Ding, J.; Abbas, S.A.; Hanmandlu, C.; Lin, L.; Lai, C.S.; Wang, P.C.; Li, L.J.; Chu, C.W.; Chang, C.C. Facile synthesis of carbon/MoO3 nanocomposites as stable battery anodes. J. Power Sources 2017, 348, 270. [Google Scholar] [CrossRef]
- Cai, G.F.; Tu, J.P.; Gu, C.D.; Zhang, J.H.; Chen, J.; Zhou, D.; Shi, S.J.; Wang, X.L. One-step fabrication of nanostructured NiO films from deep eutectic solvent with enhanced electrochromic performance. J. Mater. Chem. A 2013, 1, 4286. [Google Scholar] [CrossRef]
- SAbbas, A.; Ibrahem, M.A.; Hu, L.H.; Lin, C.N.; Fang, J.; Boopathi, K.M.; Wang, P.C.; Li, L.J.; Chu, C.W. Bifunctional separator as a polysulfide mediator for highly stable Li–S batteries. J. Mater. Chem. A 2016, 4, 9661. [Google Scholar]
- Liang, L.; Zhang, J.; Zhou, Y.; Xie, J.; Zhang, X.; Guan, M.; Pan, B.; Xie, Y. High-performance flexible electrochromic device based on facile semiconductor-to-metal transition realized by WO3 2H2O ultrathin nanosheets. Sci. Rep. 2013, 3, 1936. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.W.; Chang, C.T.; Ali, M.M.; Wu, J.Y.; Li, Y.C.; Chen, M.H.; Jen, D.J.; Yuan, C.T. Tantalum oxide film deposited by vacuum cathodic arc plasma with improved electrochromic performance. Sol. Energy Mater. Sol. Cells 2018, 182, 188. [Google Scholar] [CrossRef]

