A Review on the High Temperature Strengthening Mechanisms of High Entropy Superalloys (HESA)
Abstract
:1. Introduction
2. The Development of High Entropy Superalloys
3. Strengthening Mechanisms in High Entropy Superalloys
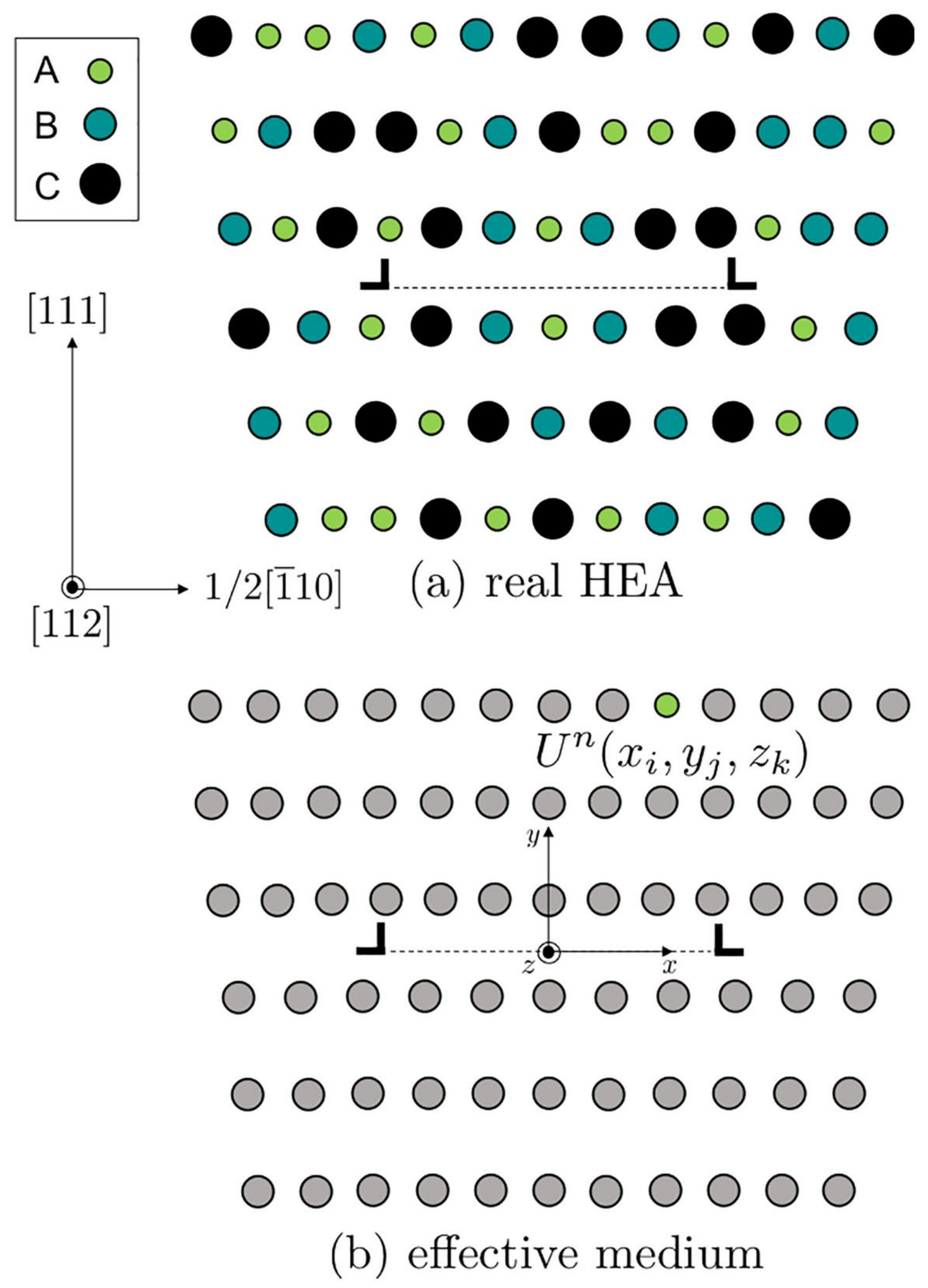
3.1. Solid Solution Strengthening
| Standard Model (Friedel) | Varvenne et al. [56] Model | Toda-Caraballo and Rivera-Díaz-Del-Castillo [58] Model | |
|---|---|---|---|
| Input Material Properties | Solute concentration, interaction parameters, Burgers vector and shear modulus of the matrix. | Elastic constants, lattice parameters, dislocation core structure, dislocation line tension, accurate elemental misfit volumes in the alloy, at composition of interest. | Lattice parameters, binary interatomic spacing, elastic constants and the dislocation line tension of the average matrix. |
| Assumptions | Only solutes atoms on the gliding plane interact with dislocation. | Solute do not alter the core geometry of the dislocation. | Solutes do not interact with each other, or their interaction is negligible. |
| The alloy is dilute, where a base element makes the host, and other small quantity elements are solutes. | Single phase FCC random alloys—thus neglect possible short range ordering effects and transformations to multiphase materials. | The general interatomic spacing between solutes i and j is independent of concentrations Xi and Xj and atoms around i and j. | |
| Individual atomic volumes are fixed. | Vegard’s law is applied to approximate the variation of cell parameter in a binary alloy. | ||
| Unique and fixed value per studied alloy for line tension is assumed. | Assume dilute-limit labusch-type analysis. | ||
| The alloy is elastically isotropic for the dislocation pressure field. | Elastic misfit contribution to strengthening. | ||
| Use generalized size and modulus misfit parameters to fit existing data. | |||
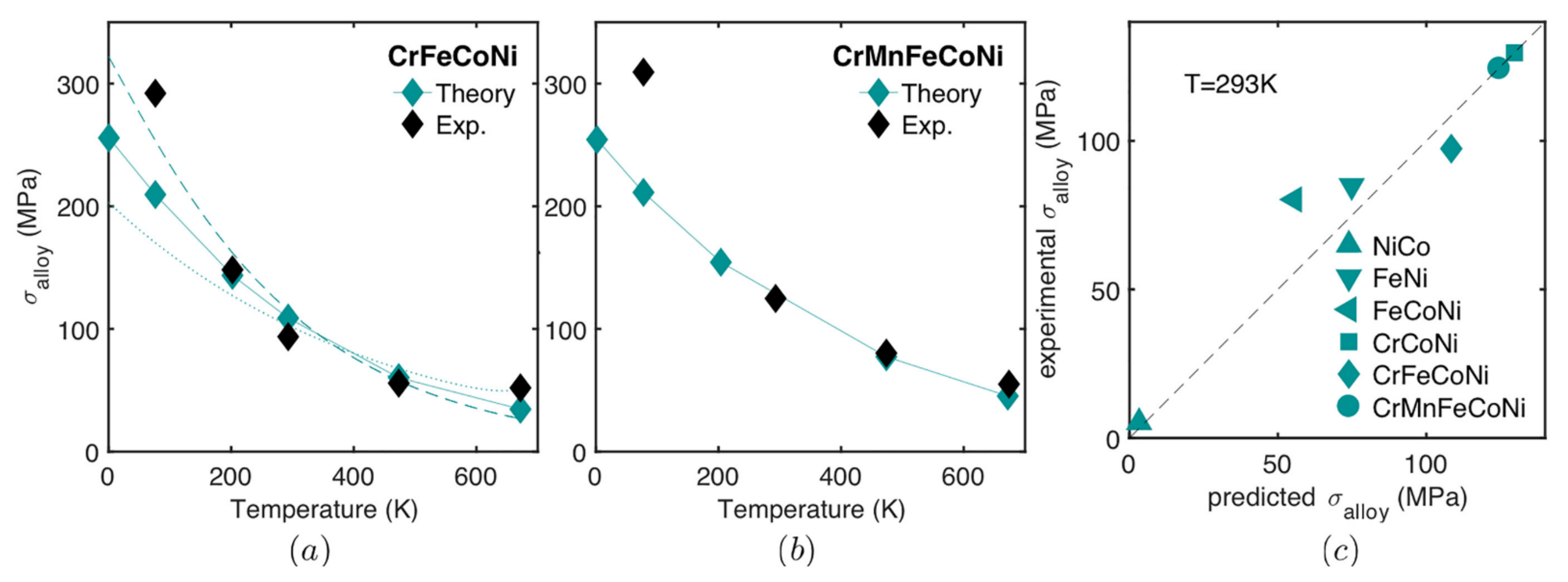
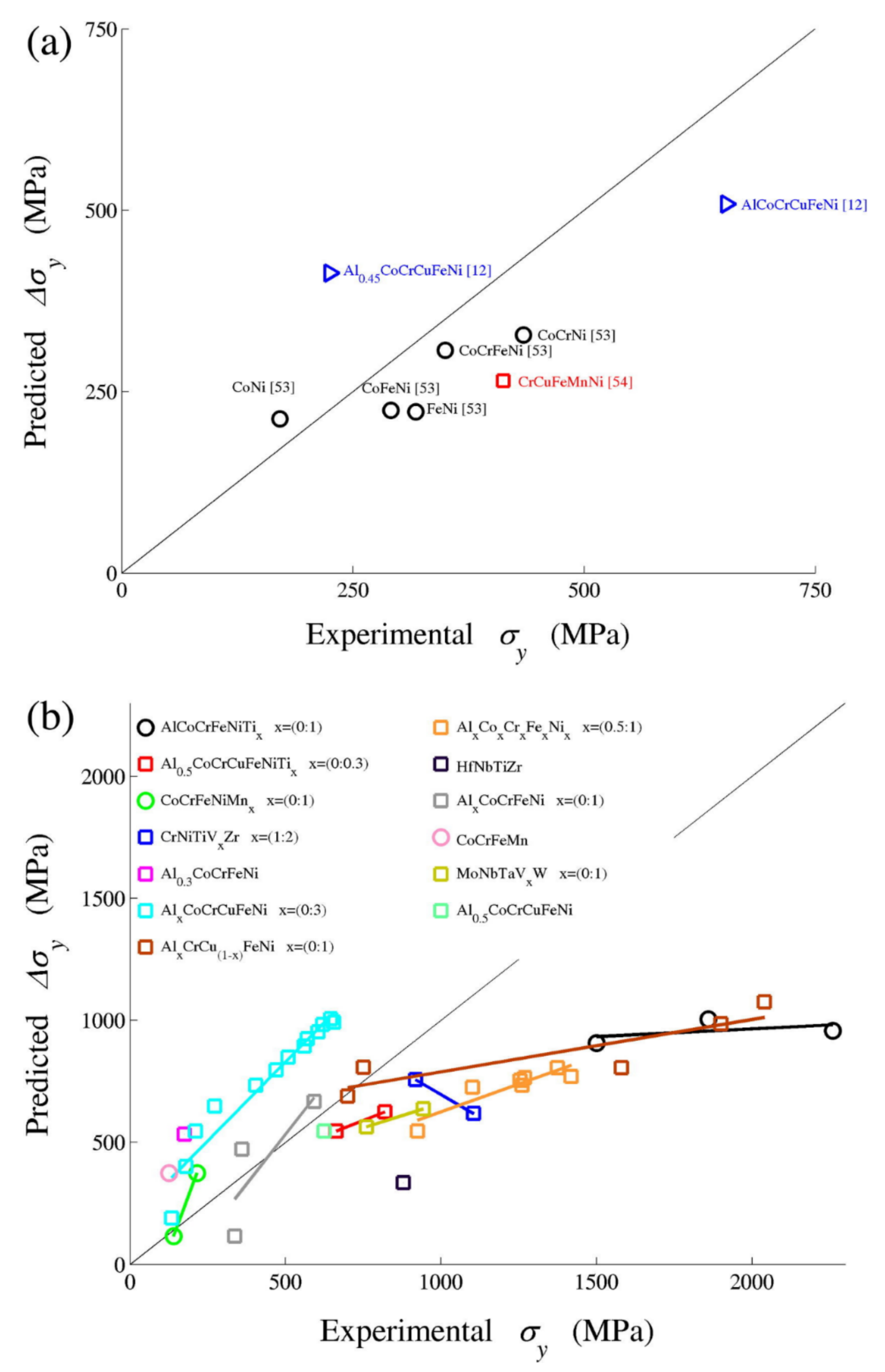
| Predictions of yield strength values relative to experimental values | The quantitative predictions are elusive [62] | The model prediction of the strength versus temperature and strain rate is very good for alloys NiCoFeCr and NiCoFeCrMn, with no fitting parameters [73]. However, the predictions are below the experiments at lowest temperatures (77 K). For the studied alloys NiCo; NiFe; NiCoFe and NiCoCr at the temperature of 293 K, the predictions are reasonably accurate, similar to those of simpler dilute binary alloys [73]. | Agreement is good for limited alloys studied, the observable deviation is attributed to accuracy of elastic misfit and for other interactions, such as stacking faults, valence, short range order and long-range order. |
| Drawbacks | Since only solutes along the glide plane are considered, the model misses the interaction energies of solutes off the glide plane, which are substantial in the vicinity of the dislocation. | The model does not consider atomic fluctuations at the scale of b < ζ, wc because the line tension concept would be invalid. Although such fluctuations are not calculable, they could generate small additional energy barriers that would contribute to strengthening at zero temperature but are ineffective at finite temperatures. | The computation of unit cell parameters of a HEA shows an overestimate for BCC HEAs and an underestimate for FCC HEAs, and thus a correction factor is involved in the calculations. |
| Application of the model at concentrations of the order of 1%, typical of engineering alloys, is questionable [62]. | The solute/dislocation interaction energies may not be easily computable in real materials. | ||
