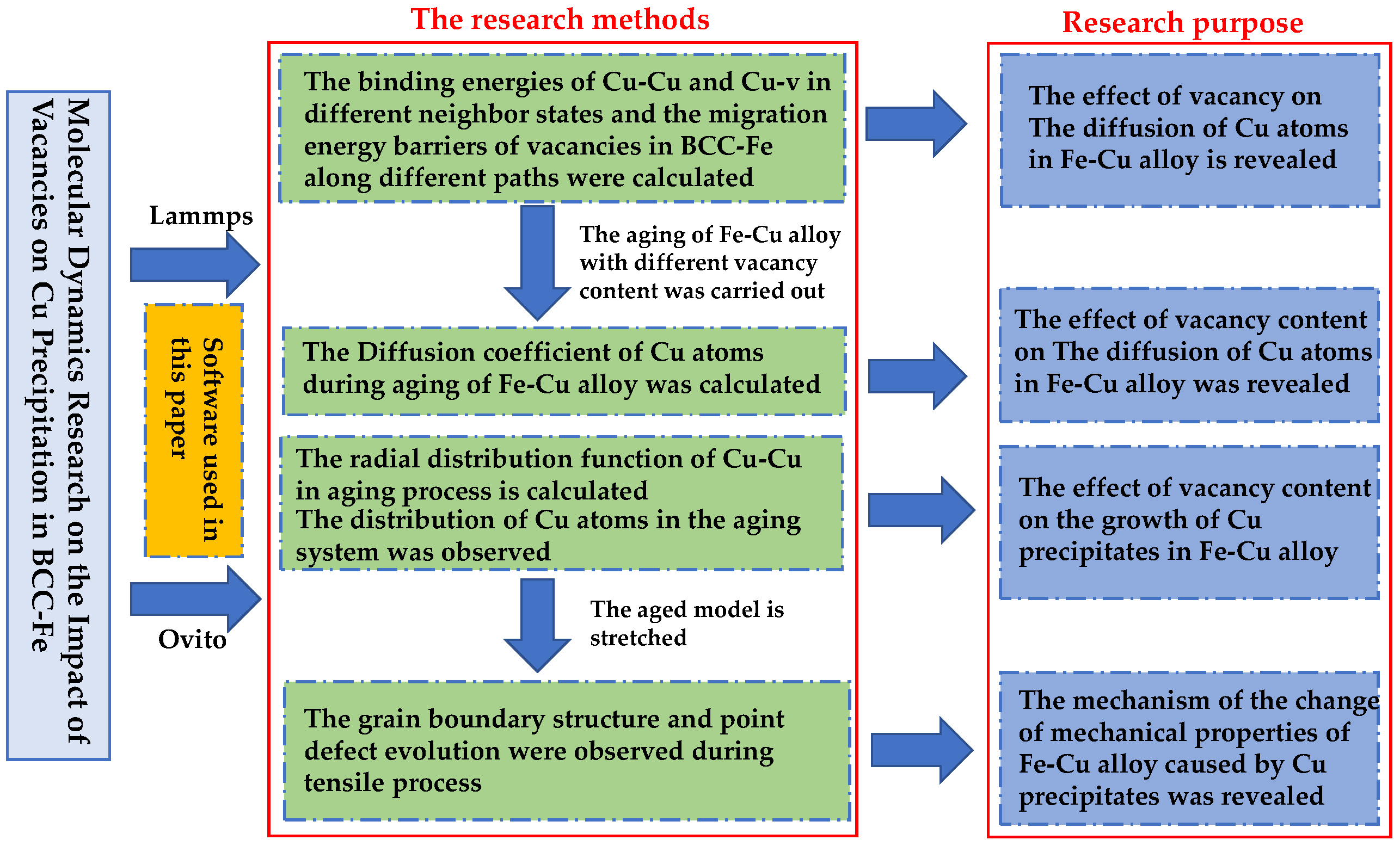
Molecular Dynamics Research on the Impact of Vacancies on Cu Precipitation in BCC-Fe
Abstract
:1. Introduction
2. Simulation Method
3. Results and Discussion
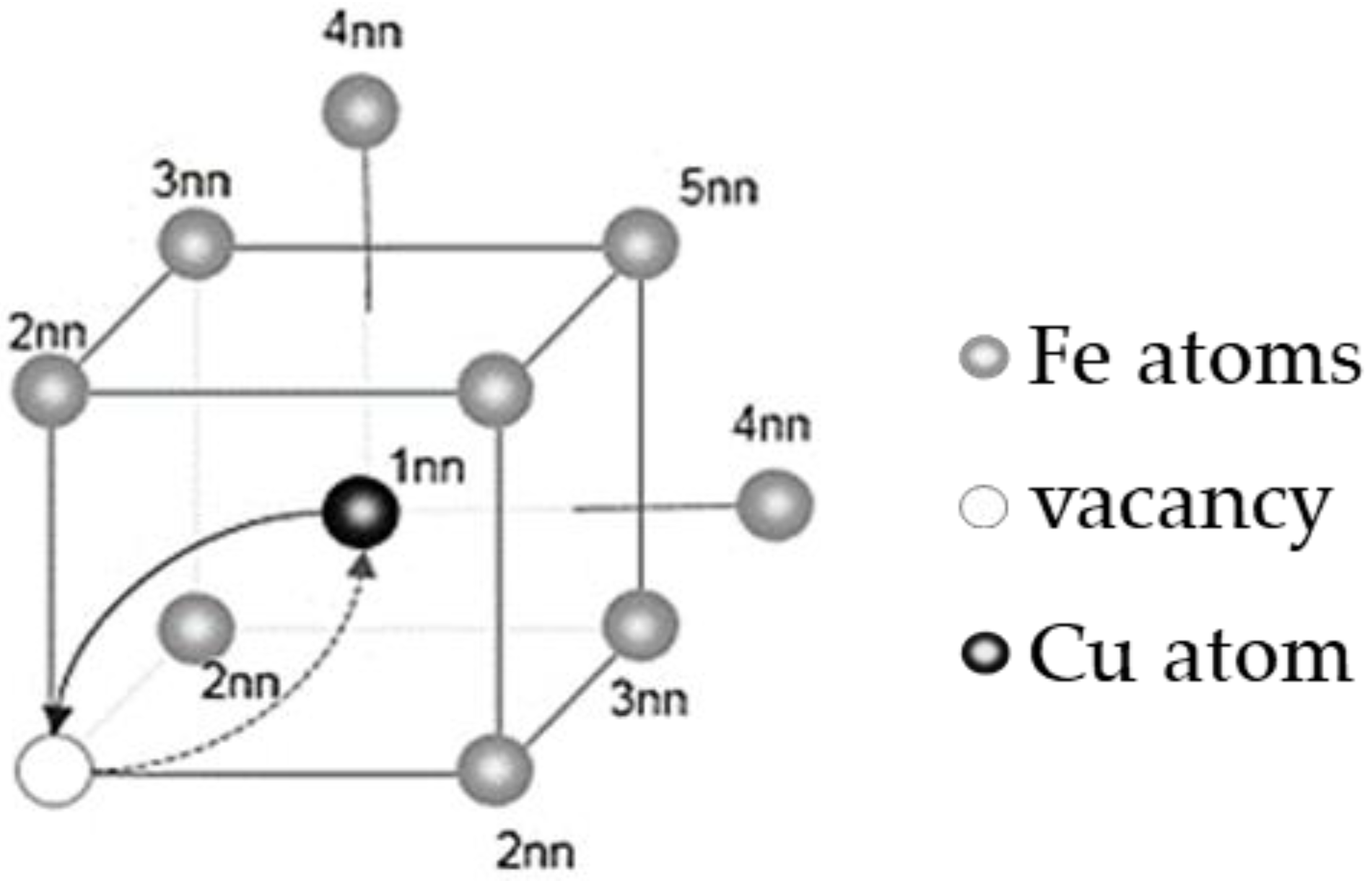
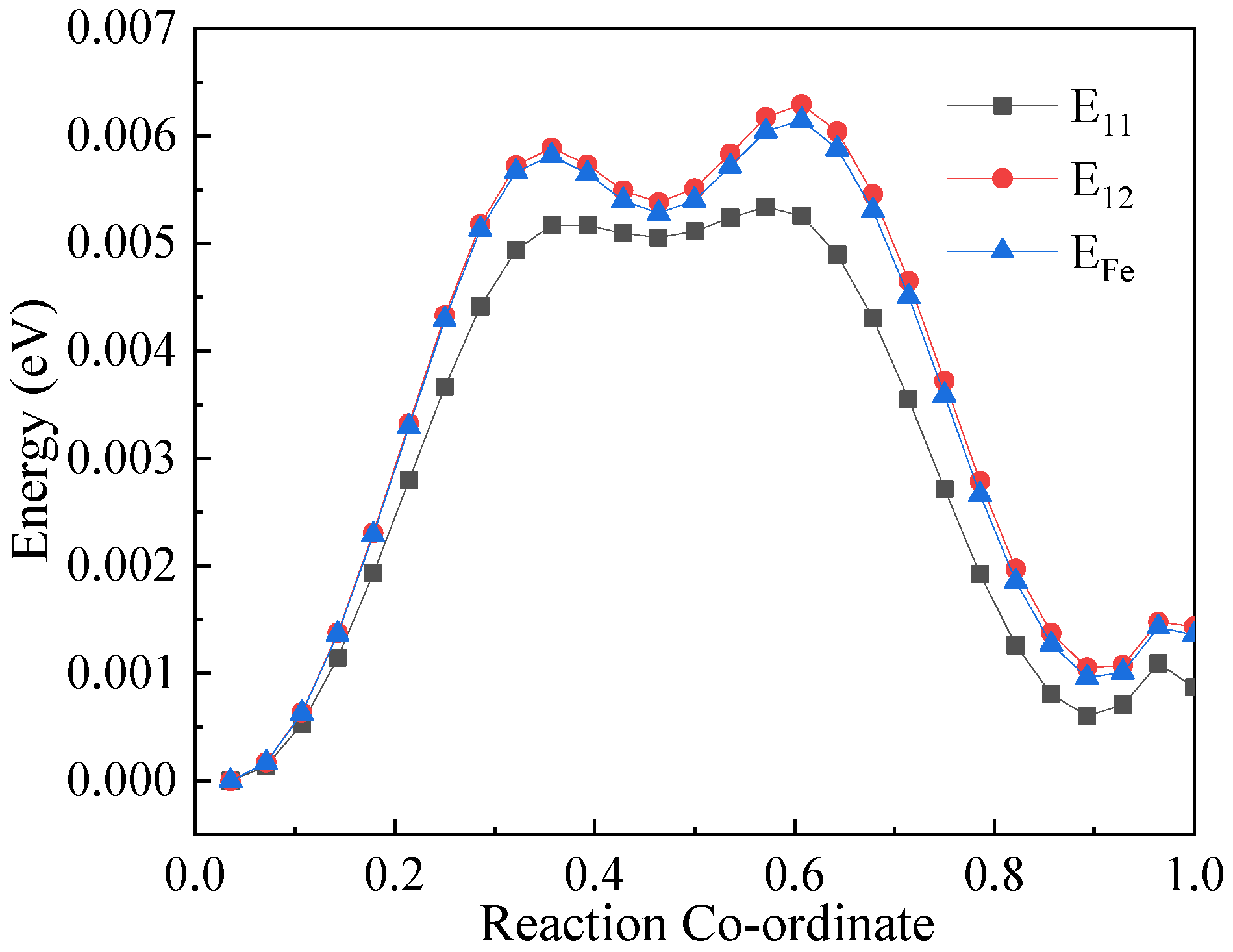
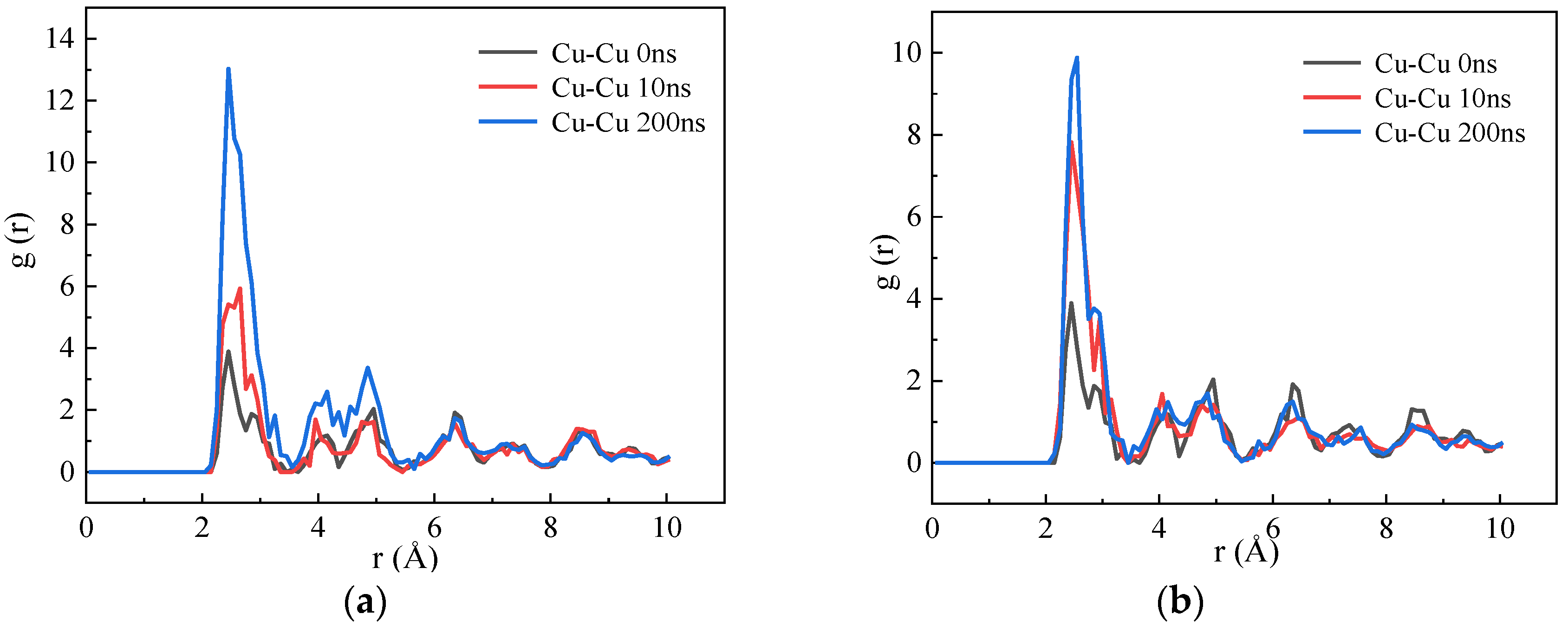
3.1. Calculation of the Binding Energy of Cu–Cu and Cu–V and the Migration Energy Barrier of Vacancies
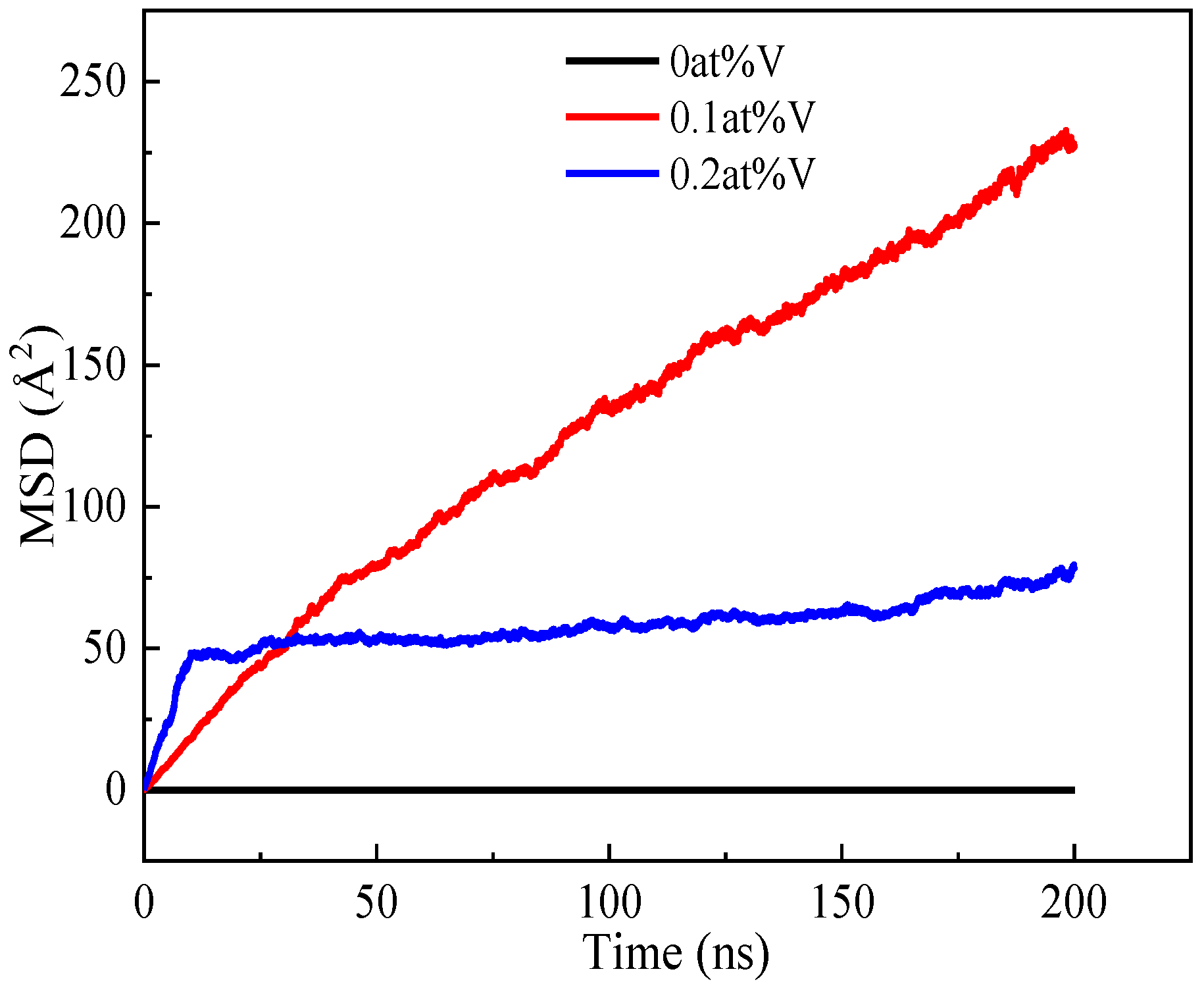
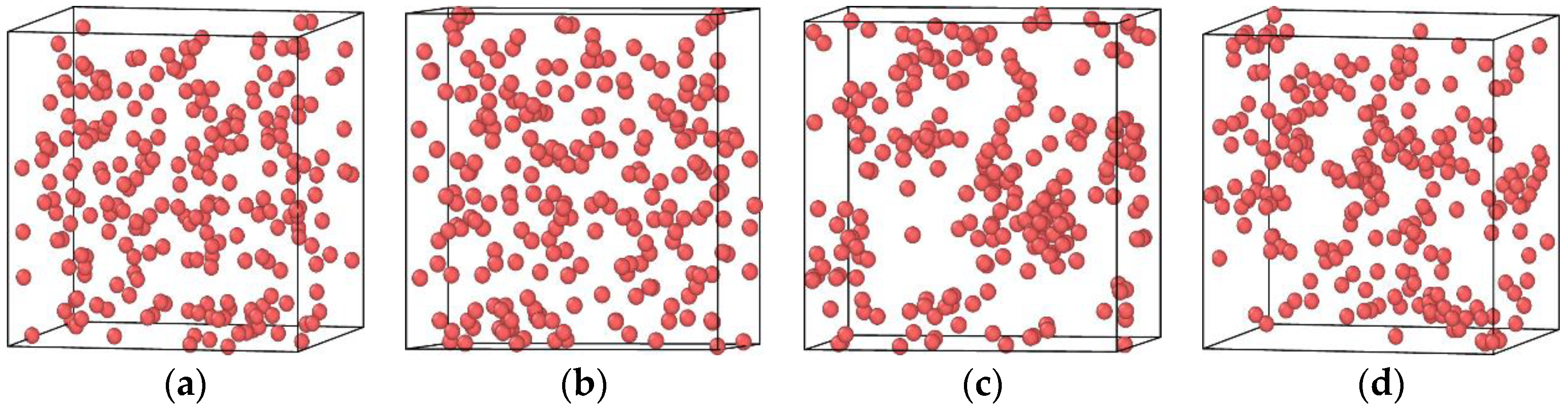
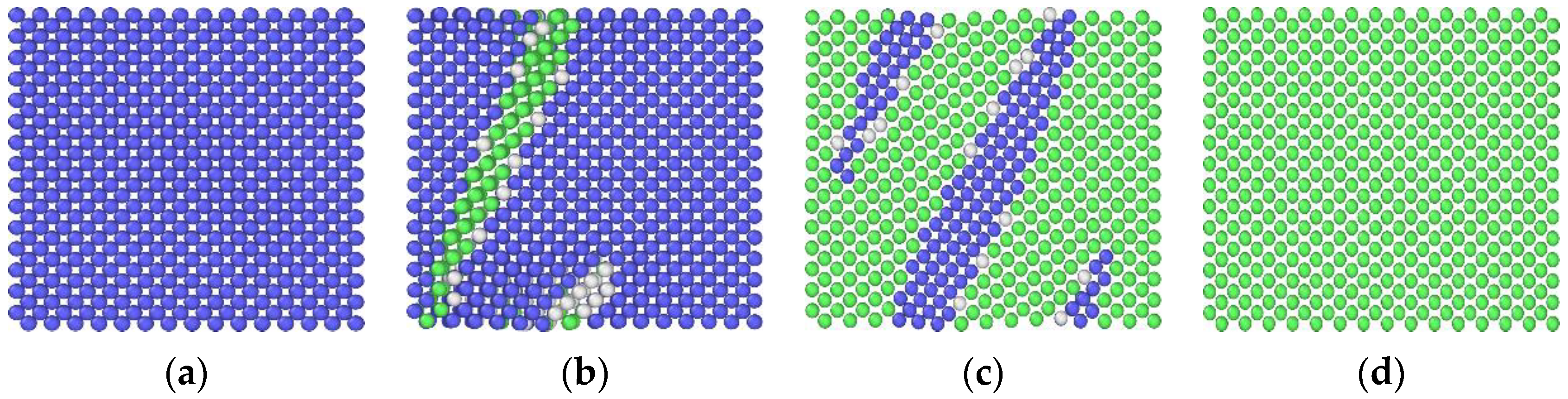
3.2. The Effect of the Concentration of Vacancies on the Diffusion and Precipitation of Cu Atoms in BCC-Fe
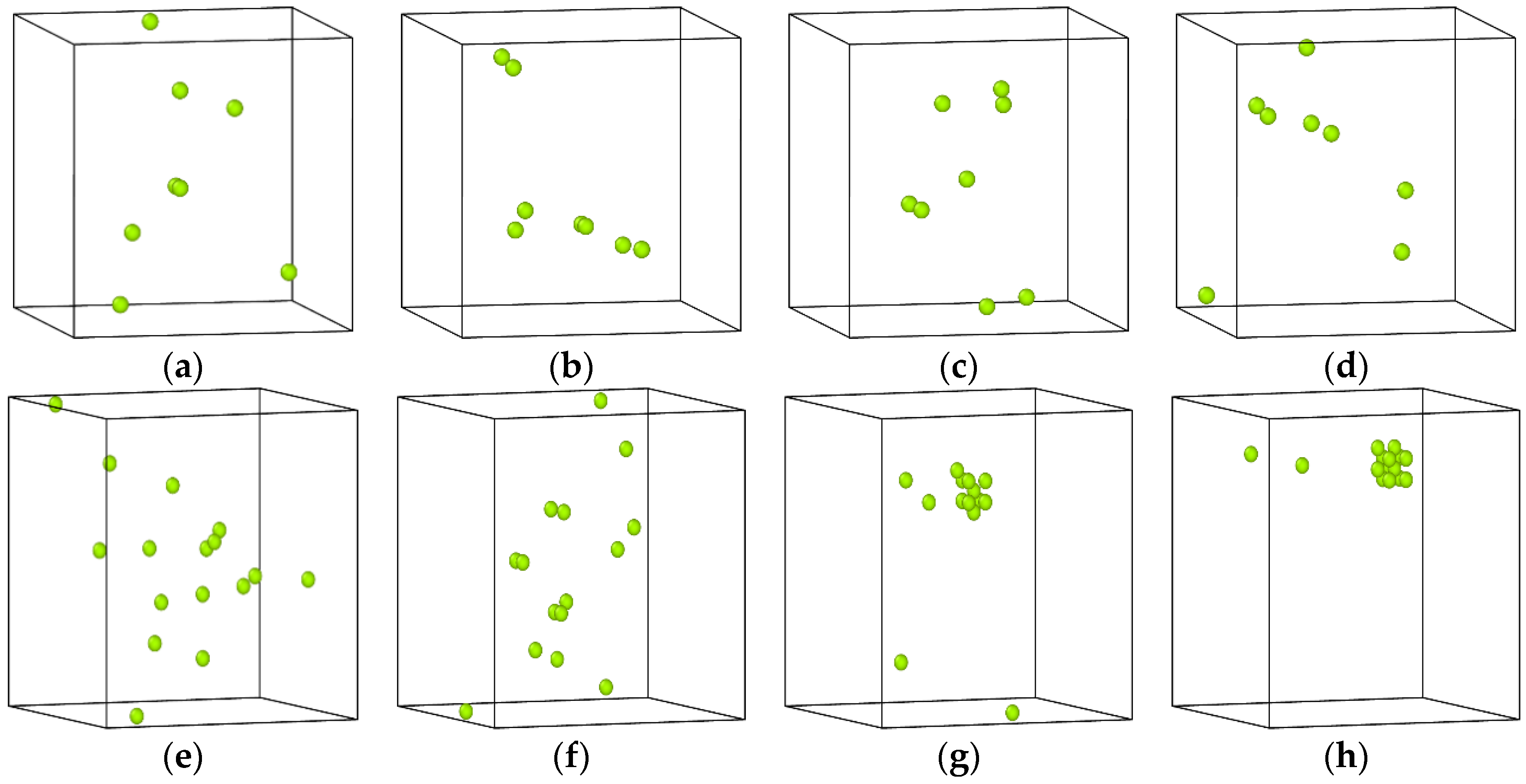
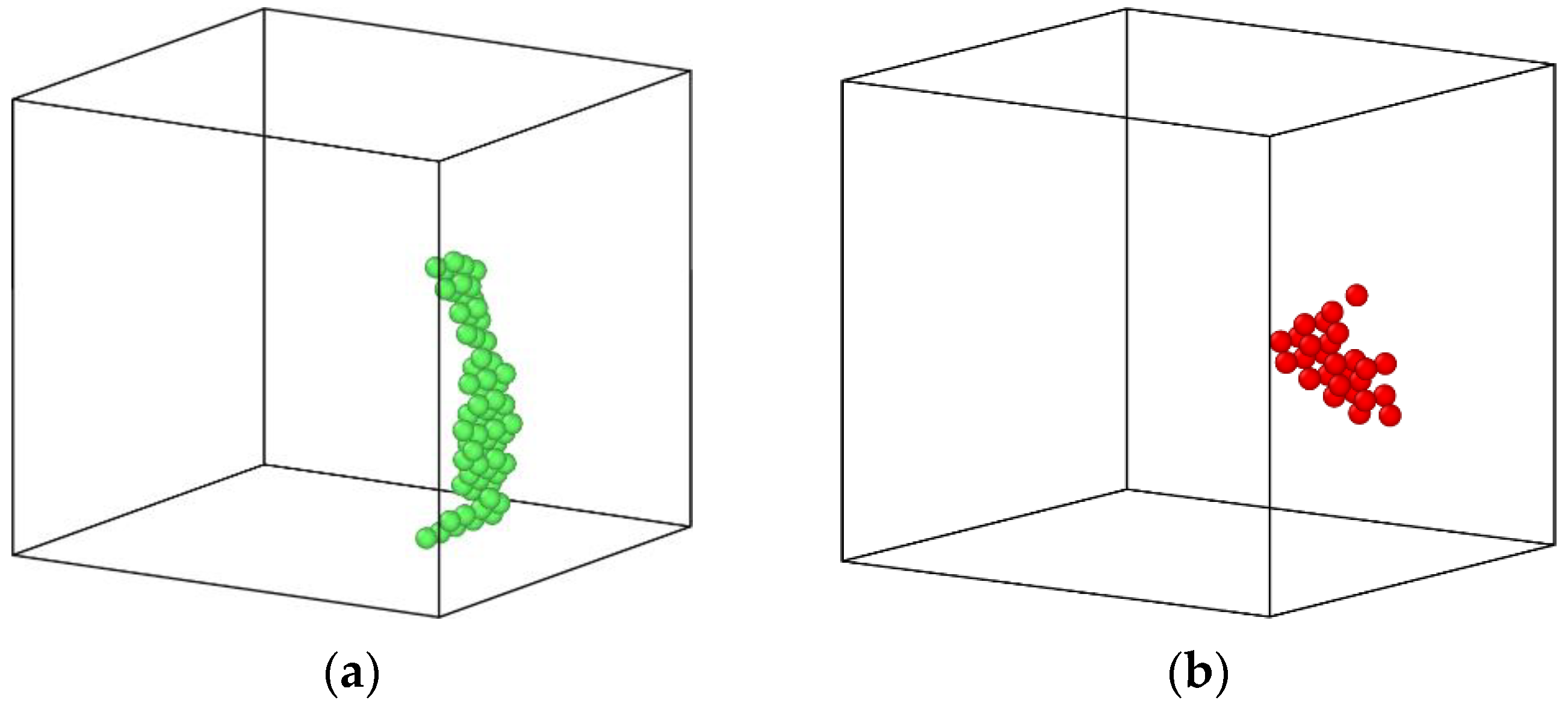
3.3. The Impact of Vacancy Concentration on the Growth of Cu Precipitates
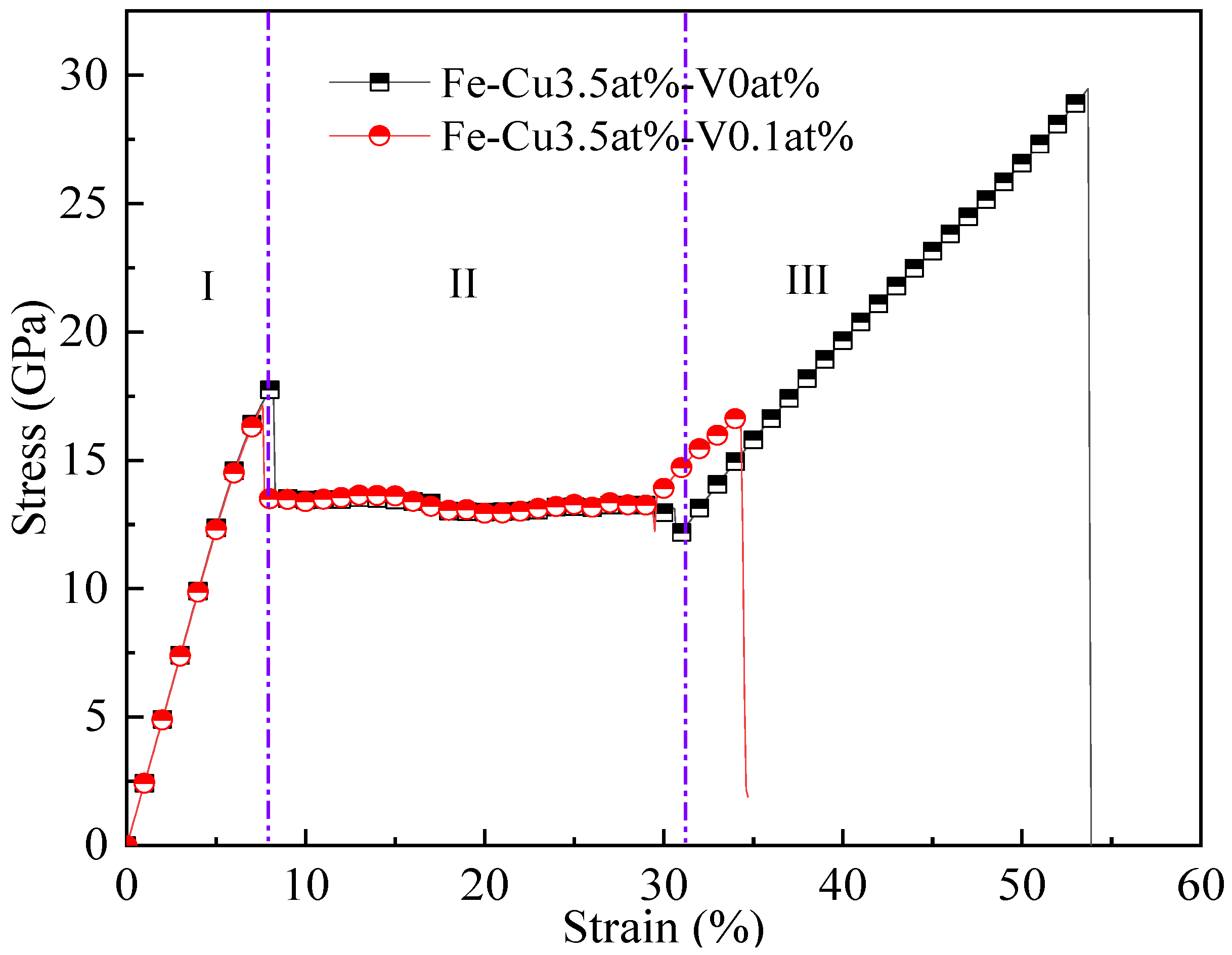
3.4. The Influence of Cu Precipitates on the Tensile Properties of Fe–Cu Alloy
4. Conclusions
