Crystal and Magnetic Structure Transitions in BiMnO3+δ Ceramics Driven by Cation Vacancies and Temperature
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
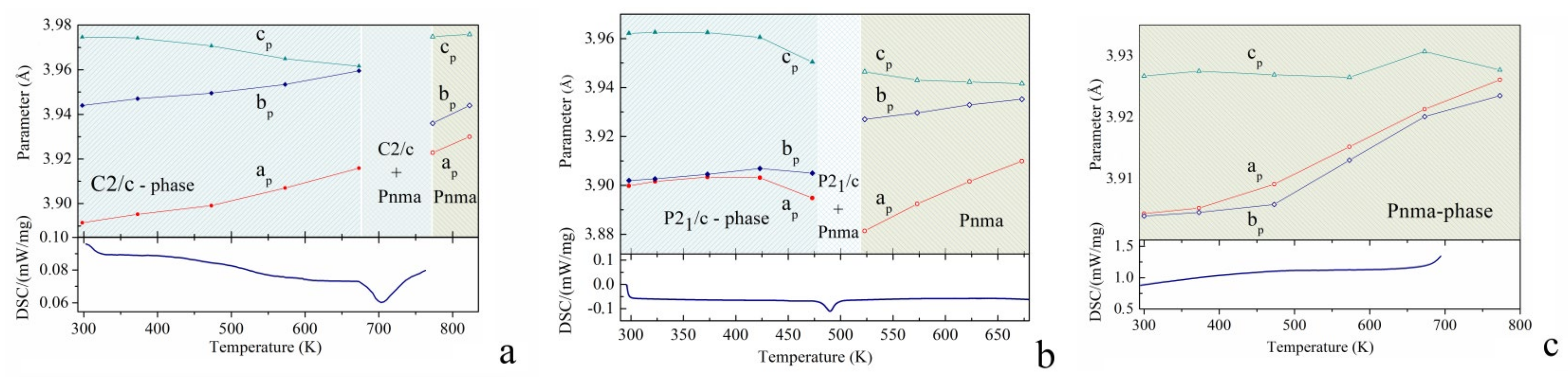
Temperature Dependent Structural Investigations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spaldin, N.A.; Ramesh, R. Advances in magnetoelectric multiferroics. Nat. Mater. 2019, 18, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Posada, C.M.; Castro, A.; Kiat, J.-M.; Porcher, F.; Peña, O.; Algueró, M.; Amorín, H. A novel perovskite oxide chemically designed to show multiferroic phase boundary with room-temperature magnetoelectricity. Nat. Commun. 2016, 7, 12772. [Google Scholar] [CrossRef]
- Ederer, C.; Spaldin, N.A. Weak ferromagnetism and magnetoelectric coupling in bismuth ferrite. Phys. Rev. B 2005, 71, 060401. [Google Scholar] [CrossRef] [Green Version]
- Belik, A.A. Local distortions in multiferroic BiMnO3 as a function of doping. Sci. Technol. Adv. Mater. 2011, 12, 044610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, T.; Kawamoto, S.; Yamada, I.; Azuma, M.; Takano, M.; Tokura, Y. Magnetocapacitance effect in multiferroic BiMnO3. Phys. Rev. B 2003, 67, 180401. [Google Scholar] [CrossRef]
- Coey, J.M.D.; Viret, M.; von Molnár, S. Mixed-valence manganites. Adv. Phys. 1999, 48, 167–293. [Google Scholar] [CrossRef]
- Karoblis, D.; Diliautas, R.; Raudonyte-Svirbutaviciene, E.; Mazeika, K.; Baltrunas, D.; Beganskiene, A.; Zarkov, A.; Kareiva, A. The Synthesis and Characterization of Sol-Gel-Derived SrTiO3-BiMnO3 Solid Solutions. Crystals 2020, 10, 1125. [Google Scholar] [CrossRef]
- Revathi, B.; Balakrishnan, L.; Chandar, N.K. Structural, morphological, optical, dielectric and magnetic field sensing characteristics of Bi1-xKxMnO3 and BiMn1-yCoyO3 nanopowders: A comparative study. Mater. Lett. 2019, 256, 126655. [Google Scholar] [CrossRef]
- Belik, A.A. Structural, magnetic, and dielectric properties of solid solutions between BiMnO3 and YMnO3. J. Solid State Chem. 2017, 246, 8–15. [Google Scholar] [CrossRef]
- Thakur, S.; Singh, K.; Pandey, O.P. Sr doped BiMO3 (M = Mn, Fe, Y) perovskites: Structure correlated thermal and electrical properties. Mater. Chem. Phys. 2017, 187, 96–103. [Google Scholar] [CrossRef]
- Kumar, P.; Dayal, V. Critical behavior and non-universal low-field magnetic scaling in La1-xBixMnO3 (x=0.4 & 0.6) perovskite manganite oxide. AIP Conf. Proc. 2015, 1665, 030014. [Google Scholar]
- Azuma, M.; Kanda, H.; Belik, A.A.; Shimakawa, Y.; Takano, M. Magnetic and structural properties of BiFe1−xMnxO3. J. Magn. Magn. Mater. 2007, 310, 1177–1179. [Google Scholar] [CrossRef]