| The model suffer difficulty to describe material with complex chemical structures, i.e., precipitates, mixed FCC plus BCC structure. | |||
| The model is applicable only when the solute obstacles are strong and have a low concentration. | Line tension effect is not precisely known [60]. | ||
| More accurate and detailed calculations of misfit volume, dislocation core structures and interaction energies with solutes are needed. | |||
| The models are describing the solid solution strengthening for substitutional elements but do not attempt to include the distinctive interstitial elements. | |||
| Models do not include a particularly important electronic contribution to solute–dislocation interaction. | |||
3.2. Precipitation and Dispersion Strengthening
3.3. Grain Boundary Strengthening
4. The Impact of Processing Route on Alloy Performance
5. The Future of High Entropy Superalloys
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| HEAs | High entropy alloys |
| HESAs | High entropy superalloys |
| FCC | Face-centered cubic |
| BCC | Body-centered cubic |
| HCP | Hexagonal close-packed |
| VEC | Valence electron concentration |
| CALPHAD | Calculations of phase diagram |
| TWIP | Twinning-induced plasticity |
| XRD | X-ray diffraction |
| EDX | Energy dispersive X-ray |
| EBSD | Electron backscatter diffraction |
| SAED | Selected area electron diffraction |
| TEM | Transmission electron microscope |
| SEM | Scanning electron microscope |
| HAADF | High-angle annular dark field |
| APT | Atom probe tomography |
| 3d-TM | 3d-transition-metals |
| L12 | Ordered FCC structure |
| A1 | Disordered FCC structure |
| A2 | Disordered BCC structure |
| B2 | Ordered BCC structure |
| CCA | Complex concentrated alloys |
| MA | Mechanical alloying |
| SPS | Spark plasma sintering |
References
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.; Knight, P.; Vincent, A. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375-377, 213–218. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, H.Y.; Xie, Y.C.; Tang, Q.H.; Dai, P.Q. Controllable fabrication of a carbide-containing FeCoCrNiMn high-entropy alloy: Microstructure and mechanical properties. Mater. Sci. Technol. 2017, 33, 2032–2039. [Google Scholar] [CrossRef]
- Gwalani, B.; Soni, V.; Choudhuri, D.; Lee, M.; Hwang, J.; Nam, S.; Ryu, H.J.; Hong, S.H.; Banerjee, R. Stability of ordered L12 and B2 precipitates in face centered cubic based high entropy alloys—Al0.3CoFeCrNi and Al0.3CuFeCrNi2. Scr. Mater. 2016, 123, 130–134. [Google Scholar] [CrossRef]
- Slone, C.; George, E.; Mills, M. Elevated temperature microstructure evolution of a medium-entropy CrCoNi superalloy containing Al,Ti. J. Alloys Compd. 2019, 817, 152777. [Google Scholar] [CrossRef]
- Stepanov, N.; Shaysultanov, D.; Tikhonovsky, M.; Zherebtsov, S. Structure and high temperature mechanical properties of novel non-equiatomic Fe-(Co, Mn)-Cr-Ni-Al-(Ti) high entropy alloys. Intermetallics 2018, 102, 140–151. [Google Scholar] [CrossRef] [Green Version]
- Sathiyamoorthi, P.; Basu, J.; Kashyap, S.; Pradeep, K.; Kottada, R.S. Thermal stability and grain boundary strengthening in ultrafine-grained CoCrFeNi high entropy alloy composite. Mater. Des. 2017, 134, 426–433. [Google Scholar] [CrossRef]
- He, J.; Wang, H.; Wu, Y.; Liu, X.; Mao, H.; Nieh, T.; Lu, Z. Precipitation behavior and its effects on tensile properties of FeCoNiCr high-entropy alloys. Intermetallics 2016, 79, 41–52. [Google Scholar] [CrossRef] [Green Version]
- Praveen, S.; Anupam, A.; Tilak, R.; Kottada, R.S. Phase evolution and thermal stability of AlCoCrFe high entropy alloy with carbon as unsolicited addition from milling media. Mater. Chem. Phys. 2018, 210, 57–61. [Google Scholar] [CrossRef]
