Rapid Evaluation of the Pozzolanic Activity of Bayer Red Mud by a Polymerization Degree Method: Correlations with Alkali Dissolution of (Si+Al) and Strength
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
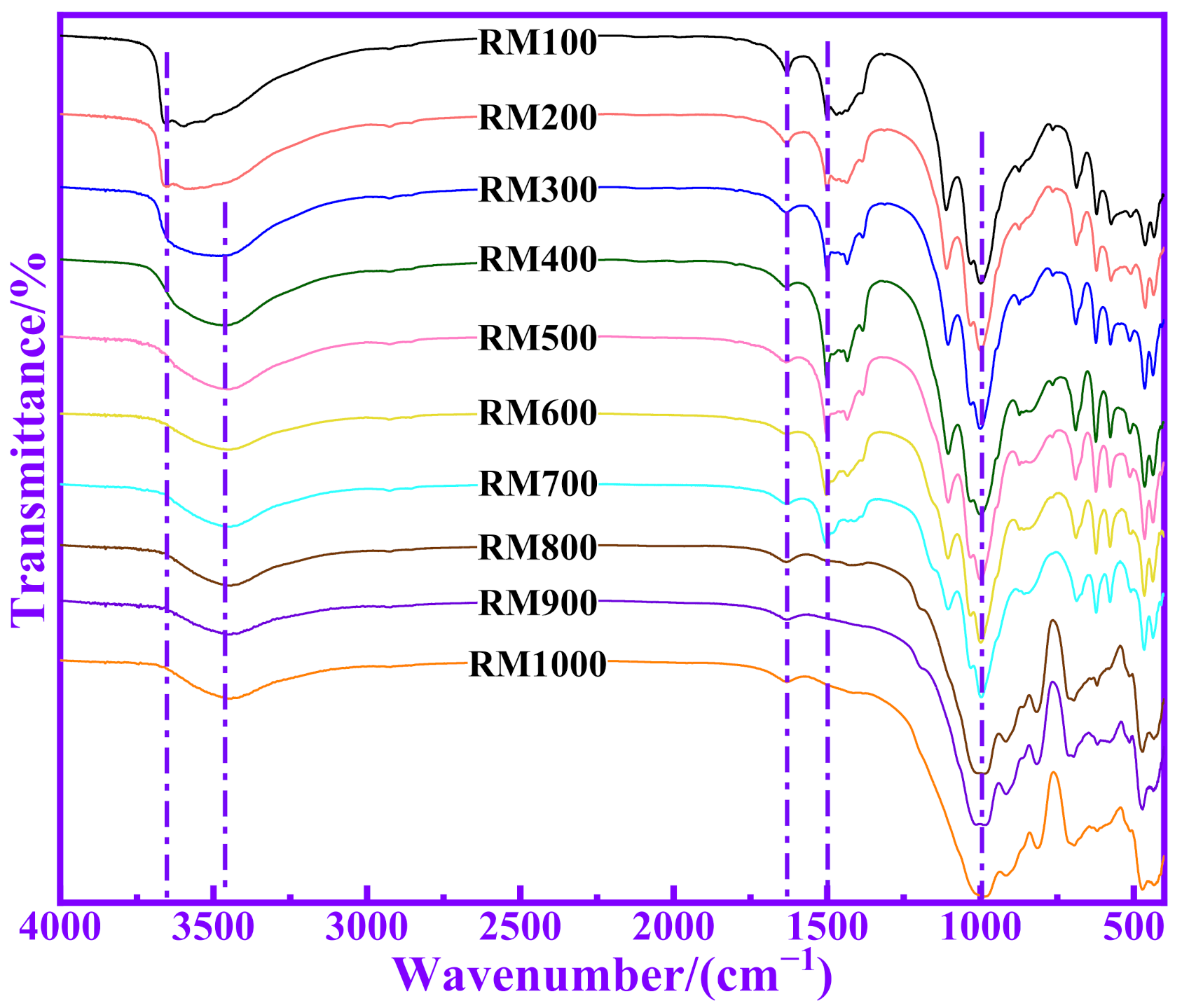
3.1. XRD of RMs with Different Pozzolanic Activities
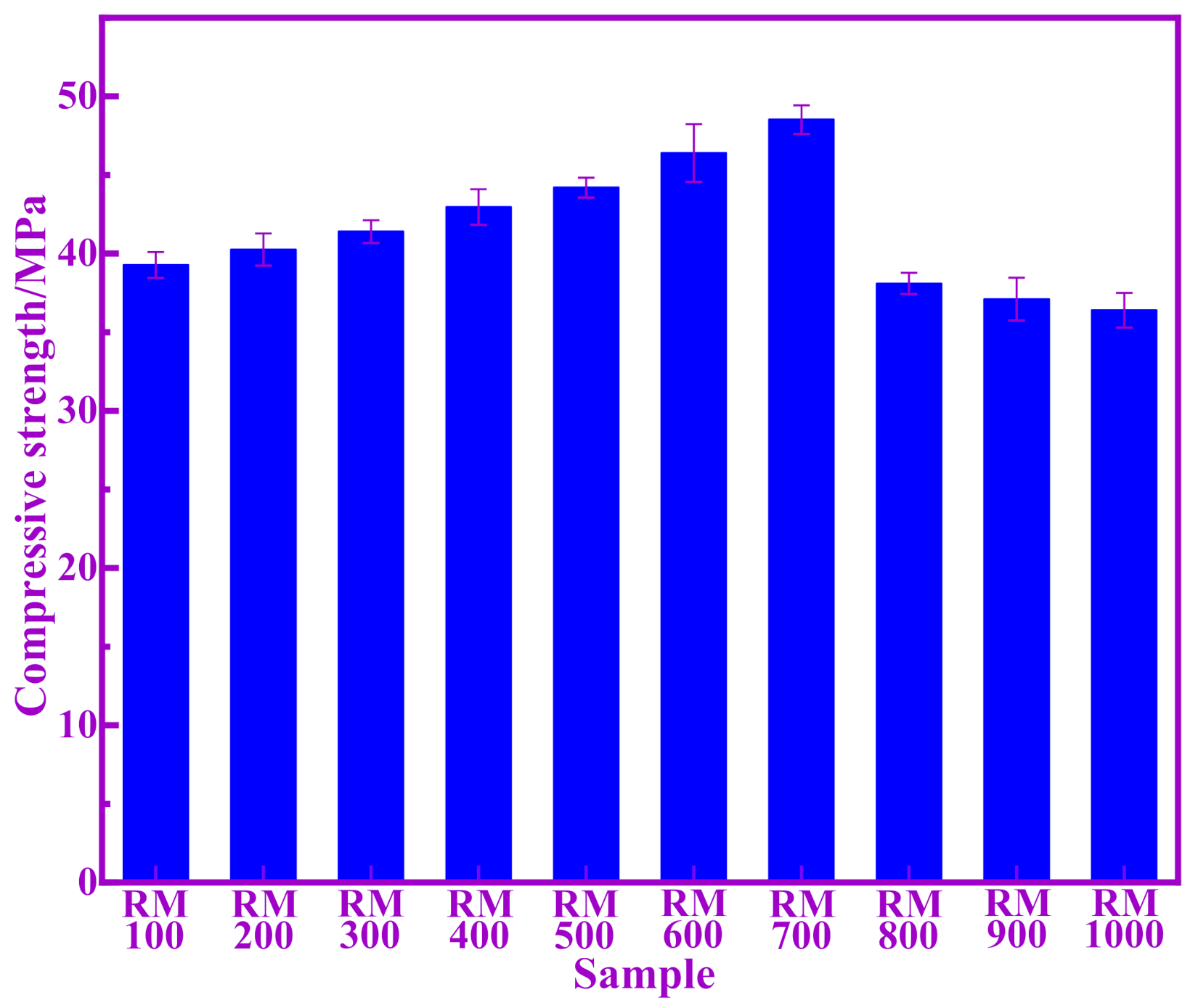
3.2. Compressive Strength Evaluation of Pozzolanic Activity
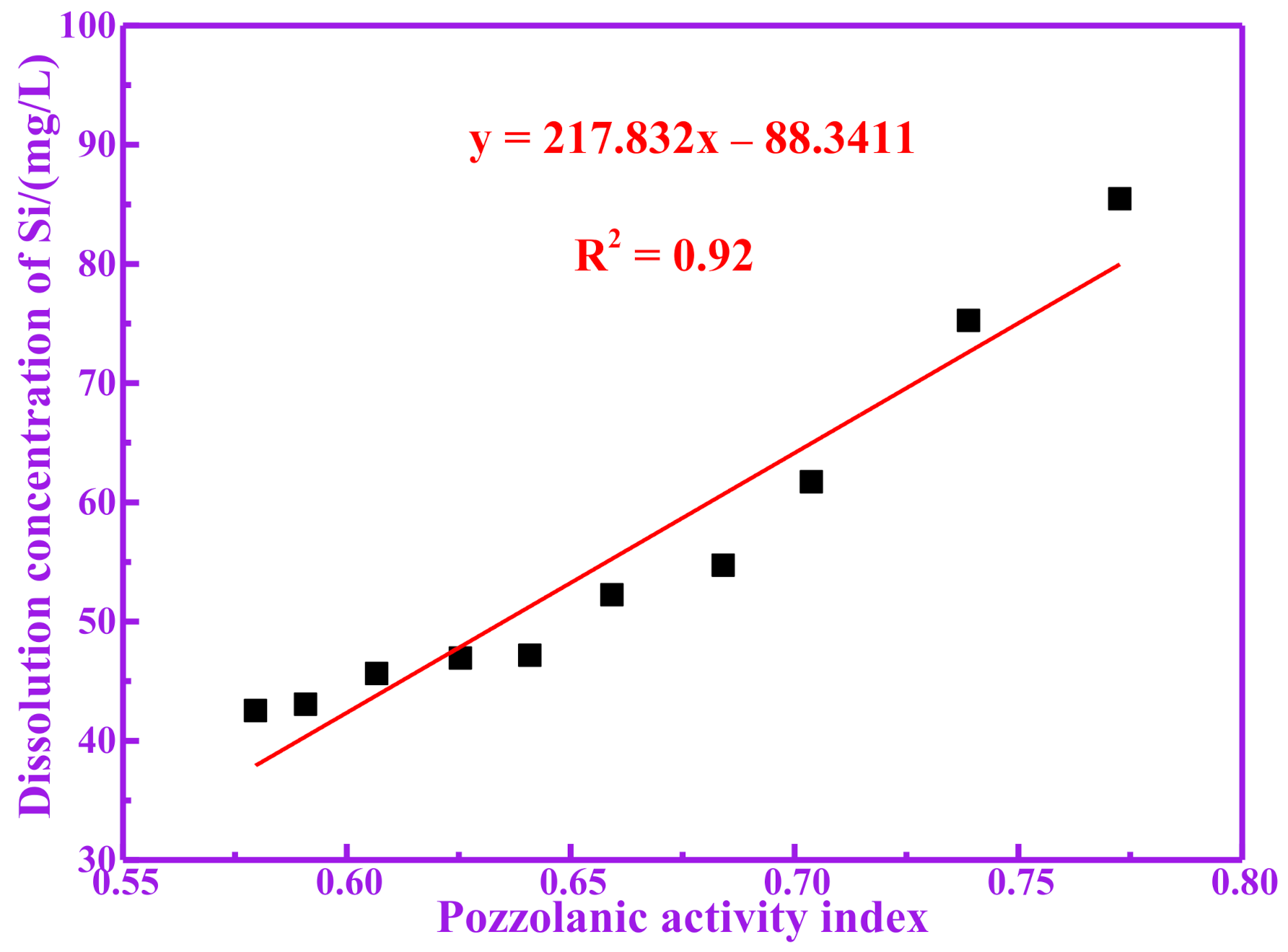
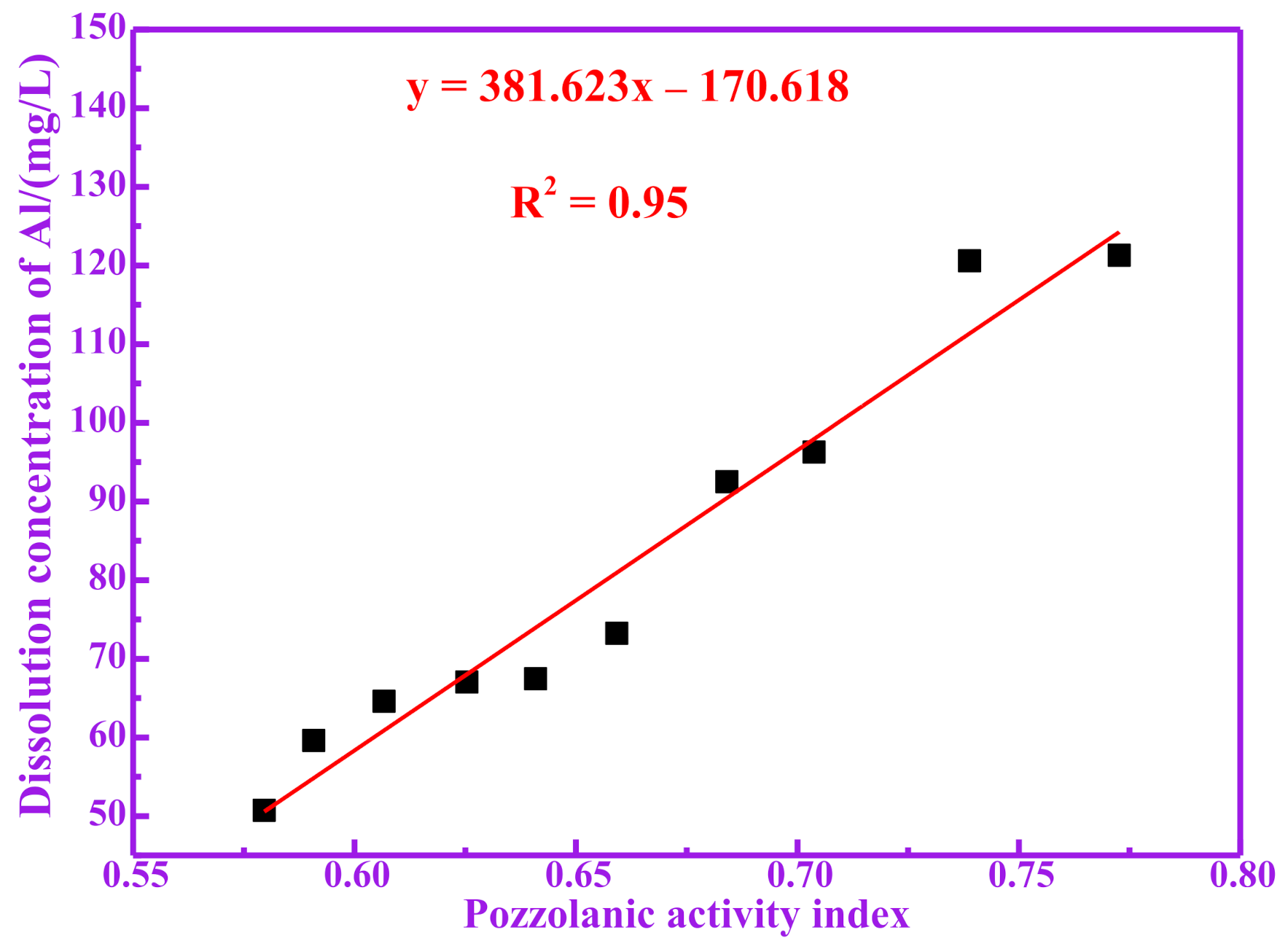
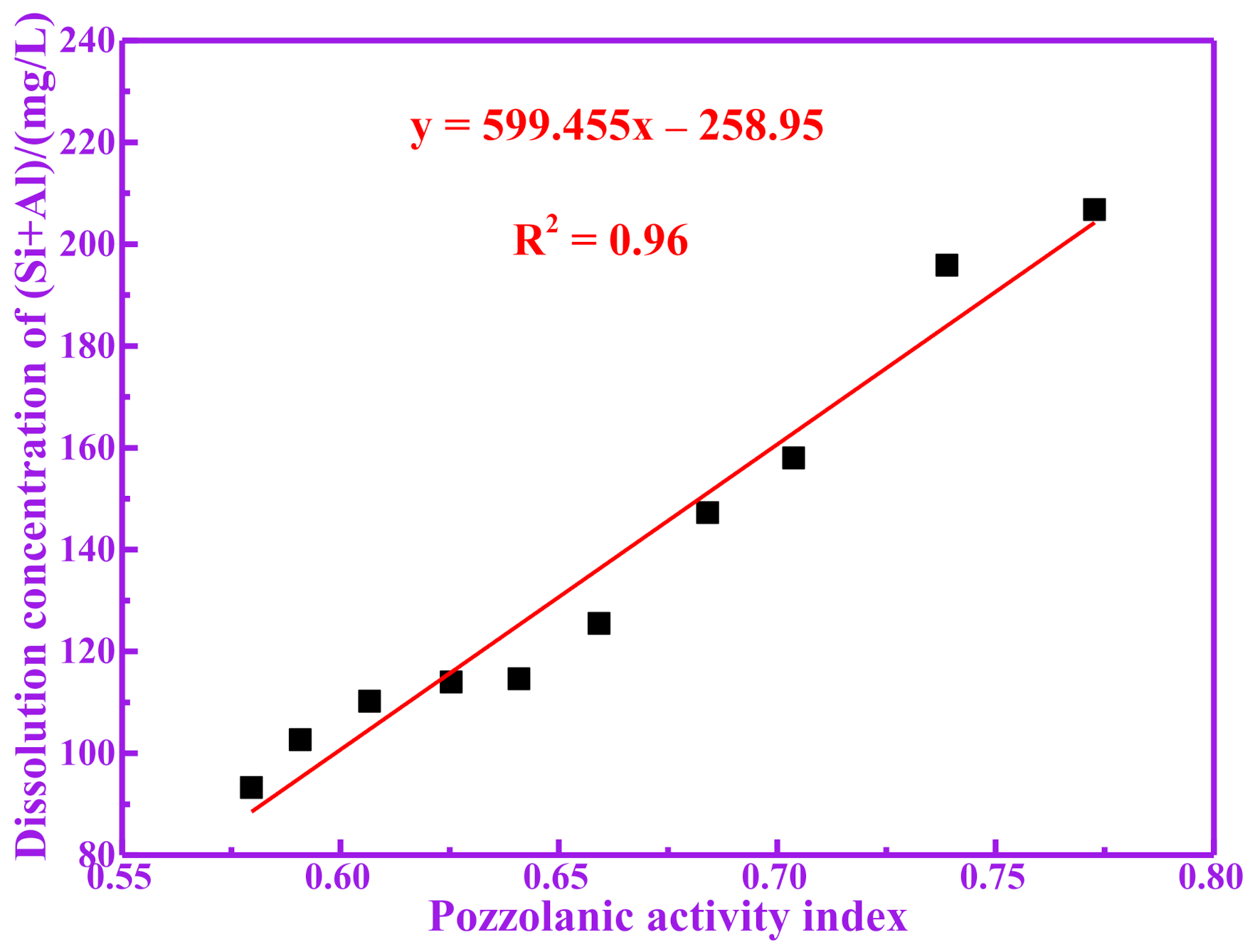
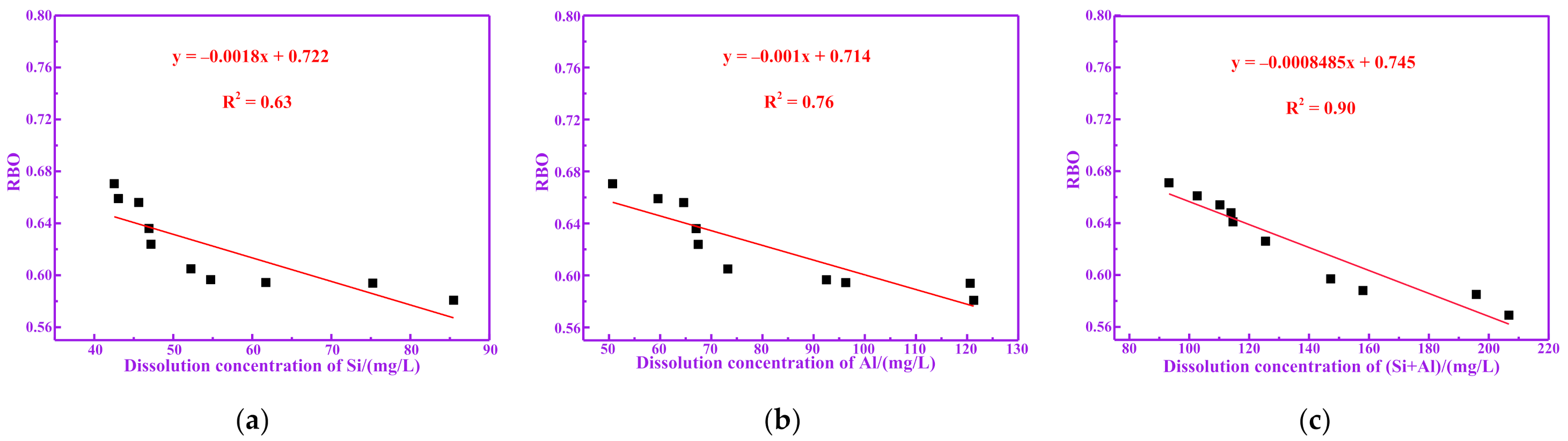
3.3. Pozzolanic Activity Evaluation for Dissolution of Silicon and Aluminum
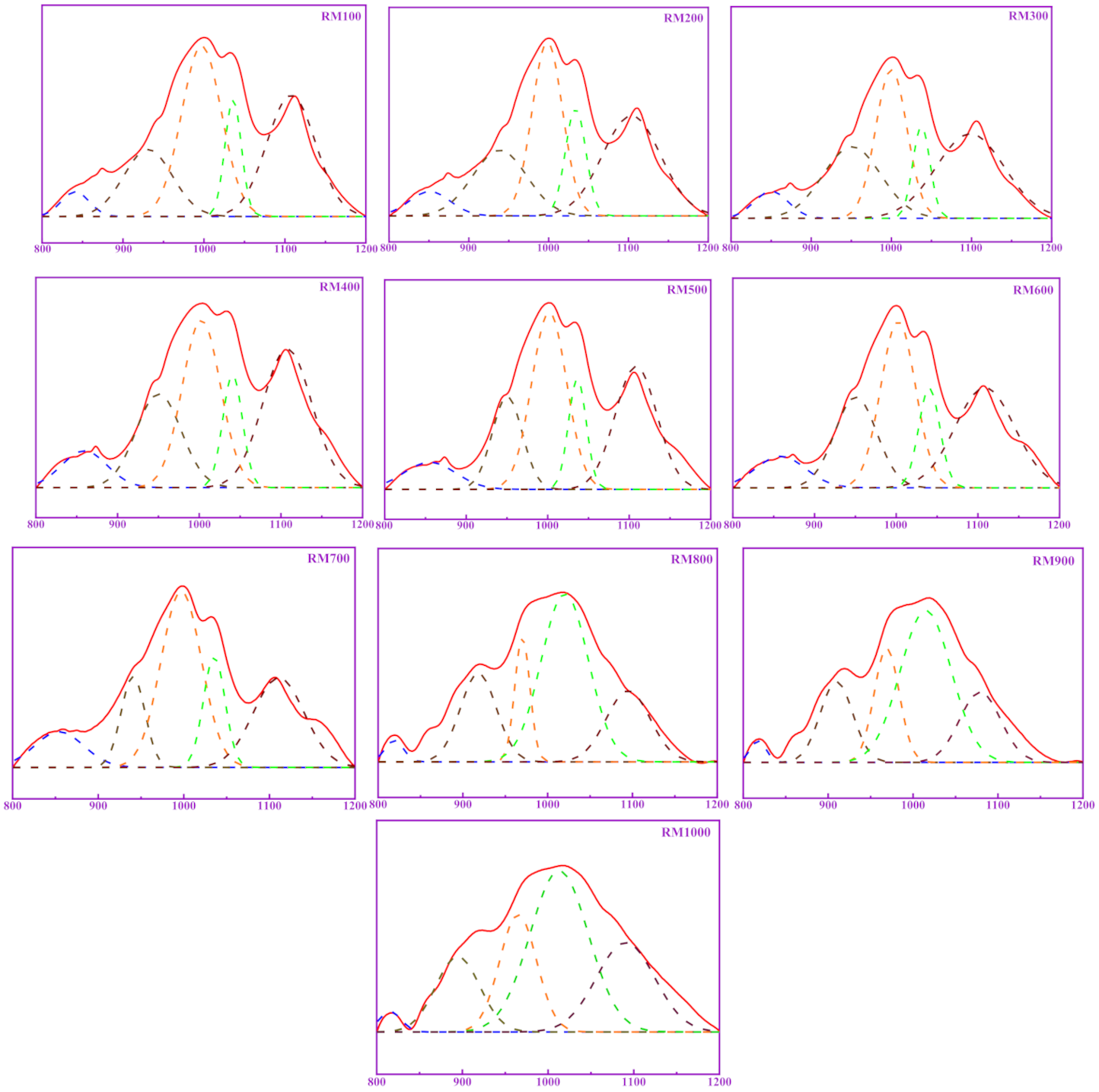
3.4. Relationship between Polymerization Degree and Pozzolanic Activity for RM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, C.; Zhang, X.; Sun, R.; Cao, Y. Neutralization of red mud using bio-acid generated by hydrothermal carbonization of waste biomass for potential soil application. J. Clean. Prod. 2020, 271, 122525. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Liu, X.; Zhang, W.; Li, Z.; Li, Y.; Ren, Y.; Li, H. Mechanism of magnetizing the Bayer red mud and meanwhile improving the cementitious activity of its tailings by using biomass. J. Clean. Prod. 2021, 287, 125016. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Li, Y.; Zhang, W.; Xue, Y. Tailings after Iron Extraction in Bayer Red Mud by Biomass Reduction: Pozzolanic Activity and Hydration Characteristics. Materials 2021, 14, 3955. [Google Scholar] [CrossRef]
- Cho, Y.K.; Jung, S.H.; Choi, Y.C. Effects of chemical composition of fly ash on compressive strength of fly ash cement mortar. Constr. Build. Mater. 2019, 204, 255–264. [Google Scholar] [CrossRef]
- Peng, D.; Wang, Y.; Liu, X.; Tang, B.; Zhang, N. Preparation, characterization, and application of an eco-friendly sand-fixing material largely utilizing coal-based solid waste. J. Hazard. Mater. 2019, 373, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, B.; Wang, Q. Application of steel slag in cement treated aggregate base course. J. Clean. Prod. 2020, 269, 121733. [Google Scholar] [CrossRef]
- Ortega, J.M.; Cabeza, M.; Tenza-Abril, A.J.; Real-Herraiz, T.; Climent, M.; Sánchez, I. Effects of red mud addition in the microstructure, durability and mechanical performance of cement mortars. Appl. Sci. 2019, 9, 984. [Google Scholar] [CrossRef] [Green Version]
- Payá, J.; Borrachero, M.V.; Monzó, J.; Peris-Mora, E.; Amahjour, F. Enhanced conductivity measurement techniques for evaluation of fly ash pozzolanic activity. Cem. Concr. Res. 2001, 31, 41–49. [Google Scholar] [CrossRef]
- Tashiro, C.; Ikeda, K.; Inoue, Y. Evaluation of pozzolanic activity by the electric resistance measurement method. Cem. Concr. Res. 1994, 24, 1133–1139. [Google Scholar] [CrossRef]
- Roszczynialski, W. Determination of pozzolanic activity of materials by thermal analysis. J. Therm. Anal. Calorim. 2002, 70, 387–392. [Google Scholar] [CrossRef]
- Shvarzman, A.; Kovler, K.; Schamban, I.; Grader, G.S.; Shter, G.E. Influence of chemical and phase composition of mineral admixtures on their pozzolanic activity. Adv. Cem. Res. 2002, 14, 35–41. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, Q.; Chen, Q.; Kero, J.; Andersson, A.; Ahmed, H.; Engström, F.; Samuelsson, C. Characterization and evaluation of the pozzolanic activity of granulated copper slag modified with CaO. J. Clean. Prod. 2019, 232, 1112–1120. [Google Scholar] [CrossRef]
- Martín, C.M.; Scarponi, N.B.; Villagrán, Y.A.; Manzanal, D.G.; Piqué, T.M. Pozzolanic activity quantification of hollow glass microspheres. Cem. Concr. Compos. 2021, 118, 103981. [Google Scholar] [CrossRef]
- Basto, P.A.; Junior, H.; Neto, A.A. Characterization and pozzolanic properties of sewage sludge ashes (SSA) by electrical conductivity. Cem. Concr. Compos. 2019, 104, 103410. [Google Scholar] [CrossRef]
- Hasani, M.; Tarighat, A. Proposing new pozzolanic activity index based on water adsorption energy via molecular dynamics simulations. Constr. Build. Mater. 2019, 213, 492–504. [Google Scholar] [CrossRef]
- Supervision, T. Pozzolanic Materials Used for Cement Production, in Chinese Code GB/T 2847-2005; Chinese Standard Press: Beijing, China, 2005. [Google Scholar]
- Zhang, J.-X.; Sun, H.-H.; Sun, Y.-M.; Zhang, N. Correlation between 29 Si polymerization and cementitious activity of coal gangue. J. Zhejiang Univ.-Sci. A 2009, 10, 1334–1340. [Google Scholar] [CrossRef]
- Zhang, J.X.; Sun, H.H.; Wan, J.H.; Zhang, N. Si polymerization degree of hydrates in coal gangue added cement. J. Cent. South Univ. Sci. Technol. 2011, 42, 329–335. [Google Scholar]
- Liu, X.; Zhang, N.; Sun, H.; Zhang, J.; Li, L. Structural investigation relating to the cementitious activity of bauxite residue—Red mud. Cem. Concr. Res. 2011, 41, 847–853. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, P.; Chen, X.; Ge, X.; Wang, Y. Study on hydration characteristics of circulating fluidized bed combustion fly ash (CFBCA). Constr. Build. Mater. 2020, 251, 118993. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Zhang, W.; Li, Z.; Zhang, Y.; Li, Y.; Ren, Y. Effects of Si/Al ratio on the efflorescence and properties of fly ash based geopolymer. J. Clean. Prod. 2020, 244, 118852. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, D.; Zhuang, S. The soundness of steel slag with different free CaO and MgO contents. Constr. Build. Mater. 2017, 151, 138–146. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Xu, Y.; Tang, B.; Wang, Y.; Mukiza, E. Preparation and characterization of cement treated road base material utilizing electrolytic manganese residue. J. Clean. Prod. 2019, 232, 980–992. [Google Scholar] [CrossRef]


| Oxides | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | SO3 | TiO2 | LOI |
|---|---|---|---|---|---|---|---|---|---|
| RM | 22.71 | 22.96 | 26.57 | 2.17 | 0.13 | 11.08 | 1.01 | 1.72 | 11.19 |
| Cement | 18.60 | 3.81 | 3.25 | 64.86 | 2.90 | 0.24 | 0.45 | 0.31 | 4.14 |
| Sample | RM100 | RM200 | RM300 | RM400 | RM500 | RM600 | RM700 | RM800 | RM900 | RM1000 |
|---|---|---|---|---|---|---|---|---|---|---|
| Kα | 62.54% | 64.09% | 65.92% | 68.41% | 70.38% | 73.89% | 77.27% | 60.67% | 59.08% | 57.96% |
| Sample | Dissolution Concentration of Si | Dissolution Concentration of Al | Dissolution Concentration of (Si+Al) |