- The presence of vacancies in the Fe-3.5Cu alloy will improve the diffusion and precipitation ability of Cu atoms, and the diffusion and precipitation ability of Cu atoms will first increase and then reduce with the increase in vacancies in the alloy. When the aging temperature is 1050 K, and the alloy contains no vacancies, 0.1 at% and 0.2 at% vacancies respectively, the diffusion coefficient of Cu atoms is 2.37 × 10−14, 1.78 × 10−8 and 2.65 × 10−9 cm2·s−1 respectively.
- During the aging process, the existence of vacancies in the Fe-3.5Cu alloy will stimulate the generation of Cu precipitates, and the concentration of vacancies has an important impact on the growth of Cu precipitates. When there are no vacancies in the alloy, aging will not produce Cu precipitates. In the alloy containing 0.1at% vacancies, the size of the Cu precipitates produced by aging will be larger than that of Cu precipitates generated in alloy with 0.2at% vacancies, but the amount of Cu precipitates produced by aging is less than that in the alloy with 0.2at% vacancies.
- Compared to the aged Fe-3.5Cu-0V alloy, during the tensile process, the aged Fe-3.5Cu-0.1V alloy will have structural phase transition earlier and vacancy defects appear in the system, so that the yield strength of the aged Fe-3.5Cu-0.1V alloy is slightly lower and the strain hardening strength is significantly reduced compared with that of the aged Fe-3.5Cu-0V alloy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, M.; Huang, L.; Li, C.; Li, J.; Li, P. Evaluation of the deformation behaviors and hot workability of a high-strength low-alloy steel. Mater. Sci. Eng. A 2021, 810, 141031–141047. [Google Scholar] [CrossRef]
- Jorge, J.C.F.; de Souza, L.F.G.; Mendes, M.C.; Bott, I.S.; Araujo, L.S.; dos Santos, V.R.; Rebello, J.M.A.; Evans, G.M. Microstructure characterization and its relationship with impact toughness of C-Mn and high strength low alloy steel weld metals—A review. J. Mater. Res. Technol. 2020, 10, 471–501. [Google Scholar] [CrossRef]
- Kong, H.J.; Xu, C.; Bu, C.C.; Da, C.; Luan, J.H.; Jiao, Z.B.; Chen, G.; Liu, C.T. Hardening mechanisms and impact toughening of a high-strength steel containing low Ni and Cu additions. Acta Mater. 2019, 172, 150–160. [Google Scholar] [CrossRef]