- Shivam, V.; Basu, J.; Pandey, V.K.; Shadangi, Y.; Mukhopadhyay, N. Alloying behaviour, thermal stability and phase evolution in quinary AlCoCrFeNi high entropy alloy. Adv. Powder Technol. 2018, 29, 2221–2230. [Google Scholar] [CrossRef]
- Yeh, A.C.; Tsao, T.K.; Chang, Y.J.; Chang, K.C.; Yeh, J.W.; Chiou, M.S.; Jian, S.R.; Kuo, C.M.; Wang, W.R.; Murakami, H. Developing New Type of High Temperature Alloys–High Entropy Superalloys. Int. J. Metall. Mater. Eng. 2015, 1, 107. [Google Scholar] [CrossRef]
- Keil, T.; Bruder, E.; Durst, K. Exploring the compositional parameter space of high-entropy alloys using a diffusion couple approach. Mater. Des. 2019, 176, 107816. [Google Scholar] [CrossRef]
- Wang, P.; Cai, H.; Zhou, S.; Xu, L. Processing, microstructure and properties of Ni1.5CoCuFeCr0.5−xVx high entropy alloys with carbon introduced from process control agent. J. Alloys Compd. 2017, 695, 462–475. [Google Scholar] [CrossRef]
- Kong, T.; Kang, B.; Ryu, H.J.; Hong, S.H. Microstructures and enhanced mechanical properties of an oxide dispersion-strengthened Ni-rich high entropy superalloy fabricated by a powder metallurgical process. J. Alloys Compd. 2020, 839, 155724. [Google Scholar] [CrossRef]
- Fang, S.; Chen, W.; Fu, Z. Microstructure and mechanical properties of twinned Al0.5CrFeNiCo0.3C0.2 high entropy alloy processed by mechanical alloying and spark plasma sintering. Mater. Des. 2014, 54, 973–979. [Google Scholar] [CrossRef]
- Yim, D.; Sathiyamoorthi, P.; Hong, S.-J.; Kim, H.S. Fabrication and mechanical properties of TiC reinforced CoCrFeMnNi high-entropy alloy composite by water atomization and spark plasma sintering. J. Alloys Compd. 2018, 781, 389–396. [Google Scholar] [CrossRef]
- Masemola, K.; Popoola, P.; Malatji, N. The effect of annealing temperature on the microstructure, mechanical and electrochemical properties of arc-melted AlCrFeMnNi equi-atomic High entropy alloy. J. Mater. Res. Technol. 2020, 9, 5241–5251. [Google Scholar] [CrossRef]
- Munitz, A.; Kaufman, M.; Nahmany, M.; Derimow, N.; Abbaschian, R. Microstructure and mechanical properties of heat treated Al1.25CoCrCuFeNi high entropy alloys. Mater. Sci. Eng. A 2018, 714, 146–159. [Google Scholar] [CrossRef]
- Senkov, O.N.; Isheim, D.; Seidman, D.N.; Pilchak, A.L. Development of a Refractory High Entropy Superalloy. Entropy 2016, 18, 102. [Google Scholar] [CrossRef]
- Detrois, M.; Jablonski, P.D.; Antonov, S.; Li, S.; Ren, Y.; Tin, S.; Hawk, J.A. Design and thermomechanical properties of a γʹ precipitate-strengthened Ni-based superalloy with high entropy γ matrix. J. Alloys Compd. 2019, 792, 550–560. [Google Scholar] [CrossRef] [Green Version]
- Waseem, O.A.; Ryu, H.J. Powder Metallurgy Processing of a WxTaTiVCr High-Entropy Alloy and Its Derivative Alloys for Fusion Material Applications. Sci. Rep. 2017, 7, 1926. [Google Scholar] [CrossRef]
- Dong, Y.; Yao, Z.; Huang, X.; Du, F.; Li, C.; Chen, A.; Wu, F.; Cheng, Y.; Zhang, Z. Microstructure and mechanical properties of AlCoxCrFeNi3-x eutectic high-entropy-alloy system. J. Alloys Compd. 2020, 823, 153886. [Google Scholar] [CrossRef]
- Tsao, T.-K.; Yeh, A.-C.; Kuo, C.-M.; Murakami, H. On The Superior High Temperature Hardness of Precipitation Strengthened High Entropy Ni-Based Alloys. Adv. Eng. Mater. 2016, 19, 1600475. [Google Scholar] [CrossRef]
- Ming, K.; Bi, X.; Wang, J. Realizing strength-ductility combination of coarse-grained Al0.2Co1.5CrFeNi1.5Ti0.3 alloy via nano-sized, coherent precipitates. Int. J. Plast. 2018, 100, 177–191. [Google Scholar] [CrossRef]