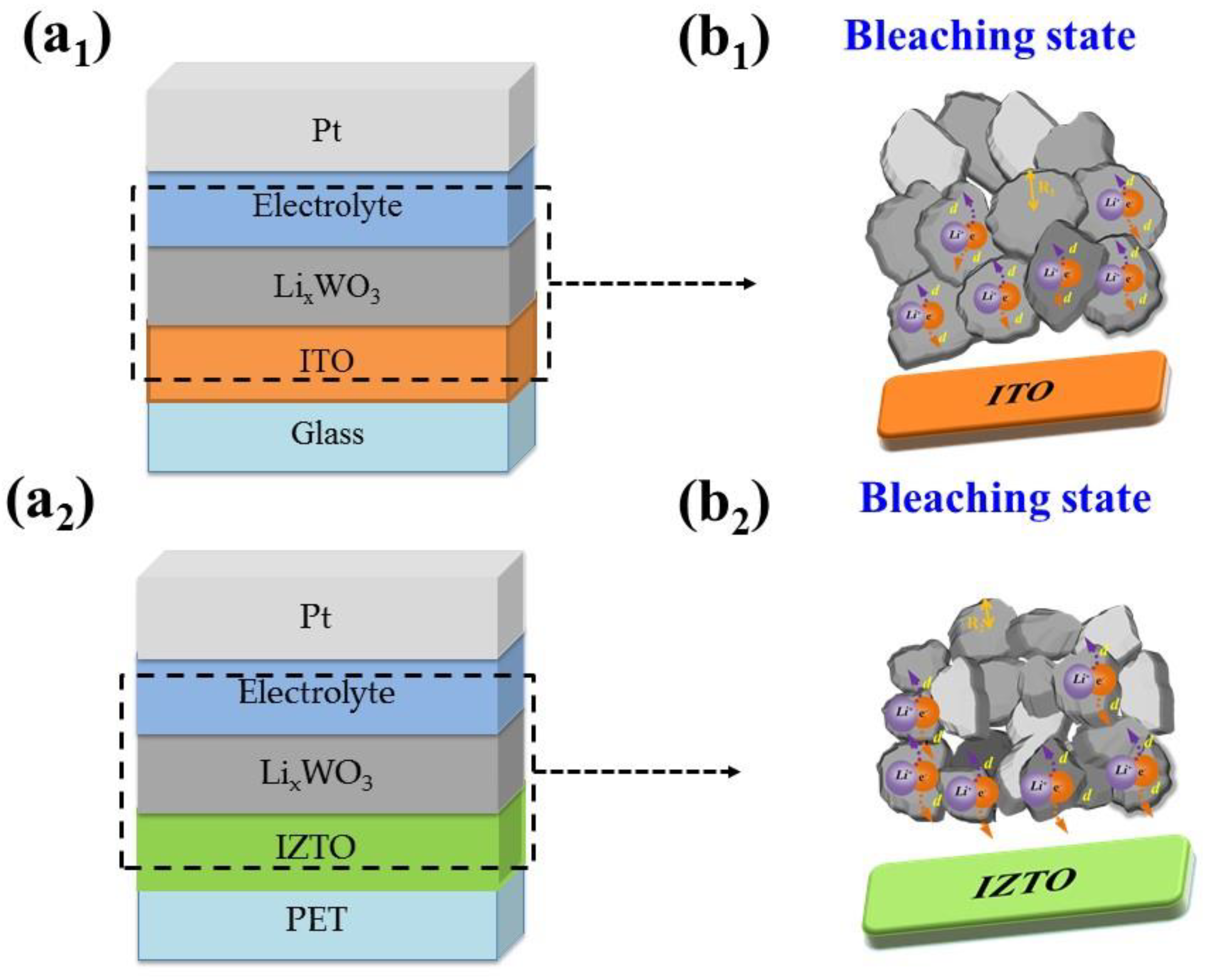
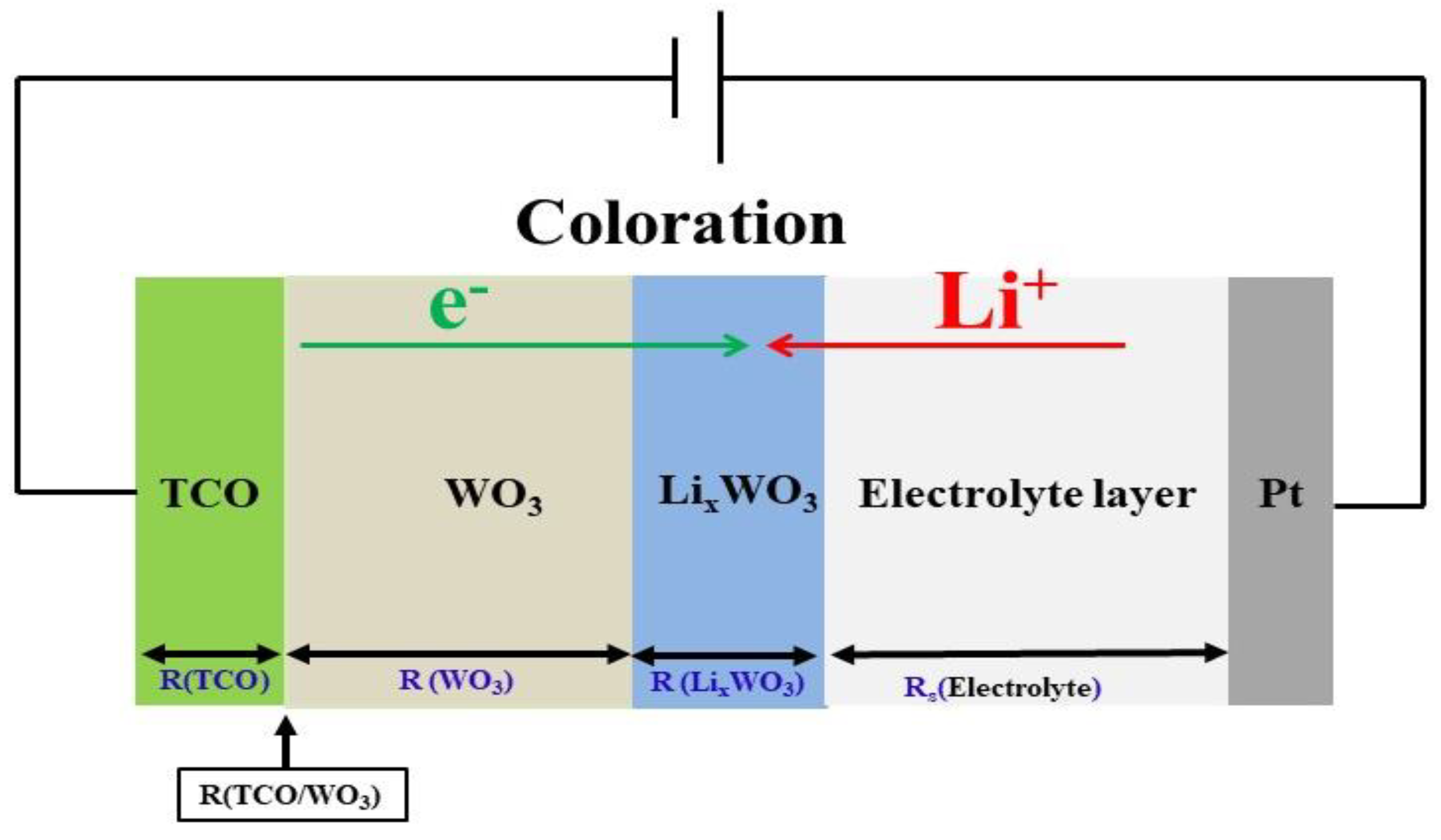

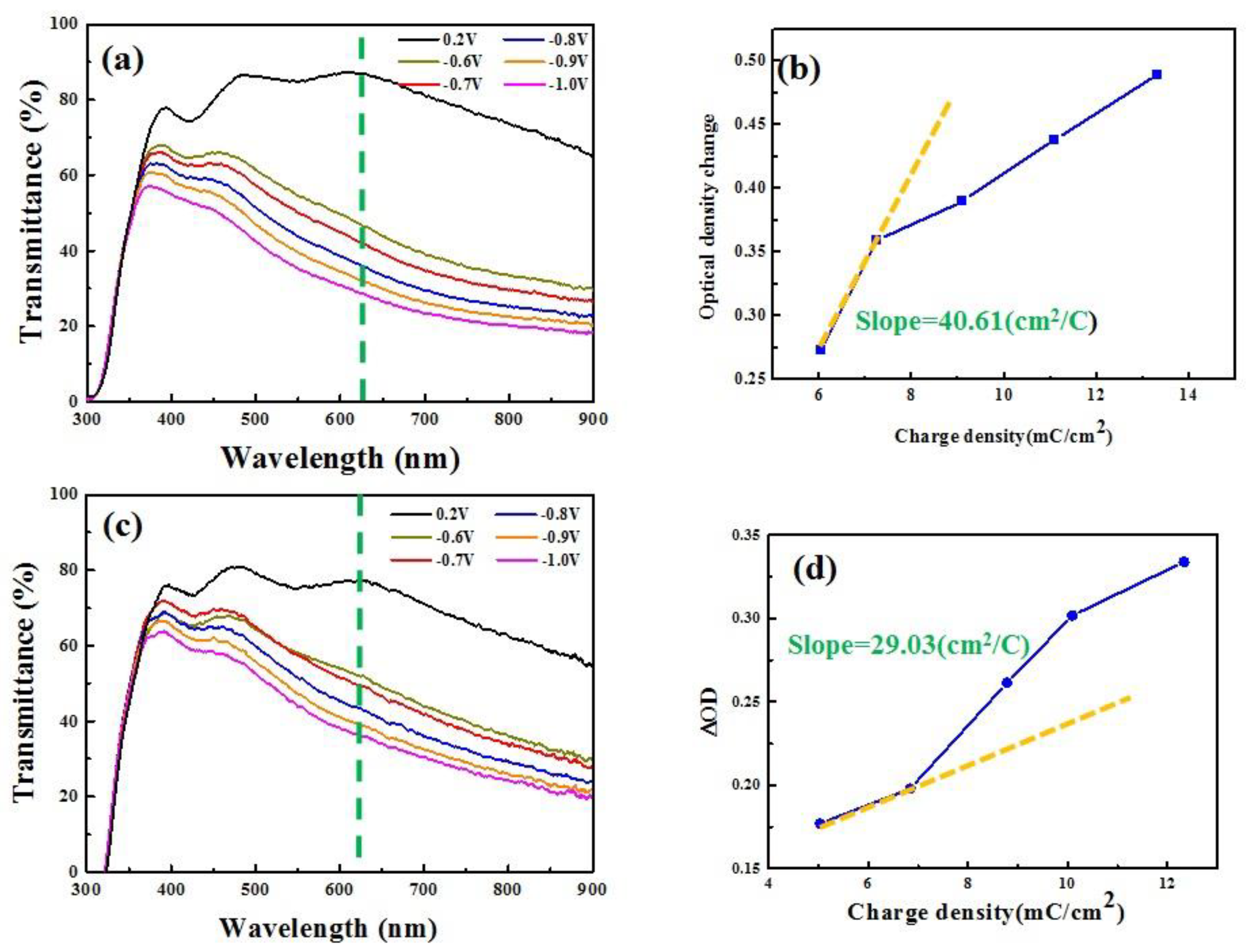

| Processing | Working Pres. (Pa) | Base Pres. (Pa) | Ar (sccm) | DC Power (W) | Thickness (nm) | Deposition Temp. (°C) |
|---|---|---|---|---|---|---|
| IZTO | 0.13 | 9.33 × 10−4 | 30 | 100 | 800 | RT |
| ITO | 0.13 | 9.33 × 10−4 | 30 | 100 | 800 | 230 |
| Target | Working Pres. (Pa) | Base Pres. (Pa) | Ar/O2 (sccm) | Power (W) | Thickness (nm) | Deposition Temp. (°C) | Deposition Rate (nm/min) |
|---|---|---|---|---|---|---|---|