- Far, A.; Anijdan, S.; Abbasi, S.M. The effect of increasing Cu and Ni on a significant enhancement of mechanical properties of high strength low alloy, low carbon steels of HSLA-100 type. Mater. Sci. Eng. A 2019, 746, 384–393. [Google Scholar] [CrossRef]
- Alipooramirabad, H.; Paradowska, A.; Nafisi, S.; Reid, M.; Ghomashchi, R. Post-Weld Heat Treatment of API 5L X70 High Strength Low Alloy Steel Welds. Materials 2020, 13, 5801. [Google Scholar] [CrossRef]
- Sun, M.X. Mechanism of Microstructure and Property Regulation of Ultra-Low Carbon Nanocu-Rich HSLA Steels; Northeastern University: Shenyang, China, 2017. [Google Scholar]
- He, Z.J.; Huang, X.F.; Le, G.M. First principles calculation of interactions between solute chromium atoms and vacancies in tungsten. Rare Metal Mater. Eng. 2020, 49, 2691–2696. [Google Scholar]
- Yang, Z.; Banhart, J. Natural and artificial ageing in aluminium alloys-the role of excess vacancies. Acta Mater. 2021, 215, 117014–117025. [Google Scholar] [CrossRef]
- Lv, G.C.; Zhang, H.; He, X.F.; Yang, W.; Su, Y.J. Vacancy enhanced formation and phase transition of Cu-rich precipitates in α-iron under neutron irradiation. AIP Adv. 2016, 6, 45004–45014. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wang, X.; Li, H.; Li, C.; Li, Y. Molecular Dynamics Study on the Impact of Cu Clusters at the BCC-Fe Grain Boundary on the Tensile Properties of Crystal. Metals 2020, 10, 1533. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Millett, P.C.; Tonks, M.R.; Bai, X.-M.; Biner, B. Preferential Cu precipitation at extended defects in BCC-Fe: An atomistic study. Comput. Mater. Sci. 2015, 101, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Duparc, H.A.H.; Doole, R.C.; Jenkins, M.L.; Barbu, A. A high-resolution electron microscopy study of copper precipitation in Fe-1.5wt%Cu under electron irradiation. Philos. Mag. Lett. 1995, 71, 325–333. [Google Scholar] [CrossRef]