- Daoud, H.M.; Manzoni, A.; Wanderka, N.; Glatzel, U. High-Temperature Tensile Strength of Al10Co25Cr8Fe15Ni36Ti6 Compositionally Complex Alloy (High-Entropy Alloy). JOM 2015, 67, 2271–2277. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, W.; Fu, L.; Wang, J.; Luo, R.; Han, X.; Chen, B.; Wang, X. Effect of coherent L12 nanoprecipitates on the tensile behavior of a fcc-based high-entropy alloy. Mater. Sci. Eng. A 2017, 696, 503–510. [Google Scholar] [CrossRef]
- Zheng, F.; Zhang, G.; Chen, X.; Yang, X.; Yang, Z.; Li, Y.; Li, J. A new strategy of tailoring strength and ductility of CoCrFeNi based high-entropy alloy. Mater. Sci. Eng. A 2020, 774, 138940. [Google Scholar] [CrossRef]
- Niu, S.; Kou, H.; Guo, T.; Zhang, Y.; Wang, J.; Li, J. Strengthening of nanoprecipitations in an annealed Al0.5CoCrFeNi high entropy alloy. Mater. Sci. Eng. A 2016, 671, 82–86. [Google Scholar] [CrossRef]
- Praveen, S.; Kim, H.S. High-Entropy Alloys: Potential Candidates for High-Temperature Applications—An Overview. Adv. Eng. Mater. 2017, 20, 1700645. [Google Scholar] [CrossRef]
- Miracle, D.; Senkov, O. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Zhang, W.; Liaw, P.K.; Zhang, Y. Science and technology in high-entropy alloys. Sci. China Mater. 2018, 61, 2–22. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.-L.; Mao, H.; Chen, Q. Database development and Calphad calculations for high entropy alloys: Challenges, strategies, and tips. Mater. Chem. Phys. 2017, 210, 279–290. [Google Scholar] [CrossRef]
- Wu, M.; Wang, S.; Huang, H.; Shu, D.; Sun, B. CALPHAD aided eutectic high-entropy alloy design. Mater. Lett. 2019, 262, 127175. [Google Scholar] [CrossRef]
- Wang, W.; Chen, H.-L.; Larsson, H.; Mao, H. Thermodynamic constitution of the Al–Cu–Ni system modeled by CALPHAD and ab initio methodology for designing high entropy alloys. Calphad 2019, 65, 346–369. [Google Scholar] [CrossRef]
- Manzoni, A.M.; Glatzel, U. New multiphase compositionally complex alloys driven by the high entropy alloy approach. Mater. Charact. 2018, 147, 512–532. [Google Scholar] [CrossRef]
- Mishra, R.S.; Haridas, R.S.; Agrawal, P. High entropy alloys—Tunability of deformation mechanisms through integration of compositional and microstructural domains. Mater. Sci. Eng. A 2021, 812, 141085. [Google Scholar] [CrossRef]
- Gao, M.C.; Yeh, J.W.; Liaw, P.K.; Zhang, Y. High-Entropy Alloys; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Miracle, D.B.; Tsai, M.-H.; Senkov, O.N.; Soni, V.; Banerjee, R. Refractory high entropy superalloys (RSAs). Scr. Mater. 2020, 187, 445–452. [Google Scholar] [CrossRef]
- Senkov, O.N.; Woodward, C.; Miracle, D.B. Microstructure and Properties of Aluminum-Containing Refractory High-Entropy Alloys. JOM 2014, 66, 2030–2042. [Google Scholar] [CrossRef]
- Senkov, O.; Senkova, S.; Woodward, C. Effect of aluminum on the microstructure and properties of two refractory high-entropy alloys. Acta Mater. 2014, 68, 214–228. [Google Scholar] [CrossRef]
- Whitfield, T.E.; Pickering, E.J.; Owen, L.R.; Senkov, O.N.; Miracle, D.B.; Stone, H.J.; Jones, N.G. An assessment of the thermal stability of refractory high entropy superalloys. J. Alloys Compd. 2020, 857, 157583. [Google Scholar] [CrossRef]
- Soni, V.; Senkov, O.N.; Gwalani, B.; Miracle, D.B.; Banerjee, R. Microstructural Design for Improving Ductility of An Initially Brittle Refractory High Entropy Alloy. Sci. Rep. 2018, 8, 8816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senkov, O.N.; Miracle, D.B.; Chaput, K.J.; Couzinie, J.-P. Development and exploration of refractory high entropy alloys—A review. J. Mater. Res. 2018, 33, 3092–3128. [Google Scholar] [CrossRef] [Green Version]