|---|---|---|---|
| RM100 | 46.93 | 67.04 | 113.97 |
| RM200 | 47.18 | 67.44 | 114.62 |
| RM300 | 52.24 | 73.24 | 125.48 |
| RM400 | 54.73 | 92.51 | 147.24 |
| RM500 | 61.72 | 96.29 | 158.01 |
| RM600 | 75.25 | 120.60 | 195.85 |
| RM700 | 85.47 | 121.30 | 206.77 |
| RM800 | 45.63 | 64.60 | 110.23 |
| RM900 | 43.06 | 59.60 | 102.66 |
| RM1000 | 42.53 | 50.71 | 93.24 |
| Sample | Relative Content/% | RBO | R2 | ||||
|---|---|---|---|---|---|---|---|
| SiQ0 | SiQ1 | SiQ2 | SiQ3 | SiQ4 | |||
| RM100 | 3.20 | 17.21 | 35.12 | 10.95 | 33.52 | 0.6360 | 0.995 |
| RM200 | 4.41 | 20.11 | 30.04 | 12.42 | 33.02 | 0.6238 | 0.995 |
| RM300 | 5.43 | 24.20 | 27.24 | 9.26 | 33.87 | 0.6049 | 0.995 |
| RM400 | 7.85 | 19.87 | 29.98 | 10.38 | 31.92 | 0.5966 | 0.995 |
| RM500 | 8.07 | 15.98 | 35.40 | 11.22 | 29.33 | 0.5944 | 0.994 |
| RM600 | 8.28 | 19.21 | 30.30 | 11.07 | 31.14 | 0.5939 | 0.994 |
| RM700 | 9.74 | 12.05 | 39.65 | 13.29 | 25.27 | 0.5808 | 0.987 |
| RM800 | 2.13 | 19.39 | 11.39 | 48.13 | 18.96 | 0.6560 | 0.992 |