- Hirel, P. Atomsk: A tool for manipulating and converting atomic data files. Comput. Phys. Commun. 2015, 197, 212–219. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. Comput. Phys. Commun. 1995, 117, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Bonny, G.; Pasianot, R.C.; Castin, N.; Malerba, L. Ternary Fe-Cu-Ni many-body potential to model reactor pressure vessel steels: First validation by simulated thermal annealing. Philos. Mag. 2009, 89, 3531–3546. [Google Scholar] [CrossRef]
- Du, Y.K.; He, X.F.; Jia, L.X. Study on the contribution mechanism of Cu precipitates to α-Fe irradiation hardening. Mater. Rep. 2018, 32, 307–312. [Google Scholar]
- Zheng, Z.X.; Zhang, L. Atomic scale calculation of structure change of Fe-Cu binary system containing Cu cluster in Fe matrix during heating. Acta Phys. Sin. 2017, 66, 282–292. [Google Scholar]
- Zhu, L.S. Molecular Dynamics Simulation Study on Early Precipitation Behavior of Fe-Cu-Ni Alloy Medium-Rich Cu Clusters; Shanghai University: Shanghai, China, 2014. [Google Scholar]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO-the Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2010, 18, 2154–2162. [Google Scholar] [CrossRef]
- Bakaev, A.; Terentyev, D.; He, X. Interaction of carbon-vacancy complex with minor alloying elements of ferritic steels. J. Nucl. Mater. 2014, 451, 82–87. [Google Scholar] [CrossRef]
- Li, R.-S.; Li, F.; Xin, D.-Q.; Luo, J.-J.; Chen, S.; Zhang, Y. A molecular dynamics simulation of energetics and diffusion of point defects in a Au-Ag alloy. Bull. Mater. Sci. 2019, 42, 113–127. [Google Scholar] [CrossRef] [Green Version]
- Doan, D.-Q.; Fang, T.-H.; Tran, A.-S.; Chen, T.-H. Residual stress and elastic recovery of imprinted Cu-Zr metallic glass films using molecular dynamic simulation. Comput. Mater. Sci. 2019, 170, 109162–109181. [Google Scholar] [CrossRef]
- Lv, G.C. Computer Simulation of the Effect of Cu Rich Precipitates on Hardening and Embrittlement of RPV Steel; University of Science and Technology Beijing: Beijing, China, 2017. [Google Scholar]