- Soni, V.; Senkov, O.; Couzinie, J.-P.; Zheng, Y.; Gwalani, B.; Banerjee, R. Phase stability and microstructure evolution in a ductile refractory high entropy alloy Al10Nb15Ta5Ti30Zr40. Materialia 2019, 9, 100569. [Google Scholar] [CrossRef]
- Yeh, A.-C.; Chang, Y.-J.; Tsai, C.-W.; Wang, Y.-C.; Yeh, J.-W.; Kuo, C.-M. On the Solidification and Phase Stability of a Co-Cr-Fe-Ni-Ti High-Entropy Alloy. Met. Mater. Trans. A 2013, 45, 184–190. [Google Scholar] [CrossRef]
- Chuang, M.; Tsai, M.; Wang, W.; Lin, S.; Yeh, J. Microstructure and wear behavior of AlxCo1. 5CrFeNi1. 5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Daoud, H.M.; Manzoni, A.; Volkl, R.; Wanderka, N.; Glatzel, U. Microstructure and Tensile Behavior of Al8Co17Cr17Cu8Fe17Ni33 (at.%) High-Entropy Alloy. JOM 2013, 65, 1805–1814. [Google Scholar] [CrossRef]
- Manzoni, A.M.; Daoud, H.M.; Voelkl, R.; Glatzel, U.; Wanderka, N. Influence of W, Mo and Ti trace elements on the phase separation in Al8Co17Cr17Cu8Fe17Ni33 based high entropy alloy. Ultramicroscopy 2015, 159, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Pickering, E.; Stone, H.; Jones, N. Fine-scale precipitation in the high-entropy alloy Al0.5CrFeCoNiCu. Mater. Sci. Eng. A 2015, 645, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Tsao, T.-K.; Yeh, A.-C.; Murakami, H. The Microstructure Stability of Precipitation Strengthened Medium to High Entropy Superalloys. Met. Mater. Trans. A 2017, 48, 2435–2442. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, H.; Lu, Z.; Nieh, T. Thermal stability and coarsening of coherent particles in a precipitation-hardened (NiCoFeCr)94Ti2Al4 high-entropy alloy. Acta Mater. 2018, 147, 184–194. [Google Scholar] [CrossRef]
- Kang, B.; Kong, T.; Ryu, H.J.; Hong, S.H. The outstanding tensile strength of Ni-rich high entropy superalloy fabricated by powder metallurgical process. Mater. Chem. Phys. 2019, 235, 121749. [Google Scholar] [CrossRef]
- Shafiee, A.; Moon, J.; Kim, H.S.; Jahazi, M.; Nili-Ahmadabadi, M. Precipitation behaviour and mechanical properties of a new wrought high entropy superalloy. Mater. Sci. Eng. A 2019, 749, 271–280. [Google Scholar] [CrossRef]
- Dieter, G.E. Mechanical Metallurgy, SI Metric; McGraw-Hill Book: Singapore, 1988. [Google Scholar]
- Varvenne, C.; Leyson, G.; Ghazisaeidi, M.; Curtin, W. Solute strengthening in random alloys. Acta Mater. 2017, 124, 660–683. [Google Scholar] [CrossRef] [Green Version]
- Varvenne, C.; Luque, A.; Curtin, W.A. Theory of strengthening in fcc high entropy alloys. Acta Mater. 2016, 118, 164–176. [Google Scholar] [CrossRef] [Green Version]
- Toda-Caraballo, I.; Rivera-Díaz-Del-Castillo, P.E. Modelling solid solution hardening in high entropy alloys. Acta Mater. 2015, 85, 14–23. [Google Scholar] [CrossRef]
- Gil Coury, F.; Wilson, P.; Clarke, K.; Kaufman, M.J.; Clarke, A.J. High-throughput solid solution strengthening characterization in high entropy alloys. Acta Mater. 2019, 167, 1–11. [Google Scholar] [CrossRef]
- LaRosa, C.R.; Shih, M.; Varvenne, C.; Ghazisaeidi, M. Solid solution strengthening theories of high-entropy alloys. Mater. Charact. 2019, 151, 310–317. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Gao, Y.; Bei, H. Thermal activation mechanisms and Labusch-type strengthening analysis for a family of high-entropy and equiatomic solid-solution alloys. Acta Mater. 2016, 120, 108–119. [Google Scholar] [CrossRef] [Green Version]
- Leyson, G.; Curtin, W. Friedel vs. Labusch: The strong/weak pinning transition in solute strengthened metals. Philos. Mag. 2013, 93, 2428–2444. [Google Scholar] [CrossRef]