- Moreira dos Santos, A.; Cheetham, A.K.; Atou, T.; Syono, Y.; Yamaguchi, Y.; Ohoyama, K.; Chiba, H.; Rao, C.N.R. Orbital ordering as the determinant for ferromagnetism in biferroic BiMnO3. Phys. Rev. B 2002, 66, 064425. [Google Scholar] [CrossRef]
- Figueiras, F.G.; Karpinsky, D.; Tavares, P.B.; Gonçalves, J.N.; Yañez-Vilar, S.; Moreira Dos Santos, A.F.; Franz, A.; Tovar, M.; Agostinho Moreira, J.; Amaral, V.S. Novel multiferroic state and ME enhancement by breaking the AFM frustration in LuMn1−xO3. Phys. Chem. Chem. Phys. 2017, 19, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Belik, A.A.; Kodama, K.; Igawa, N.; Shamoto, S.-i.; Kosuda, K.; Takayama-Muromachi, E. Crystal and Magnetic Structures and Properties of BiMnO3+δ. J. Am. Chem. Soc. 2010, 132, 8137–8144. [Google Scholar] [CrossRef] [PubMed]
- Többens, D.M.; Zander, S. KMC-2: An X-ray beamline with dedicated diffraction and XAS endstations at BESSY II. JLSRF 2016, 2, A49. [Google Scholar] [CrossRef] [Green Version]
- Fischer, P.; Frey, G.; Koch, M.; Könnecke, M.; Pomjakushin, V.; Schefer, J.; Thut, R.; Schlumpf, N.; Bürge, R.; Greuter, U.; et al. High-resolution powder diffractometer HRPT for thermal neutrons at SINQ. Physica B 2000, 276–278, 146–147. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Cowley, J.M.; Moodie, A.F.; Miyake, S.; Takagi, S.; Fujimoto, F. The extinction rule for reflexions in symmetrical electron-diffraction spot patterns. Acta Crystallogr. Sect. A 1961, 14, 87–88. [Google Scholar] [CrossRef]
- Sundaresan, A.; Mangalam, R.V.K.; Iyo, A.; Tanaka, Y.; Rao, C.N.R. Crucial role of oxygen stoichiometry in determining the structure and properties of BiMnO3. J. Mater. Chem. C 2008, 18, 2191–2193. [Google Scholar] [CrossRef]
- Figueiras, F.G.; Karpinsky, D.; Tavares, P.B.; Das, S.; Leitão, J.V.; Brück, E.H.; Moreira, J.A.; Amaral, V.S. Breaking the geometric magnetic frustration in controlled off-stoichiometric LuMn1+zO3+δ compounds. Phys. Chem. Chem. Phys. 2016, 18, 13519–13523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markovich, V.; Rozenberg, E.; Gorodetsky, G.; Jung, G.; Fita, I.; Puzniak, R.; Wisniewski, A.; Martin, C.; Hebert, S.; Raveau, B. Vacancies at Mn-sites in LaMn1-xO3 manganites: Interplay between ferromagnetic interactions and hydrostatic pressure. J. Appl. Phys. 2004, 95, 7112–7114. [Google Scholar] [CrossRef]
- Shames, A.I.; Auslender, M.; Rozenberg, E.; Gorodetsky, G.; Hébert, S.; Martin, C. Ferromagnetic ordering in LaMn1−xO3 manganites: EMR probing. J. Magn. Magn. Mater. 2007, 316, e640–e643. [Google Scholar]