- Marian, J.; Wirth, B.D.; Odette, G.R.; Perlado, J.M. Cu diffusion in α-Fe: Determination of solute diffusivities using atomic-scale simulations. Comput. Mater. Sci. 2004, 31, 347–367. [Google Scholar] [CrossRef]
- Li, F.B.; Gao, N.; Li, Y.P. Molecular dynamics study of the effect of he on the tensile properties of W Σ3{112} symmetric grain boundaries. J. Mater. Heat Treat. 2019, 40, 96–102. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Chen, Y.; Wang, X.; Li, H.; Li, Y. Molecular Dynamics Research on the Impact of Vacancies on Cu Precipitation in BCC-Fe. Materials 2021, 14, 5029. https://doi.org/10.3390/ma14175029
Zhang H, Chen Y, Wang X, Li H, Li Y. Molecular Dynamics Research on the Impact of Vacancies on Cu Precipitation in BCC-Fe. Materials. 2021; 14(17):5029. https://doi.org/10.3390/ma14175029
Chicago/Turabian StyleZhang, Haichao, Yinli Chen, Xufeng Wang, Huirong Li, and Yungang Li. 2021. "Molecular Dynamics Research on the Impact of Vacancies on Cu Precipitation in BCC-Fe" Materials 14, no. 17: 5029. https://doi.org/10.3390/ma14175029