- He, J.; Wang, H.; Huang, H.; Xu, X.; Chen, M.; Wu, Y.; Liu, X.; Nieh, T.; An, K.; Lu, Z. A precipitation-hardened high-entropy alloy with outstanding tensile properties. Acta Mater. 2016, 102, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Gao, B.; Wang, Y.; Chen, X.; Xin, Y.; Tang, S.; Liu, B.; Liu, Y.; Song, M. Microstructures and mechanical properties of nano carbides reinforced CoCrFeMnNi high entropy alloys. J. Alloys Compd. 2019, 792, 170–179. [Google Scholar] [CrossRef]
- Basu, I.; De Hosson, J.T. Strengthening mechanisms in high entropy alloys: Fundamental issues. Scr. Mater. 2020, 187, 148–156. [Google Scholar] [CrossRef]
- Bracq, G.; Laurent-Brocq, M.; Varvenne, C.; Perrière, L.; Curtin, W.; Joubert, J.-M.; Guillot, I. Combining experiments and modeling to explore the solid solution strengthening of high and medium entropy alloys. Acta Mater. 2019, 177, 266–279. [Google Scholar] [CrossRef] [Green Version]
- Leyson, G.P.M.; Curtin, W.A. Solute strengthening at high temperatures. Model. Simul. Mater. Sci. Eng. 2016, 24, 065005. [Google Scholar] [CrossRef] [Green Version]
- Toda-Caraballo, I. A general formulation for solid solution hardening effect in multicomponent alloys. Scr. Mater. 2017, 127, 113–117. [Google Scholar] [CrossRef]
- Yoshida, S.; Ikeuchi, T.; Bhattacharjee, T.; Bai, Y.; Shibata, A.; Tsuji, N. Effect of elemental combination on friction stress and Hall-Petch relationship in face-centered cubic high/medium entropy alloys. Acta Mater. 2019, 171, 201–215. [Google Scholar] [CrossRef]
- Okamoto, N.; Yuge, K.; Tanaka, K.; Inui, H.; George, E. Atomic displacement in the CrMnFeCoNi high-entropy alloy—A scaling factor to predict solid solution strengthening. AIP Adv. 2016, 6, 125008. [Google Scholar] [CrossRef]
- George, E.; Curtin, W.; Tasan, C. High entropy alloys: A focused review of mechanical properties and deformation mechanisms. Acta Mater. 2019, 188, 435–474. [Google Scholar] [CrossRef]
- Walbrühl, M.; Linder, D.; Ågren, J.; Borgenstam, A. Modelling of solid solution strengthening in multicomponent alloys. Mater. Sci. Eng. A 2017, 700, 301–311. [Google Scholar] [CrossRef]
- Varvenne, C.; Curtin, W.A. Strengthening of high entropy alloys by dilute solute additions: CoCrFeNiAl x and CoCrFeNiMnAl x alloys. Scr. Mater. 2017, 138, 92–95. [Google Scholar] [CrossRef] [Green Version]
- Yin, B.; Maresca, F.; Curtin, W. Vanadium is an optimal element for strengthening in both fcc and bcc high-entropy alloys. Acta Mater. 2020, 188, 486–491. [Google Scholar] [CrossRef]
- Gladman, T. Precipitation hardening in metals. Mater. Sci. Technol. 1999, 15, 30–36. [Google Scholar] [CrossRef]
- Abd El-Aty, A.; Xu, Y.; Guo, X.; Zhang, S.H.; Ma, Y.; Chen, D. Strengthening mechanisms, deformation behavior, and anisotropic mechanical properties of Al-Li alloys: A review. J. Adv. Res. 2018, 10, 49–67. Available online: https://www.researchgate.net/figure/Schematic-representation-of-the-interaction-modes-between-ordered-precipitates-of-Al-3-Li_fig5_322073696 (accessed on 6 September 2021). [CrossRef] [PubMed]
- Tsao, T.-K.; Yeh, A.-C.; Kuo, C.-M.; Kakehi, K.; Murakami, H.; Yeh, J.-W.; Jian, S.-R. The High Temperature Tensile and Creep Behaviors of High Entropy Superalloy. Sci. Rep. 2017, 7, 12658. [Google Scholar] [CrossRef]
- Tsao, T.K.; Yeh, A.C.; Kuo, C.M.; Murakami, H. High temperature oxidation and corrosion properties of high entropy superalloys. Entropy. 2016, 18, 62. [Google Scholar] [CrossRef] [Green Version]
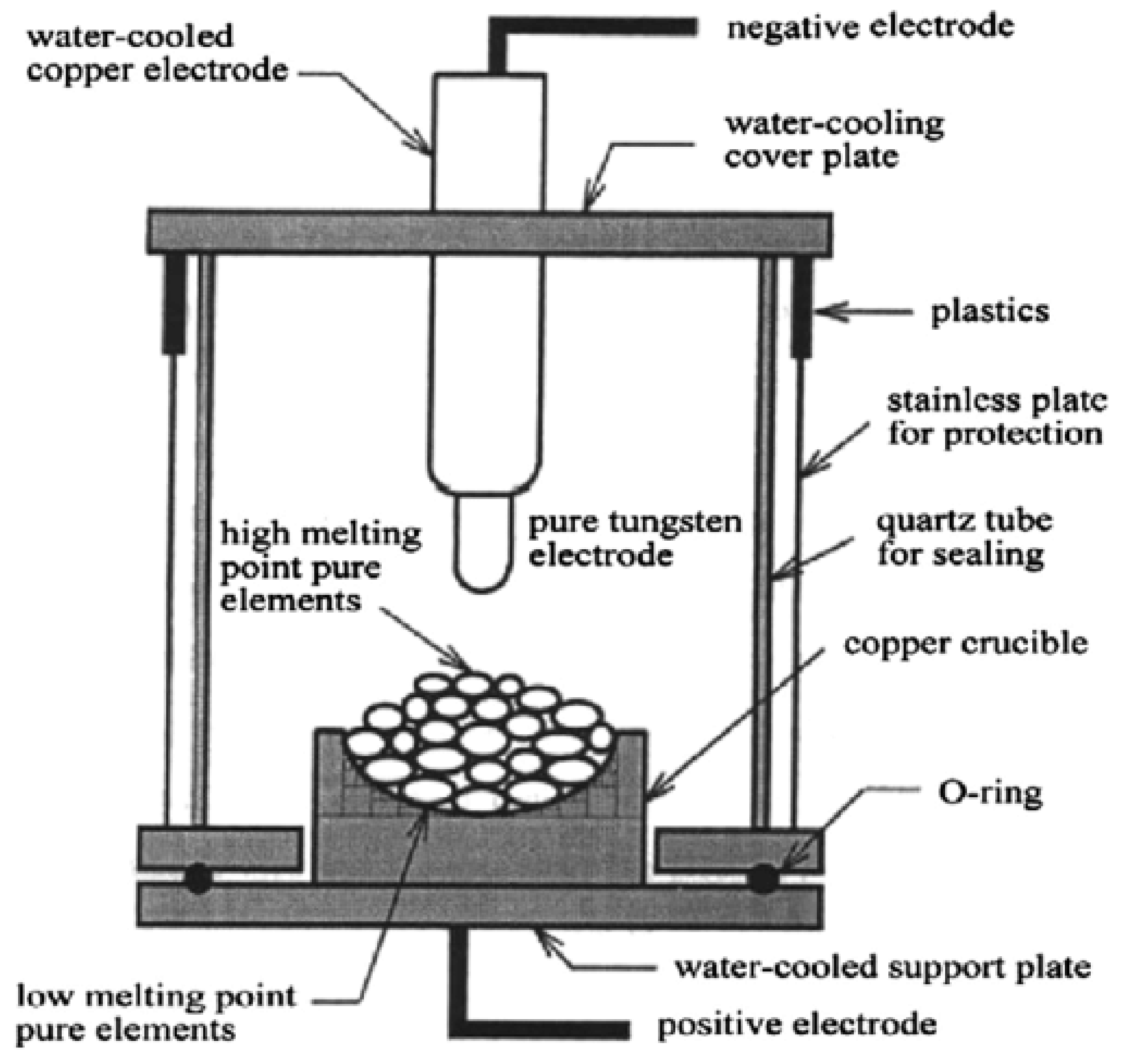
- Microstructures and Properties of High Entropy Alloys, Scientific Figure on Researchgate. Available online: https://www.researchgate.net/figure/A-schematic-diagram-of-the-arc-melting-method-99_fig17_259887707 (accessed on 6 September 2021).