- Goodenough, J.B. An interpretation of the magnetic properties of the perovskite-type mixed crystals La1-xSrxCoO3−λ. J. Phys. Chem. Solids 1958, 6, 287–297. [Google Scholar] [CrossRef]
- Kanamori, J. Superexchange interaction and symmetry properties of electron orbitals. J. Phys. Chem. Solids 1959, 10, 87–98. [Google Scholar] [CrossRef]
- Paraskevopoulos, M.; Mayr, F.; Hemberger, J.; Loidl, A.; Heichele, R.; Maurer, D.; Müller, V.; Mukhin, A.A.; Balbashov, A.M. Magnetic properties and the phase diagram of La1-xSrxMnO3 for x <=0.2. J. Phys. Condens. Matter. 2000, 12, 3993–4011. [Google Scholar] [CrossRef]



| Sample | SG | Mn-O-Mn/deg. | Magn. Order | Mn-O/Å |
|---|---|---|---|---|
| BiMnO3.02 | C2/c | Mn(1)-O(1)-Mn(2) (148.05) | FM | Mn(1)-O(1): 1.955(7); Mn(1)-O(2): 2.037(5); Mn(1)-O(3): 2.076(4); Mn(2)-O(1): 2.125(8); Mn(2)-O(2): 2.081(5); Mn(2)-O(3): 1.899(7); |
| Mn(1)-O(2)-Mn(2) (158.96) | AFM | |||
| Mn(1)-O(3)-Mn(2) (152.32) | FM | |||
| BiMnO3.08 | P21/c | Mn(1)-O(1)-Mn(2) (144.08) | AFM | Mn(1)-O(1): 2.045(7); Mn(1)-O(2): 1.983(5); Mn(1)-O(3): 1.922(6); Mn(1)-O(4): 2.098(6); Mn(1)-O(5): 1.993(6); Mn(1)-O(6): 1.944(5); Mn(2)-O(1): 2.008(8); Mn(2)-O(3): 2.121(2); Mn(2)-O(5): 1.900(4); Mn(3)-O(2): 2.091(6); Mn(3)-O(4): 2.029(2); Mn(3)-O(6): 1.965(3); |
| Mn(1)-O(2)-Mn(3) (160.10) | FM | |||
| Mn(1)-O(3)-Mn(2) (154.28) | FM | |||
| Mn(1)-O(4)-Mn(3) (157.91) | FM | |||
| Mn(1)-O(5)-Mn(2) (153.26) | FM | |||
| Mn(1)-O(6)-Mn(3) (163.87) | AFM | |||
| BiMnO3.14 | Pnma | Mn-O(1)-Mn (155.87) | AFM | Mn-O(1): 1.995(3); Mn-O(2): 1.935(2); Mn-O(2): 2.027(7). |
| Mn-O(2)-Mn (172.38) | FM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karpinsky, D.V.; Silibin, M.V.; Zhaludkevich, D.V.; Latushka, S.I.; Sikolenko, V.V.; Többens, D.M.; Sheptyakov, D.; Khomchenko, V.A.; Belik, A.A. Crystal and Magnetic Structure Transitions in BiMnO3+δ Ceramics Driven by Cation Vacancies and Temperature. Materials 2021, 14, 5805. https://doi.org/10.3390/ma14195805
Karpinsky DV, Silibin MV, Zhaludkevich DV, Latushka SI, Sikolenko VV, Többens DM, Sheptyakov D, Khomchenko VA, Belik AA. Crystal and Magnetic Structure Transitions in BiMnO3+δ Ceramics Driven by Cation Vacancies and Temperature. Materials. 2021; 14(19):5805. https://doi.org/10.3390/ma14195805
Chicago/Turabian StyleKarpinsky, Dmitry V., Maxim V. Silibin, Dmitry V. Zhaludkevich, Siarhei I. Latushka, Vadim V. Sikolenko, Daniel M. Többens, Denis Sheptyakov, Vladimir A. Khomchenko, and Alexei A. Belik. 2021. "Crystal and Magnetic Structure Transitions in BiMnO3+δ Ceramics Driven by Cation Vacancies and Temperature" Materials 14, no. 19: 5805. https://doi.org/10.3390/ma14195805