| Metal W | 2.7 | 1.3 × 10−3 | 0.2 | 1350 | 220 | RT | 14.67 |
| Processing | Transmittance (%) | Sheet Resistance (Ω/□) | Electrical Resistivity (Ω·cm) | Mobility (cm2·V−1) | Carrier Concentration (cm−3) | Figure of Merit (Ω−1) |
|---|---|---|---|---|---|---|
| IZTO | 86.8 | 7.8 | 6.2 × 10−4 | 6.622 | 8.40 × 1020 | 31.1 × 10−3 |
| ITO | 88.4 | 7.2 | 5.7 × 10−4 | 2.95 | 2.08 × 1021 | 40 × 10−3 |
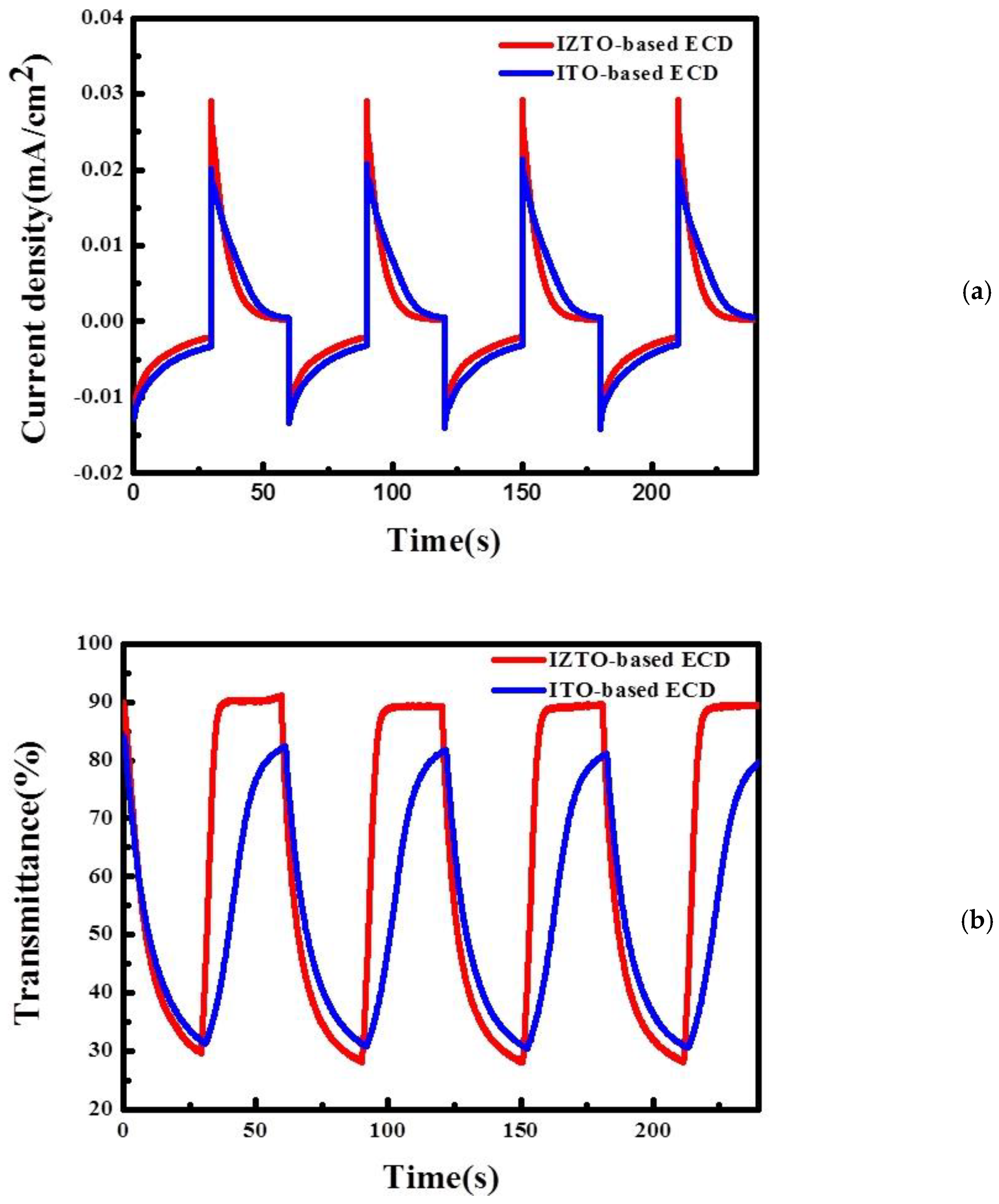
| Samples | Bleaching Time, tb (s) | Coloration Time, tc (s) |
|---|---|---|
| ITO based ECDs | 22.32 | 20.13 |
| IZTO based ECDs | 4.7 | 21.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.-D.; Chen, P.-W.; Chang, K.-S. Low-Temperature Deposition of Transparent Conducting Films Applied to Flexible Electrochromic Devices. Materials 2021, 14, 4959. https://doi.org/10.3390/ma14174959
Li K-D, Chen P-W, Chang K-S. Low-Temperature Deposition of Transparent Conducting Films Applied to Flexible Electrochromic Devices. Materials. 2021; 14(17):4959. https://doi.org/10.3390/ma14174959
Chicago/Turabian StyleLi, Ke-Ding, Po-Wen Chen, and Kao-Shuo Chang. 2021. "Low-Temperature Deposition of Transparent Conducting Films Applied to Flexible Electrochromic Devices" Materials 14, no. 17: 4959. https://doi.org/10.3390/ma14174959