- Senkov, O.; Couzinie, J.-P.; Rao, S.; Soni, V.; Banerjee, R. Temperature dependent deformation behavior and strengthening mechanisms in a low density refractory high entropy alloy Al10Nb15Ta5Ti30Zr40. Materialia 2020, 9, 100627. [Google Scholar] [CrossRef]
- Journal of Material Science. Adopted from: Flexible Scanner-Based Laser Surface Treatment,” Scientific Figure on ResearchGate. Available online: https://www.researchgate.net/figure/Principle-of-the-laser-cladding-process_fig1_239028642 (accessed on 6 September 2021).
- Shaysultanov, D.; Salishchev, G.; Ivanisenko, Y.; Zherebtsov, S.; Tikhonovsky, M.; Stepanov, N. Novel Fe36Mn21Cr18Ni15Al10 high entropy alloy with bcc/B2 dual-phase structure. J. Alloys Compd. 2017, 705, 756–763. [Google Scholar] [CrossRef]
- Moravcikova-Gouvea, L.; Moravcik, I.; Omasta, M.; Veselý, J.; Cizek, J.; Minárik, P.; Cupera, J.; Záděra, A.; Jan, V.; Dlouhy, I. High-strength Al0.2Co1.5CrFeNi1.5Ti high-entropy alloy produced by powder metallurgy and casting: A comparison of microstructures, mechanical and tribological properties. Mater. Charact. 2019, 159, 110046. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, W.; Wen, H.; Zhang, D.Z.; Chen, Z.; Zheng, B.; Zhou, Y.; Lavernia, E.J. Microstructure and strengthening mechanisms in an FCC structured single-phase nanocrystalline Co25Ni25Fe25Al7.5Cu17.5 high-entropy alloy. Acta Mater. 2016, 107, 59–71. [Google Scholar] [CrossRef]
- Moravcik, I.; Cizek, J.; Zapletal, J.; Kovacova, Z.; Vesely, J.; Minarik, P.; Kitzmantel, M.; Neubauer, E.; Dlouhy, I. Microstructure and mechanical properties of Ni1,5Co1,5CrFeTi0,5 high entropy alloy fabricated by mechanical alloying and spark plasma sintering. Mater. Des. 2017, 119, 141–150. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, S.; Qiao, J.; Wang, Z.; Gao, M.; Jiao, Z.; Yang, H.; Zhang, Y. Superior high tensile elongation of a single-crystal CoCrFeNiAl0.3 high-entropy alloy by Bridgman solidification. Intermetallics 2014, 54, 104–109. [Google Scholar] [CrossRef]
- Zuo, T.; Li, R.; Ren, J.; Zhang, A. Effects of Al and Si addition on the structure and properties of CoFeNi equal atomic ratio alloy. J. Magn. Magn. Mater. 2014, 371, 60–68. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Wang, Y.; Chen, G. Microstructure and compressive properties of multicomponent Alx(TiVCrMnFeCoNiCu)100−x high-entropy alloys. Mater. Sci. Eng. A 2007, 454-455, 260–265. [Google Scholar] [CrossRef]
- Senkov, O.; Senkova, S.; Woodward, C.; Miracle, D. Low-density, refractory multi-principal element alloys of the Cr–Nb–Ti–V–Zr system: Microstructure and phase analysis. Acta Mater. 2013, 61, 1545–1557. [Google Scholar] [CrossRef]
- Couzinié, J.-P.; Senkov, O.; Miracle, D.; Dirras, G. Comprehensive data compilation on the mechanical properties of refractory high-entropy alloys. Data Brief 2018, 21, 1622–1641. [Google Scholar] [CrossRef]
- Wu, Y.; Cai, Y.; Chen, X.; Wang, T.; Si, J.; Wang, L.; Wang, Y.; Hui, X. Phase composition and solid solution strengthening effect in TiZrNbMoV high-entropy alloys. Mater. Des. 2015, 83, 651–660. [Google Scholar] [CrossRef]
- Miracle, D.; Majumdar, B.; Wertz, K.; Gorsse, S. New strategies and tests to accelerate discovery and development of multi-principal element structural alloys. Scr. Mater. 2017, 127, 195–200. [Google Scholar] [CrossRef]
- Ma, E. Unusual dislocation behavior in high-entropy alloys. Scr. Mater. 2020, 181, 127–133. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, X.; Wang, W.; Liu, B.; Liu, B.; Yang, W.; Xu, D.; Liu, Y. A review on fundamental of high entropy alloys with promising high–temperature properties. J. Alloys Compd. 2018, 760, 15–30. [Google Scholar] [CrossRef]





| Particle-Matrix Coherency (Δσcs) | Modulus Mismatch (Δσms) | Atomic Ordering (Δσos) |
|---|---|---|
| Alloy | Processing | Phases | ε˙(s−1) | Test | T (°C) | σy (MPa) | UTS (MPa) | εf (%) | Hv | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Al0.3CoCrFeNi | BC | SX [001] | 4 × 10 − 4 | T | 23 | 185 | 399 | ~80 | - | [30] |
| AC | FCC + L12 | 4 × 10 − 4 | 23 | 224 ± 51 | 434 ± 94 | 48 ± 10 | - | |||
| Al0.3CrCuFeNi2 | AC | FCC + L12 + Cu-rich phase | - | - | - | - | - | - | 171 ± 05 | [4] |
| 550 °C/150 h | - | - | - | - | - | - | 294 ± 09 | |||
| 700 °C/50 h | - | - | - | - | - | - | 273 ± 11 | |||