| RM900 | 2.07 | 15.83 | 17.03 | 46.56 | 18.51 | 0.6590 | 0.995 |
| RM1000 | 1.93 | 14.75 | 19.23 | 41.39 | 22.7 | 0.6705 | 0.988 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, X.; Xie, Z.; Wang, H.; Zhang, W.; Xue, Y. Rapid Evaluation of the Pozzolanic Activity of Bayer Red Mud by a Polymerization Degree Method: Correlations with Alkali Dissolution of (Si+Al) and Strength. Materials 2021, 14, 5546. https://doi.org/10.3390/ma14195546
Wang Y, Liu X, Xie Z, Wang H, Zhang W, Xue Y. Rapid Evaluation of the Pozzolanic Activity of Bayer Red Mud by a Polymerization Degree Method: Correlations with Alkali Dissolution of (Si+Al) and Strength. Materials. 2021; 14(19):5546. https://doi.org/10.3390/ma14195546
Chicago/Turabian StyleWang, Yaguang, Xiaoming Liu, Zhiqing Xie, Huimin Wang, Wei Zhang, and Yang Xue. 2021. "Rapid Evaluation of the Pozzolanic Activity of Bayer Red Mud by a Polymerization Degree Method: Correlations with Alkali Dissolution of (Si+Al) and Strength" Materials 14, no. 19: 5546. https://doi.org/10.3390/ma14195546