| Al0.3CoCrFeNi | AC | FCC + L12 | - | - | - | - | - | - | 152 ± 11 | [4] |
| 550 °C/150 h | FCC + B2 | - | - | - | - | - | - | 216 ± 07 | ||
| 700 °C/50 h | - | - | - | - | - | - | 259 ± 02 | |||
| Al0.5CoCrCu0.5FeNi2 | CR 43%, 1100 °C/24 | FCC + L12 | 1 × 10 − 3 | T | 23 | 360 ± 100 | 639 ± 5 | 3.4 ± 0.4 | - | [30] |
| Al0.5CoCrCu0.5FeNi2 | AC | FCC + L12 | 3.3 × 10 − 3 | T | RT | 357 | 459 | 9 | 275 | [48] |
| 700 °C/5 h | 365 | 365 | 0.1 | - | ||||||
| 1150 °C/5 h | 215 | 489 | 39 | - | ||||||
| AC | 500 | 315 ± 12 | 334 ± 1.0 | 0.7 ± 0.3 | - | |||||
| 700 °C/5 h | 310 ± 2.0 | 310 ± 2.0 | <0.02 | - | ||||||
| 1150 °C/5 h | 215 ± 11 | 248 ± 10 | 6.0 ± 3.0 | - | ||||||
| Al0.5CoCrCuFeNi | 1000 °C/6h, CR 84% | FCC + L12 | 1 × 10 − 3 | T | 23 | 1248 | 1344 | 7.6 | - | [30] |
| Al0.2CrCoCu0.2FeNi2 | 700 °C/20 h | FCC + L12 | 1.7 × 10 − 3 | T | RT | 719 | 1048 | 30.4 | - | [26] |
| 800 °C/1 h | 460 | 732 | 31.7 | - | ||||||
| Al0.7Co1.7Cr0.5FeNi2.4Ti0.4 | 1220 °C/20 h–900 °C/5 h | FCC + L12 | 0.83 × 10 − 4 | T | RT | 786 | 568 | 12 | - | [25] |
| 600 | 674 | 501 | 26 | - | ||||||
| 700 | 702 | 487 | 18 | - | ||||||
| 800 | 672 | 535 | 27 | - | ||||||
| 1000 | 148 | – | 92 | - | ||||||
| 1220 °C/20 h–900 °C/50 h | FCC + L12 | 0.83 × 10 − 4 | T | RT | 1039 | 596 | 20 | - | ||
| 600 | 809 | 509 | 27 | - | ||||||
| 700 | 624 | 486 | 11 | - | ||||||
| 800 | 687 | 581 | 9 | - | ||||||
| Al8Co17Cr14Cu8Fe17Ni34.8W0.1Mo0.1Ti1 | AC | FCC + L12 | - | - | - | - | - | - | 225 | [49] |
| 700 °C/24 h | FCC + L12 | - | - | - | - | - | - | - | ||
| (FeCoNiCr)94Ti2Al4 | CR 70%, 650 °C/4 h | FCC + L12 + L21 | 1 × 10 − 3 | T | RT | 1005 | 1273 | 17 | [8] | |
| CR 30%, 1000 °C/2h, 800 °C/18 h | FCC + L12 + L21 | T | RT | 645 | 1094 | 39 | ||||
| Co1.5CrFeNi1.5Ti0.5 | MA & SPS | FCC + η + L12 | 0.25 × 10 − 4 | T | After SPS | 1289 ± 6.5 | 1569 ± 11.5 | 6.15 ± 0.59 | 472 ± 27 | [85] |
| 700 °C | 1388 ± 36.8 | 1661 ± 13.5 | 4.35 ± 0.19 | 515 ± 19 | ||||||
| 900 °C | 886 ± 3.5 | 1236 ± 0.5 | 9.73 ± 0.04 | 386 ± 13 | ||||||
| 1100 °C | 1048 | 1467 | 14.43 | 384 ± 88 | ||||||
| Co1.5CrFeNi1.5Ti | AC | FCC + L12 | - | - | - | - | - | - | 654 | [47] |
| Ni45(FeCoCr)40(AlTi)15 | AC | FCC + L12 | 1 × 10 − 3 | C | RT | 1110 | - | - | - | [32] |
| 750 | 855 | 1569 | - | - | ||||||
| 850 | 796 | 951 | - | - | ||||||
| 950 | 560 | 597 | - | - | ||||||
| Al0.2Co1.5CrFeNi1.5Ti0.3 | 1150 °C/3 h | FCC + L12 | 1 × 10 − 3 | T | RT | 540 | 917 | 50 | - | [24] |
| 1150 °C/3 h + 800 °C/5 h | 760 | 1160 | 40 | - | ||||||
| CoFeNiSi0.5 | AC | FCC + Ni3Si | 2 × 10 − 4 | C | RT | 476 | 2250 − 2500 | 40 − 50 | 287 | [87] |
| CoFeNiSi0.75 | AC | FCC + Ni3Si | 2 × 10 − 4 | C | RT | 1301 | 2000 | 0 − 5 | 570 | |
| Al0.3Cr0.5Mn0.6FeNi0.4 | AC | BCC + B2 + Minor FCC | 10 − 4 | C | 25 | 750 | 880 | 2.5 | 420 ± 10 | [82] |
| 400 | 640 | 900 | 20 | - | ||||||
| 500 | 515 | 715 | 42 | - | ||||||
| 600 | 310 | 404 | 55 | - | ||||||
| Al40(CoCrCuFeMnNiTiV)60 | AC | BCC + B2 | 1 × 10−4 | C | RT | 1461 | 1461 | <1 | - | [88] |
| AlMo0.5NbTa0.5TiZr | HIP 1400 °C/207 MPa/2 h, 1400 °C/24 h | BCC + B2 | 10 − 3 | C | 23 | 2000 | 880 | - | - | [89] |
| 800 | 1597 | 900 | - | - | ||||||
| 1000 | 745 | 715 | - | - | ||||||
| 1200 | 255 | 405 | - | - | ||||||
| NbTiVZr | AC | BCC + B2 | 2 × 10 − 4 | C | RT | 1105 | - | >50 | 335 | [90] |
| TiZrNbVMo1.3. | AC | BCC + B2 | 2 × 10 − 4 | C | RT | 1496 | - | 30 | - | [91] |
| TiZrNbVMo1.5 | AC | BCC + B2 | 2 × 10 − 4 | RT | 1603 | - | 20 | - | ||
| TiZrNbVMo1.7 | AC | BCC + B2 | 2 × 10 − 4 | RT | 1645 | - | 15 | - | ||
| TiZrNbVMo2.0 | AC | BCC + B2 | 2 × 10 − 4 | RT | 1765 | - | 12 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joele, M.; Matizamhuka, W.R. A Review on the High Temperature Strengthening Mechanisms of High Entropy Superalloys (HESA). Materials 2021, 14, 5835. https://doi.org/10.3390/ma14195835
Joele M, Matizamhuka WR. A Review on the High Temperature Strengthening Mechanisms of High Entropy Superalloys (HESA). Materials. 2021; 14(19):5835. https://doi.org/10.3390/ma14195835
Chicago/Turabian StyleJoele, Malefane, and Wallace Rwisayi Matizamhuka. 2021. "A Review on the High Temperature Strengthening Mechanisms of High Entropy Superalloys (HESA)" Materials 14, no. 19: 5835. https://doi.org/10.3390/ma14195835






